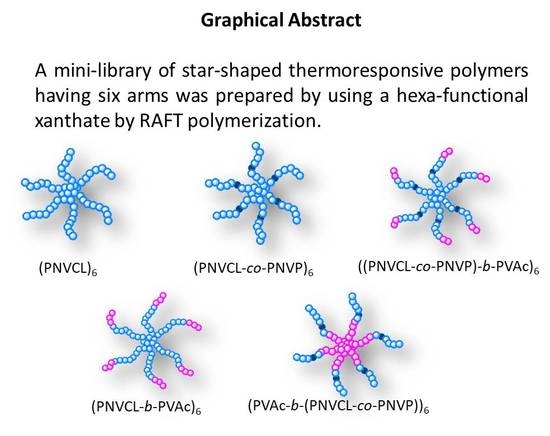
Preparation of a Mini-Library of Thermo-Responsive Star (NVCL/NVP-VAc) Polymers with Tailored Properties Using a Hexafunctional Xanthate RAFT Agent
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Measurements
2.3. Synthetic Methods
2.3.1. Preparation of the Hexafunctional Chain Transfer Agent (CTA-1 or R-RAFT Agent)
2.3.2. Preparation of Star (PNVCL) with Six Arms
2.3.3. Preparation of Star PNVCL-b-PVAc Block Copolymers with Six Arms
2.3.4. Preparation of Star PNVCL-co-PNVP Copolymers with Six Arms
2.3.5. Preparation of Star [(PNVCL-co-PNVP)-b-(PVAc))] Block Copolymers with Six Arms
2.3.6. Preparation of Star (PVAc) Polymers with Six Arms
2.3.7. Preparation of Star (PVAc-b-PNVCL) Block Copolymers with Six Arms
2.3.8. Preparation of Star [PVAc-b-(PNVCL-co-PNVP)] Block Copolymers with Six Arms
2.3.9. Removal of Xanthate End Groups from Star (PNVCL)6 Polymers
2.3.10. Preparation of Aggregates from Star Block Copolymers
2.3.11. Preparation of MTX Loaded Aggregates
2.3.12. In Vitro Drug Release
3. Results and Discussion
3.1. Polymerization of NVCL Using R-Hexafunctional Xanthate RAFT Agent
3.2. Solution Properties and Thermosensitivity of Star (PNVCL)6 Polymers
3.3. Synthesis of Star Poly(N-vinylcaprolactam)-b-poly(vinyl acetate) Block Copolymers
3.4. Synthesis of Star {[Poly(N-vinylcaprolactam)-co-poly(N-vinylpirrolydone]-b-poly(vinyl acetate)} Copolymers
3.5. Synthesis of Star Poly(vinyl acetate)-b-poly(N-vinylcaprolactam) Block Copolymers
3.6. Drug Loading and Thermosensitive MTX Release Using [PVAc-b-(PNVCL-co-PNVP)]6 and (PNVCL-b-PVAc)6 Copolymers
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schaefgen, J.R.; Flory, P.J. Synthesis of Multichain Polymers and Investigation of their Viscosities. J. Am. Chem. Soc. 1948, 70, 2709–2718. [Google Scholar] [CrossRef]
- Li, W.; Matyjaszewski, K. Star Polymers via Cross-Linking Amphiphilic Macroinitiators by AGET ATRP in Aqueous Media. J. Am. Chem. Soc. 2009, 131, 10378–10379. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.M.; McKenzie, T.G.; Fu, Q.; Wong, E.H.; Xu, J.; An, Z.; Shanmugam, S.; Davis, T.P.; Boyer, C.; Qiao, G.G. Star Polymers. Chem. Rev. 2016, 116, 6743–6836. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, A.; Traverso, G.; Farokhzad, O.C.; Weissleder, R.; Langer, R. Evolution of macromolecular complexity in drug delivery systems. Nat. Rev. Chem. 2017, 1, 0063. [Google Scholar] [CrossRef]
- Wiltshire, J.T.; Qiao, G.G. Recent Advances in Star Polymer Design: Degradability and the Potential for Drug Delivery. Aust. J. Chem. 2007, 60, 699–705. [Google Scholar] [CrossRef]
- Bukhryakov, K.V.; Mugemana, C.; Vu, K.B.; Rodionov, V.O. Palladium N-Heterocyclic Carbene Precatalyst Site Isolated in the Core of a Star Polymer. Org. Lett. 2015, 17, 4826–4829. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Peng, H.; Thurecht, K.J.; Puttick, S.; Whittaker, A.K. Biodegradable Core Crosslinked Star Polymer Nanoparticles as 19f Mri Contrast Agents for Selective Imaging. Polym. Chem. 2014, 5, 1760–1771. [Google Scholar] [CrossRef]
