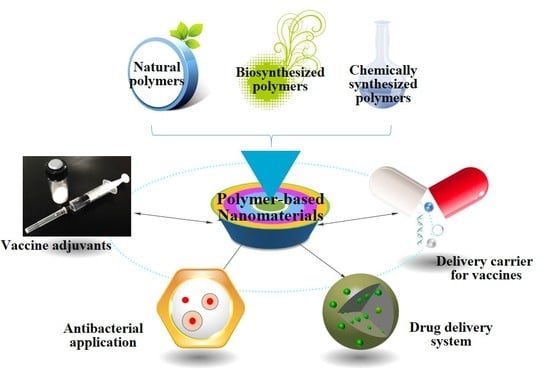
Polymer-Based Nanomaterials and Applications for Vaccines and Drugs
Abstract
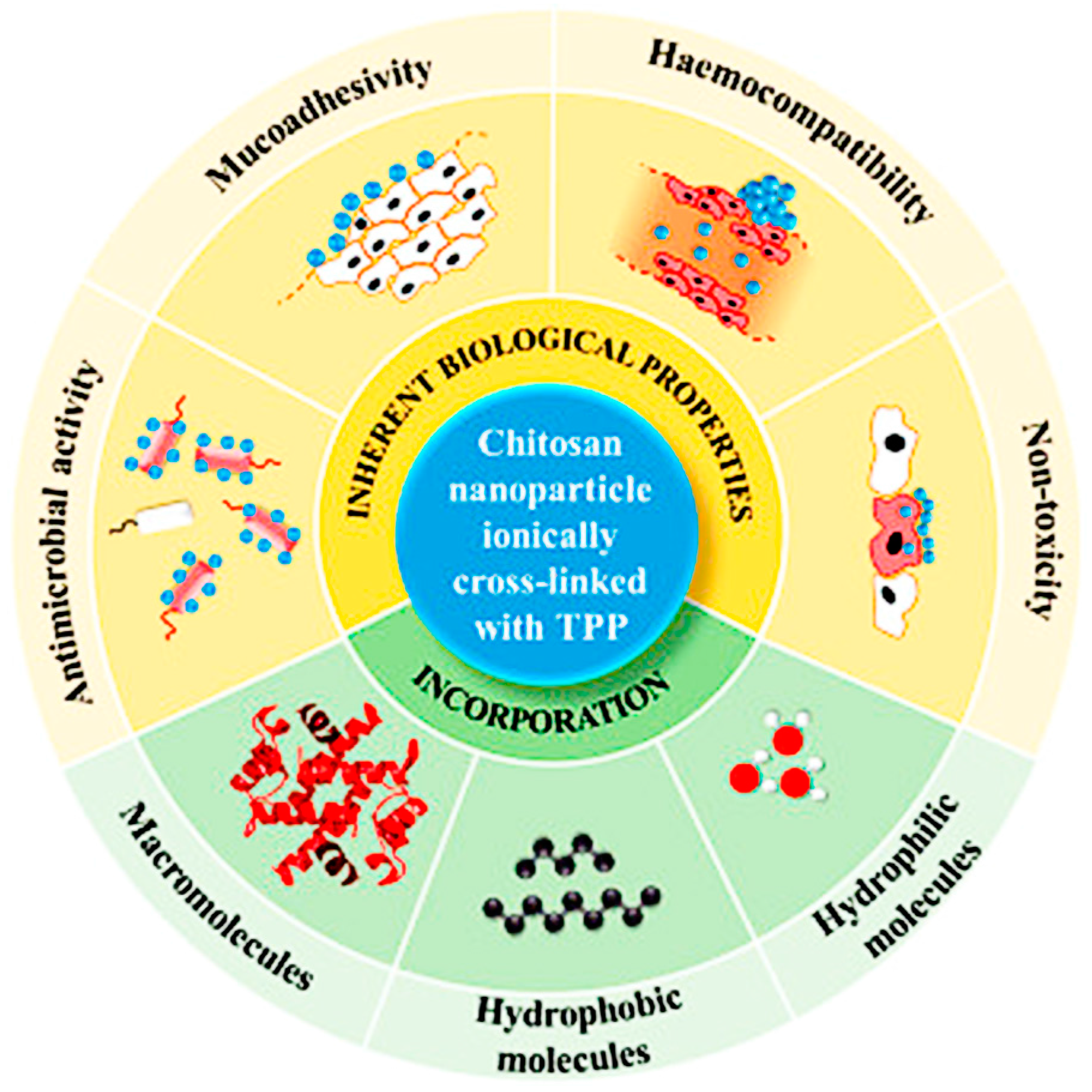
:1. Introduction
2. Polymeric Nanomaterials
2.1. Natural Polymer-Based Nanomaterials
2.2. Biosynthesized Polymer Materials
2.3. Chemically Synthesized Polymer Materials
3. Application of Polymer-Based Nanomaterials
3.1. Polymer-Based Nanomaterials as a Delivery Carrier for Vaccines
3.2. Polymer-Based Nanomaterials as Vaccine Adjuvants
3.3. Nanoparticle-Based Drug Delivery System
3.4. Antibacterial Application of Polymer-Based Nanomaterials
4. Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Marano, N.N.; Rupprecht, C.E.; Regnery, R. Vaccines for emerging infections. Rev. Sci. Tech. 2007, 26, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R.; Mandl, C.W.; Black, S.; De Gregorio, E. Vaccines for the twenty-first century society. Nat. Rev. Immunol. 2011, 11, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Mamo, T.; Poland, G.A. Nanovaccinology: The next generation of vaccines meets 21st century materials science and engineering. Vaccine 2012, 30, 6609–6611. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.; Laher, F.; Lazarus, E.; Ensoli, B.; Corey, L. Approaches to preventative and therapeutic HIV vaccines. Curr. Opin. Virol. 2016, 17, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Fauci, A.S. An HIV vaccine: Mapping uncharted territory. JAMA 2016, 316, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, K.S.; Zn, A.S. Status of hepatitis C virus vaccination: Recent update. World J. Gastroenterol. 2016, 22, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Gisterek, I.; Frydecka, I.; Świątoniowski, G.; Fidler, S.; Kornafel, J. Tumour-infiltrating CD4 and CD8 T lymphocytes in breast cancer. Rep. Pract. Oncol. Radiother. 2008, 13, 206–209. [Google Scholar] [CrossRef]
- Sheridan, B.S.; Knickelbein, J.E.; Hendricks, R.L. CD8 T cells and latent herpes simplex virus type 1: Keeping the peace in sensory ganglia. Expert Opin. Biol. Ther. 2007, 7, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, T.; Takehara, T.; Miyagi, T.; Nakazuru, S.; Mita, E.; Kanto, T.; Hiramatsu, N.; Hayashi, N. Hepatitis C virus-specific CD8+ T cell frequencies are associated with the responses of pegylated interferon-α and ribavirin combination therapy in patients with chronic hepatitis C virus infection. Hepatol. Res. 2011, 41, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, D.; Meschino, S.; Guan, L.; Clements, D.E.; Meulen, J.T.; Casimiro, D.R.; Coller, B.; Bett, A.J. Preclinical development of a dengue tetravalent recombinant subunit vaccine: Immunogenicity and protective efficacy in nonhuman primates. Vaccine 2015, 33, 4105–4116. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, J.; Xu, S.; Shi, J.; Xu, S.; Wu, X.; Fu, F.; Peng, Z.; Zhang, L.; Zheng, S. Immunogenicity of porcine circovirus type 2 nucleic acid vaccine containing CpG motif for mice. Virol. J. 2016, 13, 185. [Google Scholar] [CrossRef] [PubMed]
