A Redox Conjugated Polymer-Based All-Solid-State Reference Electrode
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Synthesis of Monomer I
2.3. Electrochemical Procedures
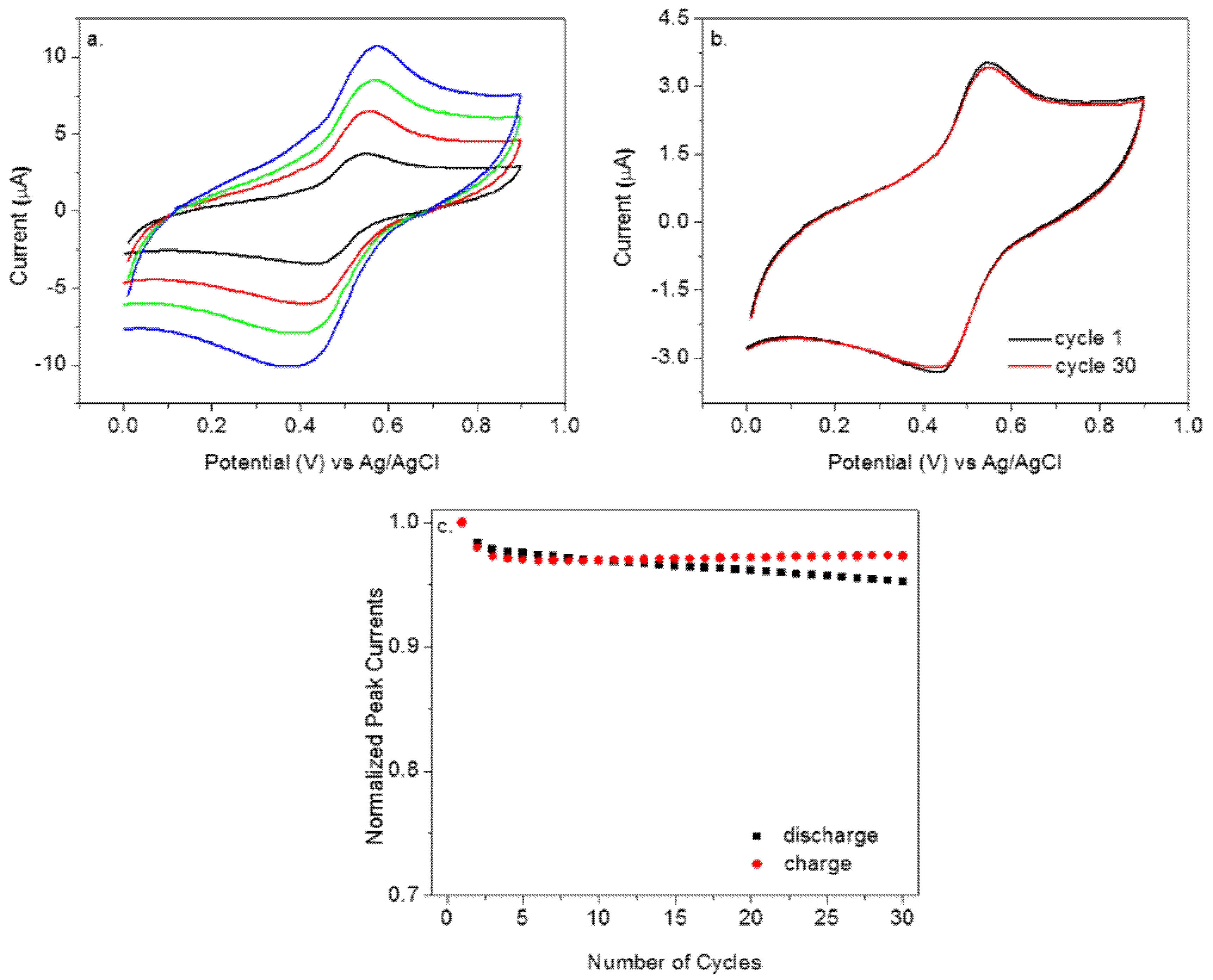
3. Results and Discussion
3.1. Electrochemical Polymerization
3.2. Electrochemical Properties of Poly(Aniline Quinone/Hydroquinone) P1
3.3. Poly (Aniline Quinone/Hydroquinone) as a Stable Reference Electrode
3.4. Various Factors to Influence Poly (Aniline Quinone/Hydroquinone) as a RE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wieckowski, A. (Ed.) Interfacial Electrochemistry: Theory, Experiment, and Applications; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 1999. [Google Scholar]
- Lewenstam, A.; Blaz, T.; Migdalski, J. All-solid-state reference electrode with heterogeneous membrane. Anal. Chem. 2017, 89, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Ruch, P.W.; Cericola, D.; Hahn, M.; Kotz, R.; Wokaun, A. On the use of activated carbon as a quasi-reference electrode in non-aqueous electrolyte solutions. J. Electroanal. Chem. 2009, 636, 128–131. [Google Scholar] [CrossRef]
- Kisiel, A.; Marcisz, H.; Michalska, A.; Maksymiuk, K. All-solid-state reference electrodes based on conducting polymers. Analyst 2015, 130, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.T.; Rosenstein, J.K. Quasi-reference electrodes in confined electrochemical cells can result in in situ production of metallic nanoparticles. Sci. Rep. 2018, 8, 1965. [Google Scholar] [CrossRef] [PubMed]
- Bentley, C.L.; Perry, D.; Unwin, P.R. Stability and placement of Ag/AgCl quasi-reference counter electrodes in confined electrochemical cells. Anal. Chem. 2018, 90, 7700–7707. [Google Scholar] [CrossRef] [PubMed]
- Michalska, A. All-solid-state ion selective and all-solid-state reference electrodes. Electroanalysis 2012, 24, 1253–1265. [Google Scholar] [CrossRef]
- Pedrotti, J.J.; Angnes, L.; Gutz, I.G.R. Miniaturized reference electrodes with microporous polymer. Electroanalysis 1996, 8, 673–675. [Google Scholar] [CrossRef]
- Shinwari, M.W.; Zhitomirsky, D.; Deen, I.A.; Selvaganapathy, P.R.; Deen, M.J.; Landdheer, D. Microfabricated reference electrodes and their biosensing applications. Sensors 2010, 10, 1679–1715. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.M.; Beck, J.R.; Brand, E.; Ziomek-Moroz, M.; Lvov, S.N. Copper-copper sulfate reference electrode for operating in high temperature and high pressure aqueous environments. Electrochim. Acta 2016, 221, 96–106. [Google Scholar] [CrossRef]
- Chen, C.C.; Chou, J.C. All-solid-state conductive polymer miniaturized reference electrode. Jpn. J. Appl. Phys. 2009, 48, 111501. [Google Scholar] [CrossRef]
- Lee, J.; Jackel, N.; Kim, D.; Widmaier, M.; Sathyamoorhi, S.; Srimuk, P.; Kim, C.; Fleischmann, S.; Zeiger, M.; Presser, V. Porous carbon as a quasi-reference electrode in aqueous electrolytes. Electrochim. Acta 2016, 222, 1800–1805. [Google Scholar] [CrossRef]