- Fukukawa, K.; Rossin, R.; Hagooly, A.; Pressly, E.D.; Hunt, J.N.; Messmore, B.W.; Wooley, K.L.; Welch, M.J.; Hawker, C.J. Synthesis and Characterization of Core−Shell Star Copolymers for in Vivo Pet Imaging Applications. Biomacromolecules 2008, 9, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, J.L.; Trollsås, M.; Hawker, C.J.; Atthoff, B.; Claesson, H.; Heise, A.; Miller, R.D.; Mecerreyes, D.; Jérôme, R.; Dubois, P. Dendrimer-like Star Block and Amphiphilic Copolymers by Combination of Ring Opening and Atom Transfer Radical Polymerization. Macromolecules 1998, 31, 8691–8705. [Google Scholar] [CrossRef]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-Catalyzed Living Radical Polymerization. Chem. Rev. 2001, 101, 3689–3745. [Google Scholar] [CrossRef] [PubMed]
- Isono, T.; Kondo, Y.; Otsuka, I.; Nishiyama, Y.; Borsali, R.; Kakuchi, T.; Satoh, T. Synthesis and Stereocomplex Formation of Star-Shaped Stereoblock Polylactides Consisting of Poly(l-lactide) and Poly(d-lactide) Arms. Macromolecules 2013, 46, 8509–8518. [Google Scholar] [CrossRef]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New Polymer Synthesis by Nitroxide Mediated Living Radical Polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef] [PubMed]
- Goseki, R.; Ito, S.; Matsuo, Y.; Higashihara, T.; Hirao, A. Precise Synthesis of Macromolecular Architectures by Novel Iterative Methodology Combining Living Anionic Polymerization with Specially Designed Linking Chemistry. Polymers 2017, 9, 470. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pitsikalis, M.; Pispas, S.; Mays, J.W. Macromolecular Architectures by Living and Controlled/Living Polymerizations. Prog. Polym. Sci. 2006, 31, 1068–1132. [Google Scholar] [CrossRef]
- Polymeropoulos, G.; Zapsas, G.; Ntetsikas, K.; Bilalis, P.; Gnanou, Y.; Hadjichristidis, N. Polymers with Complex Architectures. Macromolecules 2017, 50, 1253–1290. [Google Scholar] [CrossRef]
- Hirao, A.; Goseki, R.; Ishizone, T. Advances in Living Anionic Polymerization: From Functional Monomers, Polymerization Systems, to Macromolecular Architectures. Macromolecules 2014, 47, 1883–1905. [Google Scholar] [CrossRef]
- Chaffey-Millar, H.; Stenzel, M.H.; Davis, T.P.; Coote, M.L.; Barner-Kowollik, C. Design Criteria for Star Polymer Formation Processes via Living Free Radical Polymerization. Macromolecules 2006, 39, 6406–6419. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Davis, T.P.; Stenzel, M.H. Synthesis of Star Polymers using RAFT Polymerization: What is Possible? Aust. J. Chem. 2006, 59, 719–727. [Google Scholar] [CrossRef]
- Stenzel, M.; Davis, T.P.; Fane, A.G.; Chen, V. Star-polymer synthesis via radical reversible addition–fragmentation chain-transfer polymerization. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2777–2783. [Google Scholar] [CrossRef]
- Carmean, N.E.; Figg, C.A.; Scheutz, G.M.; Kubo, T.; Sumerlin, B.S. Catalyst-Free Photoinduced End-Group Removal of Thiocarbonylthio Functionality. ACS Macro Lett. 2017, 6, 185–189. [Google Scholar] [CrossRef]
