Role of Electrostatic Interactions on Supramolecular Organization in Calf-Thymus DNA Solutions under Flow
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Solutions Preparation
2.2. Rheometric Measurements
2.3. Flow Birefringence Visualizations
2.4. Small-Angle X-Ray Scattering (SAXS) Measurements
3. Results and Discussion
3.1. X-Ray Scattering
3.2. Birefringence and Texture under Shear
3.3. Rheological Behavior
3.3.1. Behavior at 4 mg/mL
Dynamic Measurements
Flow Measurements
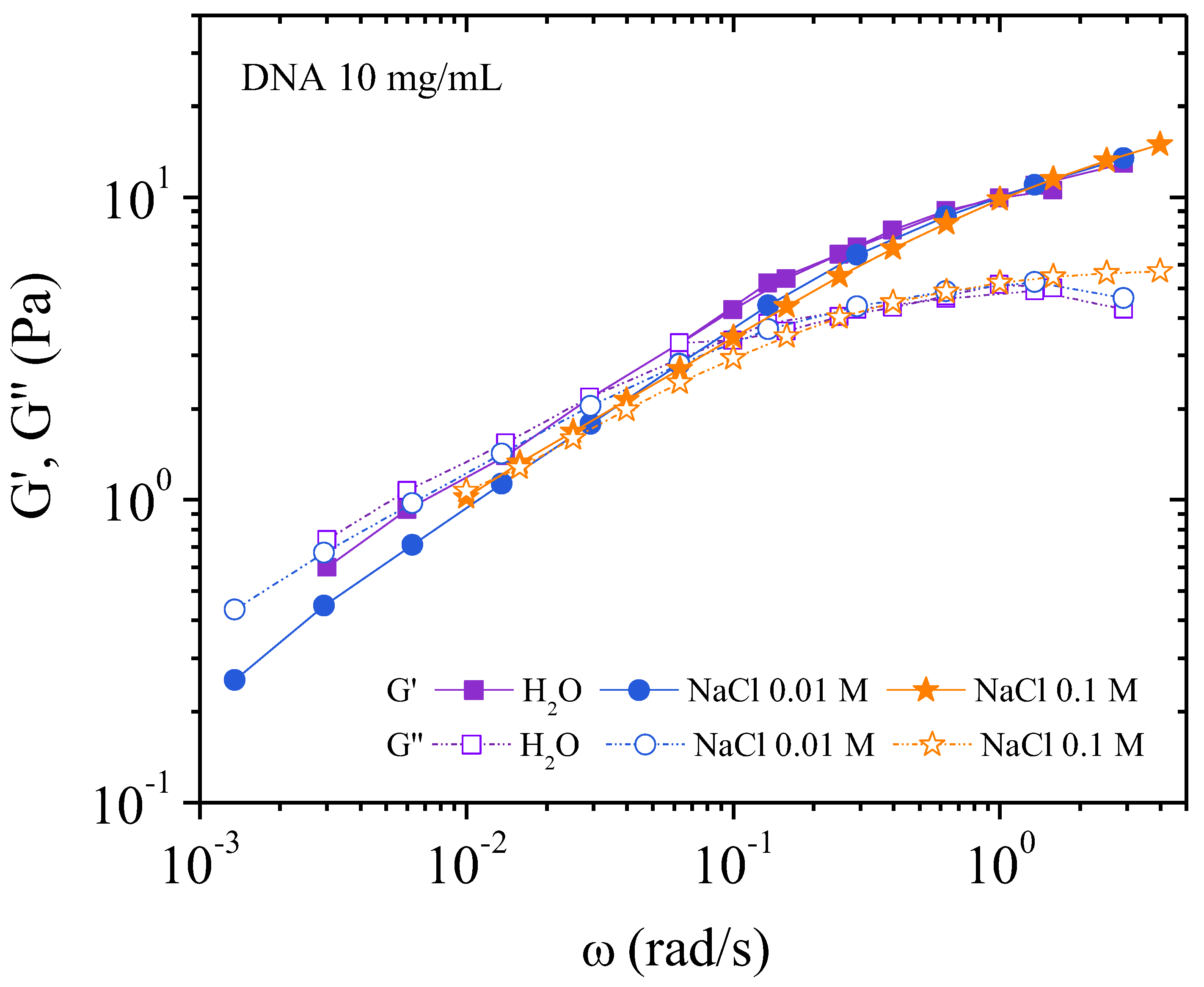
3.3.2. Behavior at 10 mg/ML
Dynamic Measurements
Flow Measurements
3.3.3. Transient Regime
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DNA | Deoxyribonucleic Acid |
| SAXS | Small-angle X-ray scattering |
References
- Maret, G.; Weill, G. Magnetic birefringence study of the electrostatic and intrinsic persistence length of DNA. Biopolymers 1983, 22, 2727–2744. [Google Scholar] [CrossRef] [PubMed]
- Odijk, T. On the Ionic-Strength Dependence of the Intrinsic Viscosity of DNA. Biopolymers 1979, 18, 3111–3113. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.S. The Persistence Length of DNA Is Reached from the Persistence Length of Its Null Isomer through an Internal Electrostatic Stretching Force. Biophys. J. 2006, 91, 3607–3616. [Google Scholar] [CrossRef] [PubMed]
- Odijk, T. Possible scaling relations for semidilute polyelectrolyte Solutions. Macromolecules 1979, 12, 688–693. [Google Scholar] [CrossRef]
- Malovikova, A.; Milas, M.; Rinaudo, M.; Borsali, R. Viscosimetric behavior of Na-polygalacturonate in the presence of low salt content. In Macro-Ion Characterization from Dilute Solutions to Complex Fluids; Schmitz, K.S., Ed.; ACS Symp. Series 548; American Chemical Society: Washington, DC, USA, 1994; pp. 315–321. ISBN 0841227705. [Google Scholar]
- Ray, J.; Manning, G.S. Formation of Loose Clusters in Polyelectrolyte Solutions. Macromolecules 2000, 33, 2901–2908. [Google Scholar] [CrossRef]
- Ray, J.; Manning, G.S. An Attractive Force between Two Rodlike Polyions Mediated by the Sharing of Condensed Counterions. Langmuir 1994, 10, 2450–2461. [Google Scholar] [CrossRef]
- Manning, G.S. Counterion condensation theory of attraction between like charges in the absence of multivalent counterions. Eur. Phys. J. E 2011, 34, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Wissenburg, P.; Odijk, T.; Cirkel, P.; Mandel, M. Multimolecular Aggregation in Concentrated Isotropic Solutions of Mononucleosomal DNA in 1 M Sodium Chloride. Macromolecules 1994, 27, 306–308. [Google Scholar] [CrossRef]