- Sergeyev, O.; Barinsky, I. Synthetic peptide vaccines. Vopr. Virusol. 2016, 61, 5–8. [Google Scholar] [PubMed]
- Apte, S.H.; Stephenson, R.J.; Simerska, P.; Groves, P.; Aljohani, S.; Eskandari, S.; Toth, I.; Doolan, D.L. Systematic evaluation of self-adjuvanting lipopeptide nano-vaccine platforms for the induction of potent CD8(+) T-cell responses. Nanomedicine 2016, 11, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.F. New approaches to HIV vaccine development. Curr. Opin. Immunol. 2015, 35, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Chablani, L. Micro- and nanoparticulate cancer vaccines: A vision for the future. AAPS Newsmag. 2016, 3, 14–18. [Google Scholar]
- Rosenthal, J.A.; Chen, L.; Baker, J.L.; Putnam, D.; Delisa, M.P. Pathogen-like particles: Biomimetic vaccine carriers engineered at the nanoscale. Curr. Opin. Biotechnol. 2014, 28, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Foged, C.; Brodin, B.; Frokjaer, S.; Sundblad, A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int. J. Pharm. 2005, 298, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.A.; Lauer, F.T.; Burchiel, S.W.; Mcdonald, J.D. Mechanisms for how inhaled multiwalled carbon nanotubes suppress systemic immune function in mice. Nat. Nanotechnol. 2009, 4, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.; Gao, J.; Wal, R.V.; Gigliotti, A.; Burchiel, S.; Mcdonald, J. Pulmonary and systemic immune response to inhaled multiwalled carbon nanotubes. Toxicol. Sci. 2007, 100, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Salatin, S.; Barar, J.; Barzegarjalali, M.; Adibkia, K.; Milani, M.A.; Jelvehgari, M. Hydrogel nanoparticles and nanocomposites for nasal drug/vaccine delivery. Arch. Pharm. Res. 2016, 39, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Prestidge, C.A. Polymer-lipid hybrid systems: Merging the benefits of polymeric and lipid-based nanocarriers to improve oral drug delivery. Expert Opin. Drug Deliv. 2016, 13, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I.; Rogge, T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W.; Hofte, M. Synthesis and fungicidal activity of new N,O-acyl chitosan derivatives. Biomacromolecules 2004, 5, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Chou, C.; Li, C. Antibacterial activity of N-alkylated disaccharide chitosan derivatives. Int. J. Food Microbiol. 2005, 97, 237–245. [Google Scholar] [CrossRef]
- Jayakumar, R.; Nwe, N.; Tokura, S.; Tamura, H. Sulfated chitin and chitosan as novel biomaterials. Int. J. Biol. Macromol. 2007, 40, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Okamoto, Y.; Kojima, K.; Miyatake, K.; Fujise, H.; Shigemasa, Y.; Minami, S. Effects of chitin and chitosan on collagen synthesis in wound healing. J. Vet. Med. Sci. 2004, 66, 1595–1598. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Guan, Y.L.; Yang, D.Z.; Li, Z.; Yao, K. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar]