- Bian, Q.; Xiao, Y.; Lang, M. R-RAFT approach for the polymerization of N-isopropylacrylamide with a star poly(ε-caprolactone) core. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 571–580. [Google Scholar] [CrossRef]
- Belal, K.; Poitras-Jolicoeur, S.; Lyskawa, J.; Pembouong, G.; Cooke, G.; Woisel, P.; Stoffelbach, F. A triple carboxylic acid-functionalized RAFT agent platform for the elaboration of well-defined telechelic 3-arm star PDMAc. Chem. Commun. 2015, 52, 1847–1850. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, Z.; Huang, Z.; Ling, J. A CTA-shuttled R-group approach: A versatile synthetic tool towards well-defined functional cylindrical polymer brushes via RAFT polymerization. Polym. Chem. 2017, 8, 2659–2665. [Google Scholar] [CrossRef]
- Benoit, D.S.W.; Srinivasan, S.; Shubin, A.D.; Stayton, P.S. Synthesis of Folate-Functionalized RAFT Polymers for Targeted siRNA Delivery. Biomacromolecules 2011, 12, 2708–2714. [Google Scholar] [CrossRef] [PubMed]
- Perrier, S.; Takolpuckdee, P.; Mars, A.C. Reversible Addition−Fragmentation Chain Transfer Polymerization: End Group Modification for Functionalized Polymers and Chain Transfer Agent Recovery. Macromolecules 2005, 38, 2033–2036. [Google Scholar] [CrossRef]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Yokoyama, M. Clinical Applications of Polymeric Micelle Carrier Systems in Chemotherapy and Image Diagnosis of Solid Tumors. J. Exp. Clin. Med. 2011, 3, 151–158. [Google Scholar] [CrossRef]
- Owen, S.C.; Chana, D.P.Y.; Shoichet, M.S. Polymeric Micelle Stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Francis, M.F.; Cristea, M.; Winnik, F.M. Polymeric micelles for oral drug delivery: Why and how. Pure Appl. Chem. 2004, 76, 1321–1335. [Google Scholar] [CrossRef]
- Nishiyama, N.; Matsumura, Y.; Kataoka, K. Development of polymeric micelles for targeting intractable cancers. Cancer Sci. 2016, 107, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Jesson, C.P.; Pearce, C.M.; Simon, H.; Werner, A.; Cunningham, V.J.; Lovett, J.R.; Smallridge, M.J.; Warren, N.J.; Armes, S.P. H2O2 Enables Convenient Removal of RAFT End-Groups from Block Copolymer Nano-Objects Prepared via Polymerization-Induced Self-Assembly in Water. Macromolecules 2017, 50, 182–191. [Google Scholar] [CrossRef]
- García-Olaiz, G.D.; Montoya-Villegas, K.A.; Licea-Claverie, A.; Cortez-Lemus, N.A. Synthesis and characterization of four- and six-arm star-shaped poly(ε-caprolactone)-b-poly(N-vinylcaprolactam): Micellar and core degradation studies. React. Funct. Polym. 2015, 88, 16–23. [Google Scholar] [CrossRef]
- Cortez-Lemus, N.A.; Licea-Claverie, A. Synthesis and characterization of “Living” star-shaped poly(N-vinylcaprolactam) with four arms and carboxylic acid end groups. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2156–2165. [Google Scholar] [CrossRef]