- Lorant, S.; Bohnke, C.; Roffat, M.; Bohnke, O. New concept of an all-solid-state reference electrode using a film of lithium lanthanum titanium oxide (LLTO). Electrochim. Acta 2012, 80, 418–425. [Google Scholar] [CrossRef]
- Migdalski, J.; Lewenstam, A. Conducting polymer-based reference electrodes. In Handbook of Reference Electrodes; Inzelt, G., Lewenstam, A., Scholz, F., Eds.; Springer: Berlin, Germany, 2013; Chapter 12. [Google Scholar]
- Kaminsky, A.; Willner, I.; Mandler, D. A reference electrode for organic solvents based on modified polyethylenimine loaded with Fe(CN)63−/4−. J. Electrochem. Soc. 1993, 140, L25–L27. [Google Scholar] [CrossRef]
- Ghilane, J.; Hapiot, P.; Bard, A.J. Metal/polypyrrole quasi-reference electrode for voltammetry in nonaqueous and aqueous solutions. Anal. Chem. 2006, 78, 6868–6872. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.N.P.; Randriamahazaka, H.; Ghilane, J. Platinum/poly(N-ferrocenylmethyl-N-allylimidazolium bromide) quasi-reference electrode for electrochemistry in non-aqueous and ionic liquid solutions. Electrochem. Commun. 2016, 73, 5–9. [Google Scholar] [CrossRef]
- Gagne, R.R.; Koval, C.A.; Lisensky, G.C. Ferrocene as an internal standard for electrochemical measurements. Inorg. Chem. 1980, 19, 2854–2855. [Google Scholar] [CrossRef]
- Heinze, J.; Frontanauribe, B.A.; Ludwigs, S. Electrochemistry of conducting polymers-persisent models and new concepts. Chem. Rev. 2010, 110, 4724–4771. [Google Scholar] [CrossRef] [PubMed]
- Casado, N.; Hernandez, G.; Sardon, H.; Mecerreyes, D. Current trends in redox polymers for energy and medicine. Prog. Polym. Sci. 2016, 52, 107–135. [Google Scholar] [CrossRef]
- Gracia, R.; Mecerreyes, D. Polymers with redox properties: Materials for batteries, biosensors and more. Polym. Chem. 2013, 4, 2206–2214. [Google Scholar] [CrossRef]
- Conte, S.; Rodriguez-Calero, G.G.; Burkhardt, S.E.; Lowe, M.A.; Abruna, H.D. Designing conducting polymer films for electrochemical energy storage technologies. RSC Adv. 2013, 3, 1957–1964. [Google Scholar] [CrossRef]
- Kunz, T.K.; Wolf, M.O. Electrodeposition and properties of TEMPO functionalized polythiophene thin films. Polym. Chem. 2011, 2, 640–644. [Google Scholar] [CrossRef]
- Ko, H.C.; Park, S.; Paik, W.; Lee, H. Electrochemistry and electrochromism of the polythiophene derivative with viologen pendant. Synth. Met. 2002, 132, 15–20. [Google Scholar] [CrossRef]
- Casado, N.; Hernandez, G.; Veloso, A.; Devaraj, S.; Mecerreyes, D.; Armand, M. PEDOT radical polymer with synergetic redox and electrical properties. ACS Macro Lett. 2016, 5, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.; Zeng, X.Q. Ionic liquid-doped polyaniline and its redox activities in the zwitterionic biological buffer MOPS. Electrochim. Acta 2016, 202, 73–83. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D.; Singha, N.K.; Lee, J.H. Progress in preparation, processing and applications of polyaniline. Progr. Polym. Sci. 2009, 34, 783–810. [Google Scholar] [CrossRef]