- Moran, J.I.; Vazquez, A.; Cyras, V.P. Bio-nanocomposites based on derivatized potato starch and cellulose, preparation and characterization. J. Mater. Sci. 2013, 48, 7196–7203. [Google Scholar] [CrossRef]
- Civalleri, D.; Scopinaro, G.; Balletto, N.; Claudiani, F.; Decian, F.; Camerini, G.; Depaoli, M.; Bonalumi, U. Changes in vascularity of liver tumours after hepatic arterial embolization with degradable starch microspheres. Br. J. Surg. 1989, 76, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Woggum, T.; Sirivongpaisal, P.; Wittaya, T. Properties and characteristics of dual-modified rice starch based biodegradable films. Int. J. Biol. Macromol. 2014, 67, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Parkes, J. A clinical in-market evaluation of an alginate fibre dressing. Br. J. Nurs. 1900, 24, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Augst, A.; Kong, H.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Rebecca, J. Alginate fiber from brown algae. Der Pharm. Lett. 2016, 8, 68–71. [Google Scholar]
- Gou, S.; Zhu, P.; Dong, C.H.; Liu, J.; Guo, D.; Amp, A.F. Selective oxidized modification of alginate fiber. Dyeing Finish. 2014, 8, 482–490. [Google Scholar]
- Wang, Z.; Sun, B.; Zhang, M.; Ou, L.; Che, Y.; Zhang, J.; Kong, D. Functionalization of electrospun poly(-caprolactone) scaffold with heparin and vascular endothelial growth factors for potential application as vascular grafts. J. Bioact. Compat. Polym. 2013, 28, 154–166. [Google Scholar] [CrossRef]
- Tao, F.; Cazeneuve, S.; Wen, Z.; Wu, L.; Wang, T. Effective recovery of poly-β-hydroxybutyrate (PHB) biopolymer from cupriavidus necator using a novel and environmentally friendly solvent system. Biotechnol. Prog. 2016, 32, 678–685. [Google Scholar]
- Fidkowski, C.; Kaazempurmofrad, M.R.; Borenstein, J.T.; Vacanti, J.P.; Langer, R.; Wang, Y. Endothelialized microvasculature based on a biodegradable elastomer. Tissue Eng. 2005, 11, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, K.; Ganguly, K.; Kulkarni, A.R.; Rudzinski, W.E.; Krauss, L.; Nadagouda, M.N.; Aminabhavi, T.M. Oral insulin delivery using deoxycholic acid conjugated PEGylated polyhydroxybutyrate co-polymeric nanoparticles. Nanomedicine 2015, 10, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.S.; Yuan, L.I.; Cao, H.L.; Peng, W. Preparation and characterization of PLA/MMT nanocomposites with microwave irradiation. J. Mater. Eng. 2012, 2, 4. [Google Scholar]
- Kim, K.; Yu, M.; Zong, X.; Chiu, J.B.; Fang, D.; Seo, Y.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Control of degradation rate and hydrophilicity in electrospun non-woven poly(d,l-lactide) nanofiber scaffolds for biomedical applications. Biomaterials 2003, 24, 4977–4985. [Google Scholar] [CrossRef]