- Cortez-Lemus, N.A.; Licea-Claverie, A. Star-shaped copolymers based on poly(N-vinylcaprolactam) and their use as nanocarriers of methotrexate. Aust. J. Chem. 2017, 70, 1291–1301. [Google Scholar] [CrossRef]
- Cortez-Lemus, N.A.; Licea-Claverie, A. Poly(N-vinylcaprolactam), a comprehensive review on a thermoresponsive polymer becoming popular. Prog. Polym. Sci. 2016, 53, 1–51. [Google Scholar] [CrossRef]
- Sun, S.; Wu, P. Infrared Spectroscopic Insight into Hydration Behavior of Poly(N-vinylcaprolactam) in Water. J. Phys. Chem. B 2011, 115, 11609–11618. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Zhou, Q.; Pu, H.; Yang, G. Controlled radical polymerization of N-vinylcaprolactam mediated by Xanthate or Dithiocarbamate. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3756–3765. [Google Scholar] [CrossRef]
- Harrisson, S.; Liu, X.; Ollagnier, J.N.; Coutelier, O.; Marty, J.D.; Destarac, M. RAFT Polymerization of Vinyl Esters: Synthesis and Applications. Polymers 2014, 6, 1437–1488. [Google Scholar] [CrossRef]
- Boschmann, D.; Vana, P. Poly(vinyl acetate) and Poly(vinyl propionate) Star Polymers via Reversible Addition Fragmentation Chain Transfer (RAFT) Polymerization. Polym. Bull. 2005, 53, 231–242. [Google Scholar] [CrossRef]
- Bernard, J.; Favier, A.; Zhang, L.; Nilasaroya, A.; Barner-Kowollik, C.; Davis, T.P.; Stenzel, M. Poly(vinyl ester) Star Polymers via Xanthate-Mediated Living Radical Polymerization: From Poly(vinyl alcohol) to Glycopolymer Stars. Macromolecules 2005, 38, 5475–5484. [Google Scholar] [CrossRef]
- Debuigne, A.; Willet, N.; Jérome, R.; Detrembleur, C. Amphiphilic Poly(vinyl acetate)-b-poly(N-vinylpyrrolidone) and Novel Double Hydrophilic Poly(vinyl alcohol)-b-poly(N-vinylpyrrolidone) Block Copolymers Prepared by Cobalt-Mediated Radical Polymerization. Macromolecules 2007, 40, 7111–7118. [Google Scholar] [CrossRef]
- Smith, A.A.; Hussmann, T.; Elich, J.; Postma, A.; Alve, M.H.; Zelikin, A.N. Macromolecular design of poly(vinyl alcohol) by RAFT polymerization. Polym. Chem. 2012, 3, 85–88. [Google Scholar] [CrossRef]
- Stenzel, M.H.; Davis, T.P.; Barner-Kowollik, C. Poly(vinyl alcohol) star polymers prepared via MADIX/RAFT polymerisation. Chem. Commun. 2004, 1546–1547. [Google Scholar] [CrossRef] [PubMed]
- Theis, A.; Davis, T.P.; Stenzel, M.H.; Barner-Kowollik, C. Probing the reaction kinetics of vinyl acetate free radical polymerization via living free radical polymerization (MADIX). Polymer 2006, 47, 999–1010. [Google Scholar] [CrossRef]
- Stenzel, M.H.; Cummins, L.; Roberts, G.E.; Davis, T.P.; Vana, P.; Barner-Kowollik, C. Xanthate Mediated Living Polymerization of Vinyl Acetate: A Systematic Variation in MADIX/RAFT Agent Structure. Macromol. Chem. Phys. 2003, 204, 1160–1168. [Google Scholar] [CrossRef]
- Chernikova, E.V.; Yulusov, V.V.; Mineeva, K.O.; Golubev, V.B.; Garina, E.S. Pseudoliving polymerization of vinyl acetate mediated by reversible addition-fragmentation chain-transfer agents. Polym. Sci. Ser. B 2011, 53, 437–447. [Google Scholar] [CrossRef]