- Wallace, G.G.; Spinks, G.M.; Kane-Mauire, L.A.P.; Teasdale, P.R. Conductive Electroactive Polymers: Intelligent Materials Systems, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Allison, L.; Hoxie, S.; Andrew, T.L. Towards seamlessly-integrated textile electronics: Methods to coat fabrics and fibers with conducting polymers for electronic applications. Chem. Commun. 2017, 53, 7182–7193. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.H.; Qu, K.; Zeng, X.Q. Investigation into the ring-substituted polyanilines and their application for the detection and adsorption of sulfur dioxide. Sens. Actuators B Chem. 2017, 249, 423–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, X.Q.; Qu, K.; Rehman, A. Glycosylated conductive polymer: A multimodal biointerfacefor studying carbohydrate-protein interactions. Acc. Chem. Res. 2016, 49, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Qu, K.; Tang, L.H.; Li, Z.L.; Moore, E.; Zeng, X.Q.; Liu, Y.; Li, J.H. Nanomaterials in carbohydrate biosensors. Trends Anal. Chem. 2014, 58, 54–70. [Google Scholar] [CrossRef]
- Janata, J.; Josowicz, M. Conducting polymers in electronic chemical sensors. Nat. Mater. 2003, 2, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wen, Z.; Li, J.H. Hydroxyl-containing antimony oxide bromide nanorods combined with chitosan for biosensors. Biomaterials 2006, 27, 5740–5747. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.R.; Dong, H.L.; Li, T.; Hviid, R.; Zou, Y.; Wei, M.; Fu, X.L.; Wang, E.J.; Zhen, Y.G.; Norgaard, K.; et al. Role of redox centre in charge transport investigated by novel self-assembled conjugated polymer molecular junctions. Nat. Commun. 2015, 6, 7478–7487. [Google Scholar] [CrossRef] [PubMed]
- Bruckenstein, S.; Krtil, P.; Hillman, A.R. Thermodynamics and kinetics of redox switching of polyvinylferrocene films in perchlorate solutions. J. Phys. Chem. B 1998, 102, 4994–5003. [Google Scholar] [CrossRef]
- Pater, E.M.; Bruckenstein, S.; Hillman, A.R. Film mass and volume changes accompanying redox-driven solvent and salt transfer during redox switching of polyvinylferrocene films. J. Chem. Soc. Faraday Trans. 1998, 94, 1097–1103. [Google Scholar] [CrossRef]
- Shundrin, L.A.; Irtegova, I.G.; Vasilieva, N.V.; Khalfina, I.A. Benzoquinone and naphthoquinone based redox-active labels for electrochemical detection of modified oligonucleotides on Au electrodes. Tetrahedron Lett. 2016, 57, 392–395. [Google Scholar] [CrossRef]
- Bolton, J.L.; Dunlap, T. Formation and biological targets of quinones: Cytotoxic versus cytoprotective effects. Chem. Res. Toxicol. 2017, 30, 13–37. [Google Scholar] [CrossRef] [PubMed]
- Malinauskas, A.; Holze, R. Electrocatalysis of the quinone/hydroquinone system by electrodes coated with substituted polyaniline. Ber. Bunsenges. Phys. Chem. 1996, 100, 1740–1745. [Google Scholar] [CrossRef]
- Karlsson, C.; Huang, H.; Strφmme, M.; Gogoll, A.; SjÖdin, M. Polymer-pendant interactions in poly(pyrrol-3-ylhydroquinone): A solution for the use of conducting polymers at stable conditions. J. Phys. Chem. C 2013, 117, 23558–23567. [Google Scholar] [CrossRef]