- Sha, L.; Chen, Z.; Chen, Z.; Zhang, A.; Yang, Z. Polylactic acid based nanocomposites: Promising safe and biodegradable materials in biomedical field. Int. J. Polym. Sci. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Noreen, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Zahoor, A.F. Bio-based polyurethane: An efficient and environment friendly coating systems: A review. Prog. Org. Coat. 2016, 91, 25–32. [Google Scholar] [CrossRef]
- He, C.; Wang, M.; Cai, X.; Huang, X.; Li, L.; Zhu, H.; Shen, J.; Yuan, J. Chemically induced graft copolymerization of 2-hydroxyethyl methacrylate onto polyurethane surface for improving blood compatibility. Appl. Surf. Sci. 2011, 258, 755–760. [Google Scholar] [CrossRef]
- Ou, C.; Su, C.; Jeng, U.; Hsu, S. Characterization of biodegradable polyurethane nanoparticles and thermally induced self-assembly in water dispersion. ACS Appl. Mater. Interfaces 2014, 6, 5685–5694. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, K.; Ding, M.; Li, J.; Tan, H.; Wang, Z.; Fu, Q. Quantitative grafting of peptide onto the nontoxic biodegradable waterborne polyurethanes to fabricate peptide modified scaffold for soft tissue engineering. J. Mater. Sci. Mater. Med. 2011, 22, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Shendi, H.K.; Omrani, I.; Ahmadi, A.; Farhadian, A.; Babanejad, N.; Nabid, M.R. Synthesis and characterization of a novel internal emulsifier derived from sunflower oil for the preparation of waterborne polyurethane and their application in coatings. Prog. Org. Coat. 2017, 105, 303–309. [Google Scholar] [CrossRef]
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Breton, A.L.; Preat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H. PEG-PLGA copolymers: Their structure and structure-influenced drug delivery applications. J. Control. Release 2014, 183, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Tan, A.; Pastorin, G.; Ho, H.K. Nanomaterial scaffolds for stem cell proliferation and differentiation in tissue engineering. Biotechnol. Adv. 2013, 31, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Mumper, R.J.; Rolland, A. Chitosan and chitosan oligomers for nucleic acid delivery: Original research article: Chitosan and depolymerized chitosan oligomers as condensing carriers for in vivo plasmid delivery, 1998. J. Control. Release 2014, 190, 46–48. [Google Scholar] [PubMed]
- Bugnicourt, L.; Ladaviere, C. Interests of chitosan nanoparticles ionically cross-linked with tripolyphosphate for biomedical applications. Prog. Polym. Sci. 2016, 60, 1–17. [Google Scholar] [CrossRef]
- Zhao, K.; Deng, Y.; Chen, J.C.; Chen, G. Polyhydroxyalkanoate (PHA) scaffolds with good mechanical properties and biocompatibility. Biomaterials 2003, 24, 1041–1045. [Google Scholar] [CrossRef]
- Obayemi, J.D.; Danyuo, Y.; Dozienwachukwu, S.; Odusanya, O.S.; Anuku, N.; Malatesta, K.; Yu, W.; Uhrich, K.E.; Soboyejo, W.O. PLGA-based microparticles loaded with bacterial-synthesized prodigiosin for Anticancer drug release: Effects of particle size on drug release kinetics and cell viability. Mater. Sci. Eng. C Mater. 2016, 66, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Javiya, C.; Jonnalagadda, S. Physicochemical characterization of spray-dried PLGA/PEG microspheres, and preliminary assessment of biological response. Drug Dev. Ind. Pharm. 2016, 42, 1504–1514. [Google Scholar] [CrossRef] [PubMed]