- Bernard, J.; Favier, A.; Davis, T.P.; Barner-Kowollik, C.; Stenzel, M.H. Synthesis of poly(vinyl alcohol) combs via MADIX/RAFT polymerization. Polymer 2006, 47, 1073–1080. [Google Scholar] [CrossRef]
- Nese, A.; Li, Y.; Averick, S.; Kwak, Y.; Konkolewicz, D.; Sheiko, S.S.; Matyjaszewski, K. Synthesis of Amphiphilic Poly(N-vinylpyrrolidone)-b-poly(vinyl acetate) Molecular Bottlebrushes. ACS Macro Lett. 2011, 1, 227–231. [Google Scholar] [CrossRef]
- Bailly, N.; Pound-Lana, G.; Klumperman, B. Synthesis, Characterization, and Self-Assembly of Poly(N-vinylpyrrolidone)-block-poly(vinyl acetate). Aust. J. Chem. 2012, 65, 1124–1131. [Google Scholar] [CrossRef]
- Etchenausia, L.; Rodrigues, A.M.; Harrisson, S.; Lejeune, E.D.; Save, M. RAFT Copolymerization of Vinyl Acetate and N-Vinylcaprolactam: Kinetics, Control, Copolymer Composition, and Thermoresponsive Self-Assembly. Macromolecules 2016, 49, 6799–6809. [Google Scholar] [CrossRef]
- Hurtgen, M.; Liu, J.; Debuigne, A.; Jerome, C.; Detrembleur, C. Synthesis of Thermo-Responsive Poly(N-vinylcaprolactam)-Containing Block Copolymers by Cobalt-Mediated Radical Polymerization. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 400–408. [Google Scholar] [CrossRef]
- Liu, J.; Detrembleur, C.; Hurtgen, M.; Debuigne, A.; De Pauw-Gillet, M.-C.; Mornet, S.; Duguet, E.; Jérôme, C. Thermo-responsive gold/poly(vinyl alcohol)-b-poly(N-vinylcaprolactam) core–corona nanoparticles as a drug delivery system. Polym. Chem. 2014, 5, 5289–5299. [Google Scholar] [CrossRef]
- Washington, K.E.; Kularatne, R.N.; Du, J.; Ren, Y.; Gillings, M.J.; Geng, M.C.; Biewer, C.X.; Stefan, M.C. Thermoresponsive star-like γ-substituted poly(caprolactone)s for micellar drug delivery. J. Mater. Chem. B 2017, 5, 5632–5640. [Google Scholar] [CrossRef]
- Yao, N.; Lin, W.J.; Zhang, X.F.; Gu, H.W.; Zhang, L.J. Amphiphilic β-cyclodextrin-based star-like block copolymer unimolecular micelles for facile in situpreparation of gold nanoparticles. J. Polym. Sci. Pol. Chem. 2016, 54, 186–196. [Google Scholar] [CrossRef]
- Blanco-Fernandez, B.; Concheiro, A.; Makwana, H.; Fernandez-Trillo, F.; Alexander, C.; Alvarez-Lorenzo, C. Dually sensitive dextran-based micelles for methotrexate delivery. RSC Adv. 2017, 7, 14448–14460. [Google Scholar] [CrossRef]
- Chen, W.-Q.; Wei, H.; Li, S.L.; Feng, J.; Nie, J.; Zhang, X.-Z.; Zhuo, R.-X. Fabrication of star-shaped, thermo-sensitive poly(N-isopropylacrylamide)-cholic acid-poly(ɛ-caprolactone) copolymers and their self-assembled micelles as drug carriers. Polymer 2008, 49, 3965. [Google Scholar] [CrossRef]
- Hira, S.K.; Ramesh, K.; Gupta, U.; Mitra, K.; Misra, N.; Ray, B.; Manna, P.P. Methotrexate-Loaded Four-Arm Star Amphiphilic Block Copolymer Elicits CD8+ T Cell Response against a Highly Aggressive and Metastatic Experimental Lymphoma. ACS Appl. Mater. Interfaces 2015, 7, 20021–20033. [Google Scholar] [CrossRef] [PubMed]
- Azo Polymerization Initiators Comprehensive Catalog. Available online: http://www.wako-chemicals.de/files/download/pdf/wako_azo_polymerization_initiators_catalog_25.pdf (accessed on 4 October 2017).