- Karlsson, C.; Gogoll, A.; Strφmme, M.; SjÖdin, M. Investigation of the redox chemistry of isoindole-4, 7-diones. J. Phys. Chem. C 2013, 117, 894–901. [Google Scholar] [CrossRef]
- Buck, R.P.; Wagoner, D.E. Selective anodic oxidation of p-alkylaryl ethers-pathways and products. J. Electroanal. Chem. 1980, 115, 89–113. [Google Scholar] [CrossRef]
- McOmie, J.F.W.; Watts, M.L.; West, D.E. Demethylation of aryl methyl ethers by boron tribromide. Tetrahedron 1968, 24, 2289–2292. [Google Scholar] [CrossRef]
- Karlsson, C.; Huang, H.; Strφmme, M.; Gogoll, A.; SjÖdin, M. Impact of linker in polypyrrole/quinone conducting redox polymers. RSC Adv. 2015, 5, 11309–11316. [Google Scholar] [CrossRef]
- Monkman, A.P.; Bloor, D.; Stevens, G.C.; Stevens, J.C.H. Electronic energy levels of polyaniline. J. Phys. D Appl. Phys. 1987, 20, 1337–1345. [Google Scholar] [CrossRef]
- Lindfors, T.; Ivaska, A. pH sensitivity of polyaniline and its substituted derivatives. J. Electroanal. Chem. 2002, 531, 43–52. [Google Scholar] [CrossRef]
- Karlsson, C.; Jāmstorp, E.; Strφmme, M.; SjÖdin, M. Computational electrochemistry study of 16 isoindole-4, 7-diones as candidates for organic cathode materials. J. Phys. Chem. C 2012, 116, 3793–3801. [Google Scholar] [CrossRef]
- Karlsson, C.; Huang, H.; Strφmme, M.; Gogoll, A.; SjÖdin, M. Quinone pendant group kinetics in poly(pyrrol-3-ylhydroquinone). J. Electroanal. Chem. 2014, 735, 95–98. [Google Scholar] [CrossRef]
- Chiang, J.C.; MacDiarmid, A.G. ‘Polyaniline’: Protonic acid doping of the emeraldine form to the metallic regime. Synth. Met. 1986, 13, 193–205. [Google Scholar] [CrossRef]
- Reiss, H. Theoretical analysis of protonic acid doping of the emeraldine form of polyaniline. J. Phys. Chem. 1988, 92, 3657–3662. [Google Scholar] [CrossRef]



| DCM | DCE | DMF | [Bmim][NTf2] | CH3CN | PBS | CH3CN:PBS (1:1) | |
|---|---|---|---|---|---|---|---|
| Eanodic | 0.312 | 0.373 | 0.319 | 0.143 | 0.189 | 0.119 | 0.0964 |
| Ecathodic | 0.0865 | 0.0533 | 0.186 | 0.0663 | 0.0898 | −0.0447 | 0.00137 |
| ΔE | 0.225 | 0.320 | 0.133 | 0.0767 | 0.0992 | 0.164 | 0.0950 |
| Average | 0.199 | 0.213 | 0.252 | 0.105 | 0.139 | 0.0371 | 0.0489 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, K.; Fang, M.; Zhang, S.; Liu, H.; Zeng, X. A Redox Conjugated Polymer-Based All-Solid-State Reference Electrode. Polymers 2018, 10, 1191. https://doi.org/10.3390/polym10111191
Qu K, Fang M, Zhang S, Liu H, Zeng X. A Redox Conjugated Polymer-Based All-Solid-State Reference Electrode. Polymers. 2018; 10(11):1191. https://doi.org/10.3390/polym10111191
Chicago/Turabian StyleQu, Ke, Mingxi Fang, Shuwei Zhang, Haiying Liu, and Xiangqun Zeng. 2018. "A Redox Conjugated Polymer-Based All-Solid-State Reference Electrode" Polymers 10, no. 11: 1191. https://doi.org/10.3390/polym10111191
APA StyleQu, K., Fang, M., Zhang, S., Liu, H., & Zeng, X. (2018). A Redox Conjugated Polymer-Based All-Solid-State Reference Electrode. Polymers, 10(11), 1191. https://doi.org/10.3390/polym10111191