- Jogala, S.; Rachamalla, S.S.; Aukunuru, J. Development of PEG-PLGA based intravenous low molecular weight heparin (LMWH) nanoparticles intended to treat venous thrombosis. Curr. Drug Deliv. 2016, 13, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Teply, B.A.; Sherifi, I.; Sung, J.; Luther, G.; Gu, F.X.; Levynissenbaum, E.; Radovicmoreno, A.F.; Langer, R.; Farokhzad, O.C. Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials 2007, 28, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; He, C.; Cheng, Y.; Li, D.; Gong, Y.; Liu, J.; Tian, H.; Chen, X. PLK1shRNA and doxorubicin co-loaded thermosensitive PLGA-PEG-PLGA hydrogels for osteosarcoma treatment. Biomaterials 2014, 35, 8723–8734. [Google Scholar] [CrossRef] [PubMed]
- Rojnik, M.; Kocbek, P.; Moret, F.; Compagnin, C.; Celotti, L.; Bovis, M.J.; Woodhams, J.H.; Macrobert, A.J.; Scheglmann, D.; Helfrich, W. In vitro and in vivo characterization of temoporfin-loaded PEGylated PLGA nanoparticles for use in photodynamic therapy. Nanomedicine 2012, 7, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lin, S.L.; Deng, L.; Liu, Z.G. Potential use of chitosan nanoparticles for oral delivery of DNA vaccine in black Seabream acanthopagrus schlegelii bleeker to protect from Vibrio parahaemolyticus. J. Fish Dis. 2013, 36, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Agrawal, U.; Mody, N.; Vyas, S.P. Polymer nanotechnology based approaches in mucosal vaccine delivery: Challenges and opportunities. Biotechnol. Adv. 2015, 33, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Uebelhoer, L.; Han, J.H.; Callendret, B.; Mateu, G.; Shoukry, N.H.; Hanson, H.L.; Rice, C.M.; Walker, C.M.; Grakoui, A. Stable cytotoxic T cell escape mutation in hepatitis C virus is linked to maintenance of viral fitness. PLoS Pathog. 2008, 4, e1000143. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, S.; Sun, L.; Huang, C. Controlled delivery of paracetamol and protein at different stages from core-shell biodegradable microspheres. Carbohydr. Polym. 2010, 79, 437–444. [Google Scholar] [CrossRef]
- Zhao, K.; Sun, Y.; Chen, G.; Rong, G.; Kang, H.; Jin, Z.; Wang, X. Biological evaluation of N-2-hydroxypropyl trimethyl ammonium chloride chitosan as a carrier for the delivery of live newcastle disease vaccine. Carbohydr. Polym. 2016, 149, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Hogenesch, H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Front. Immunol. 2013, 3, 406. [Google Scholar] [CrossRef] [PubMed]