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Xia, Y.; Burke, A.D.; Stover, H.D.H. End Group Effect on the Thermal Response of Narrow-Disperse Poly(N-isopropylacrylamide) Prepared by Atom Transfer Radical Polymerization. Macromolecules 2006, 39, 2275–2283. [Google Scholar] [CrossRef]
- Li, X.; ShamsiJazeyi, H.; Pesek, S.L.; Agrawal, A.; Hammouda, B.; Verduzco, R. Thermoresponsive PNIPAAM bottlebrush polymers with tailored side-chain length and end-group structure. Soft Matter 2014, 10, 2008–2015. [Google Scholar] [CrossRef] [PubMed]
- Plummer, R.; Hill, D.J.T.; Whittaker, A.K. Solution Properties of Star and Linear Poly(N-isopropylacrylamide). Macromolecules 2006, 39, 8379–8388. [Google Scholar] [CrossRef]
- Arndt, K.F.; Müller, G. Polymercharakterisierung; Carl Hanser Verlag: München, Germany, 1996. [Google Scholar]
- Burchard, W. Solution Properties of Branched Macromolecules. Adv. Polym. Sci. 1999, 143, 113–194. [Google Scholar]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. Polymer Handbook, 4th ed.; Wiley-Interscience: New York, NY, USA, 1999. [Google Scholar]
- Wang, A.; Cao, Y.; Zhang, X.; Wang, D.; Liu, M.; Xie, Z.; Wang, Y. Rapid Self-Assembly of Block Copolymers for Flower-Like Particles with High Throughput. Langmuir 2016, 32, 13517–13524. [Google Scholar] [CrossRef] [PubMed]
- Huo, F.; Gao, C.; Dan, M.; Xiao, X.; Sua, Y.; Zhang, W. Seeded dispersion RAFT polymerization and synthesis of well-defined ABA triblock copolymer flower-like nanoparticles. Polym. Chem. 2014, 5, 2736–2746. [Google Scholar] [CrossRef]
- Pang, X.; Zhao, L.; Akinc, M.; Kim, J.K.; Lin, Z. Novel Amphiphilic Multi-Arm, Star-Like Block Copolymers as Unimolecular Micelles. Macromolecules 2011, 44, 3746–3752. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Z.; Jiang, X.; Rui, L.; Gao, Y.; Zhang, W. Unimolecular micelles from POSS-based star-shaped block copolymers for photodynamic therapy. Polymer 2017, 118, 268–279. [Google Scholar] [CrossRef]
- Charbonneau, C.; Souza-Lima, M.; Chassenieux, C.; Colombani, O.; Nicolai, T. Structure of pH sensitive self-assembled amphiphilic di- and triblock copolyelectrolytes: Micelles, aggregates and transient networks. Phys. Chem. Chem. Phys. 2013, 15, 3955–3964. [Google Scholar] [CrossRef] [PubMed]
- González-Ayón, M.A.; Sañudo-Barajas, J.A.; Picos-Corrales, L.A.; Licea-Claverie, A. PNVCL-PEGMA nanohydrogels with tailored transition temperature for controlled delivery of 5-fluorouracil. J. Polym. Sci. A Polym. Chem. 2015, 53, 2662–2672. [Google Scholar] [CrossRef]
- Fandrich, N.; Falkenhagen, J.; Weidner, S.M.; Pfeifer, D.; Staal, B.; Thünemann, A.F.; Laschewsky, A. Characterization of New Amphiphilic Block Copolymers of N-Vinyl Pyrrolidone and Vinyl Acetate, 1—Analysis of Copolymer Composition, End Groups, Molar Masses and Molar Mass Distributions. Macromol. Chem. Phys. 2010, 211, 869–878. [Google Scholar] [CrossRef]













| Entry | Sample a | [NVCL]o:[CTA]o | Time (h) | Conv. b (%) | Mn theo c (g/mol) | Mn GPC d (g/mol) | Ð d |
|---|---|---|---|---|---|---|---|
| 1 | (PNVCL151)6 | 2320 | 10 | 55 | 178,684 | 125,800 | 1.02 |
| 2 | (PNVCL99)6 | 2320 | 6 | 44 | 143,200 | 82,200 | 1.05 |
| 3 | (PNVCL34)6 | 2320 | 3 | 18 | 59,366 | 28,100 | 1.06 |