- Hogenesch, H.; Dunham, A.; Burlet, E.; Lu, F.; Mosley, Y.Y.C.; Morefield, G.L. Preclinical safety study of a recombinant streptococcus pyogenes vaccine formulated with aluminum adjuvant. J. Appl. Toxicol. 2017, 37, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhang, Y.; Zhang, X.; Shi, C.; Wang, X.; Wang, X.; Jin, Z.; Cui, S. Chitosan-coated poly(lactic-co-glycolic) acid nanoparticles as an efficient delivery system for Newcastle disease virus DNA vaccine. Int. J. Nanomed. 2014, 9, 4609–4619. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Kamm, W.; Breitenbach, A.; Kaiserling, E.; Xiao, J.X.; Kissel, T. Biodegradable nanoparticles for oral delivery of peptides: Is there a role for polymers to affect mucosal uptake? Eur. J. Pharm. Biopharm. 2000, 50, 147–160. [Google Scholar] [CrossRef]
- Illum, L. Nanoparticulate systems for nasal delivery of drugs: A real improvement over simple systems? J. Pharm. Sci. 2007, 96, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Shan, X.; Hao, L.; Feng, Q.; Zhang, Z. Copper sulfide nanoparticle-based localized drug delivery system as an effective cancer synergistic treatment and theranostic platform. Acta Biomater. 2017, 54, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Romer, L.; Scheibel, T. Polymeric materials based on silk proteins. Polymer 2008, 49, 4309–4327. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, J.; Ren, X.R.; Mook, R.A.; Wang, J.; Spasojevic, I.; Premont, R.T.; Li, X.; Chilkoti, A.; Chen, W. Niclosamide-conjugated polypeptide nanoparticles inhibit Wnt signaling and colon cancer growth. Nanoscale 2017, 9, 12709–12717. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M.; Mano, J.F. Chitosan-based particles as controlled drug delivery systems. Drug Deliv. 2005, 12, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Von Nussbaum, F.; Brands, M.; Hinzen, B.; Weigand, S.; Habich, D. Antibacterial natural products in medicinal chemistry-exodus or revival? Angew. Chem. Int. Ed. Engl. 2006, 45, 5072–5129. [Google Scholar] [CrossRef] [PubMed]
- Witte, W. International dissemination of antibiotic resistant strains of bacterial pathogens. Infect. Genet. Evol. 2004, 4, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Millard, J.D.; Ugartegil, C.; Moore, D.A. Multidrug resistant tuberculosis. BMJ 2015, 350, h882. [Google Scholar] [CrossRef] [PubMed]
- Bakeraustin, C.; Wright, M.S.; Stepanauskas, R.; Mcarthur, J.V. Co-selection of antibiotic and metal resistance. Trends Microbiol. 2006, 14, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Huh, A.J.; Kwon, Y.J. Nanoantibiotics: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. Nanoscience, nanotechnology, and chemistry. Small 2005, 1, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Sanpui, P.; Murugadoss, A.; Prasad, P.V.; Ghosh, S.S.; Chattopadhyay, A. The antibacterial properties of a novel chitosan-Ag-nanoparticle composite. Int. J. Food Microbiol. 2008, 124, 142–146. [Google Scholar] [CrossRef] [PubMed]

| Classification | Materials | Application Areas | Advantages | Disadvantages |
|---|---|---|---|---|