| 4 | (PNVCL18)6 | 2320 | 2 | 10 | 33,558 | 15,000 | 1.10 |
| 5 | (PNVCL5)6 | 2320 | 1 | 2 | 7780 | 4500 | 1.08 |
| Sample a | Precursor Homopolymer | Corresponding Block Copolymer | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Star (PNVCL)6 | Star (PNVCL-b-PVAc)6 | ||||||||
| Mn GPC (g/mol) b | Ð b | LCST (°C) c | Dh d (nm) | Mn GPC (g/mol) e | Ð e | PVAc (mol %) f | Dh d (nm) | LCST (°C) c | |
| (PNVCL23-b-PVAc2)6 | 19,100 | 1.08 | 38 | 7.1 (1.1) | 20,200 | 1.07 | 8 | 7.8 (1.3) | 30 |
| (PNVCL23-b-PVAc11)6 | 24,700 | 1.05 | 26 | 16.9 (3.5) | 24 | ||||
| (PNVCL51-b-PVAc2)6 | 42,200 | 1.02 | 35 | 9 (2.3) | 43,100 | 1.03 | 5 | 9.8 (2.6) | 28 |
| (PNVCL99-b-PVAc21)6 | 82,600 | 1.01 | 33 | 13.3 (3.6) | 94,300 | 1.04 | 20 | 38 (12) | 28 |
| (PNVCL99-b-PVAc18)6 | 93,700 | 1.05 | 13 | 25 (11.6) | 29 | ||||
| Sample | (PNVCL0.83-co-PNVP0.17)6 a | ((PNVCL-co-PNVP)-b-PVAc)6 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mn GPC (g/mol) b | Ð b | Dh c (nm) | LCST (°C) d | Mn GPC (g/mol) e | Ð e | PVAc (mol %) f | Dh c (nm) | LCST (°C) d | |
| ((PNVCL-co-PNVP)-b-PVAc)6-1 | 28,000 | 1.01 | 8.5 (1.8) | 41 | 37,460 | 1.1 | 13.5 | 8.4 (1.8) | 27 |
| ((PNVCL-co-PNVP)-b-PVAc)6-2 | 33,800 | 1,1 | 10 | 9.1 (1.5) | 34 | ||||
| ((PNVCL-co-PNVP)-b-PVAc)6-3 | 51,930 | 1.1 | 48.0 | * | * | ||||
| Entry | (PVAc)6-macroCTA Mn GPC (g/mol) a | Copolymer b | Mn GPC (g/mol) c | Ð c | PVAc:PNVCL:PNVP (mol %) d | LCST (°C) e | Dh f (nm) | DAFM g (nm) |
|---|---|---|---|---|---|---|---|---|
| 1 | 9700 | (PVAc22-b-PNVCL11)6 | 17,420 | 1.2 | 11:89:0 | 24 | 240 | 388 |
| 2 | 11,980 | (PVAc30-b-(PNVCL28-co-PNVP17))6 | 42,290 | 1.1 | 42:47.5:10.5 | 36 | 234 | 245 |
| 3 | 6600 | (PVAc17-b-(PNVCL10-co-PNVP7))6 | 36,480 | 1.1 | 47:41.4:11.6 | 40 | 115 | 99 |
| Sample | Dh (nm) a | LCST (°C) b | LC (%) c | EE (%) d | Cumulative Drug Release (%) | ||
|---|---|---|---|---|---|---|---|
| 33 °C | 37 °C | 40 °C | |||||
| 24 h | |||||||
| (PVAc30-b-(PNVCL28-co-PNVP17))6 | 244 | 36 | 4.3 | 40 | 49 | 35 | 32 |
| (PVAc17-b-(PNVCL10-co-PNVP7))6 | 123 | 40 | 7.0 | 66 | 33 | 41 | 40 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortez-Lemus, N.A.; Licea-Claverie, A. Preparation of a Mini-Library of Thermo-Responsive Star (NVCL/NVP-VAc) Polymers with Tailored Properties Using a Hexafunctional Xanthate RAFT Agent. Polymers 2018, 10, 20. https://doi.org/10.3390/polym10010020
Cortez-Lemus NA, Licea-Claverie A. Preparation of a Mini-Library of Thermo-Responsive Star (NVCL/NVP-VAc) Polymers with Tailored Properties Using a Hexafunctional Xanthate RAFT Agent. Polymers. 2018; 10(1):20. https://doi.org/10.3390/polym10010020
Chicago/Turabian StyleCortez-Lemus, Norma Aidé, and Angel Licea-Claverie. 2018. "Preparation of a Mini-Library of Thermo-Responsive Star (NVCL/NVP-VAc) Polymers with Tailored Properties Using a Hexafunctional Xanthate RAFT Agent" Polymers 10, no. 1: 20. https://doi.org/10.3390/polym10010020
APA StyleCortez-Lemus, N. A., & Licea-Claverie, A. (2018). Preparation of a Mini-Library of Thermo-Responsive Star (NVCL/NVP-VAc) Polymers with Tailored Properties Using a Hexafunctional Xanthate RAFT Agent. Polymers, 10(1), 20. https://doi.org/10.3390/polym10010020