| Natural polymeric material | Chitosan | Hemostasis material, medical dressing, hydrogel, drug delivery carrier, gene transfer [22] | Biocompatibility, antimicrobial, innocuous, easily degradable, adsorbability, film formation [23,24,25,26,27] | Poor spinnability, poor strength, low water-solubility [23,25] |
| Starch | Hemostasis material, tissue-engineered scaffold, drug delivery carrier, bone repair material [28,29] | Extensive sources, low price, degradation products safe and non-toxic, non-antigenic [28,30] | Poor mechanical properties, resistance to water, poor blocking performance [28,30] | |
| Alginate | Pharmaceutical excipient, pepcid complete, medical dressing [31,32,33,34,35] | Hypotoxicity, biocompatibility, suppresses tumor growth, enhances immunity [31,32,33,34,35] | Bad biodegradability, cell attachment poor [31,32,33,34,35] | |
| Cellulose | Pharmaceutical adjuvant [28] | Extensive sources, low price [28] | Rare adverse reactions [28] | |
| Biosynthesis material | Poly β-hydroxybutyrate (PHB) | Drug-delivery carrier, tissue engineering material [36,37,38] | Biodegradable, safe, non-toxic, good physical and chemical properties [37,39] | High crystallinity, bad thermal stability [37,39] |
| Chemosynthes material (Copolymer) | Polylactic (PLA) | Anti-adhesion materials, patch, drug-delivery carrier, bone-fixing device, suture, tissue-engineered scaffold [40,41,42] | Biocompatibility, good mechanical properties, safe, non-toxic [40,41,42] | Poor toughness, degradation speed slow, hydrophobicity, lack of reactive side chain groups [40,41,42] |
| Polyurethane | Excipients, medical bandage [43,44,45] | Low cost, rich resource, good mechanical properties [43,44,46,47] | Degradation speed slow [43,46,47] | |
| Poly(lactic-glycolic acid) (PLGA) | Absorbable suture, drug delivery, bone screw fixation, tissue repair [48,49,50,51] | Controllable biodegradability, biocompatibility [48,49,50] | Higher cost, drug-loading capacity and stability can be improved [48,49,50] | |
| Polymethyl methacrylate resin (PMMA) | Bone-fixation materials, dental materials, artificial crystal [52] | Easy operation, good biocompatibility | Monomer has cytotoxicity, easy oxidation |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Zhao, D.; Li, D.; Wang, X.; Jin, Z.; Zhao, K. Polymer-Based Nanomaterials and Applications for Vaccines and Drugs. Polymers 2018, 10, 31. https://doi.org/10.3390/polym10010031
Han J, Zhao D, Li D, Wang X, Jin Z, Zhao K. Polymer-Based Nanomaterials and Applications for Vaccines and Drugs. Polymers. 2018; 10(1):31. https://doi.org/10.3390/polym10010031
Chicago/Turabian StyleHan, Jinyu, Dandan Zhao, Dan Li, Xiaohua Wang, Zheng Jin, and Kai Zhao. 2018. "Polymer-Based Nanomaterials and Applications for Vaccines and Drugs" Polymers 10, no. 1: 31. https://doi.org/10.3390/polym10010031
APA StyleHan, J., Zhao, D., Li, D., Wang, X., Jin, Z., & Zhao, K. (2018). Polymer-Based Nanomaterials and Applications for Vaccines and Drugs. Polymers, 10(1), 31. https://doi.org/10.3390/polym10010031