- Wissenburg, P.; Odijk, T.; Cirkel, P.; Mandel, M. Multimolecular Aggregation of Mononucleosomal DNA in Concentrated Isotropic Solutions. Macromolecules 1995, 28, 2315–2328. [Google Scholar] [CrossRef]
- Bravo-Anaya, L.M.; Macias, E.R.; Pérez-López, J.H.; Galliard, H.; Roux, D.; Landazuri, G.; Carvajal Ramos, F.; Rinaudo, M.; Pignon, F.; Soltero, J.F.A. Supramolecular organization in calf-thymus DNA solutions under flow in dependence with DNA concentration. Macromolecules 2017, 50, 8245–8257. [Google Scholar] [CrossRef]
- Nierlich, M.; Williams, C.E.; Boue, F.; Cotton, J.P.; Daoud, M.; Farnoux, B.; Jannink, G.; Picot, C.; Moan, M.; Wolff, C.; et al. Small angle neutron scattering by semi-dilute solutions of polyelectrolyte. J. Phys. 1979, 40, 701–704. [Google Scholar] [CrossRef] [Green Version]
- Morfin, I.; Reed, W.F.; Rinaudo, M.; Borsali, R. Further evidence of liquid-like correlations in polyelectrolyte solutions. J. Phys. II 1994, 4, 1001–1019. [Google Scholar] [CrossRef]
- Borsali, R.; Rinaudo, M.; Noirez, L. Light scattering and small-angle neutron scattering from polyelectrolyte solutions: The succinoglycan. Macromolecules 1995, 28, 1085–1088. [Google Scholar] [CrossRef]
- Milas, M.; Lindner, P.; Rinaudo, M.; Borsali, R. Influence of the shear rate on the small-angle neutron scattering pattern of polyelectrolyte solutions: The xanthan example. Macromolecules 1996, 29, 473–474. [Google Scholar] [CrossRef]
- Pringle, O.A.; Schmidt, P.W. Small-angle X-ray scattering from helical macromolecules. J. Appl. Crystallogr. 1971, 4, 290–293. [Google Scholar] [CrossRef] [Green Version]
- Potemkin, I.I.; Vasilevskaya, V.V.; Khokhlov, A.R. Associating polyelectrolytes: Finite size cluster stabilization versus physical gel formation. J. Chem. Phys. 1999, 111, 2809–2817. [Google Scholar] [CrossRef]
- Moan, M.; Wolff, C. Study of the conformational rigidity of polyelectrolytes by elastic neutron scattering: 1. Carboxymethylcelluloses in the intermediate momentum range. Polymer 1975, 16, 776–780. [Google Scholar] [CrossRef]
- Larson, R.G. Liquid-crystalline Polymers. In The Structure and Rheology of Complex Fluids; Oxford University Press: New York, NY, USA, 1999; Chapter 11; pp. 503–550. [Google Scholar]
- Yamanaka, J.; Matsuoka, H.; Kitano, H.; Hasegawa, M.; Ise, N. Revisit to the intrinsic viscosity-molecular weight relationship of ionic polymers. 2. Viscosity behavior of salt-free aqueous solutions of sodium poly (styrenesulfonates). J. Am. Chem. Soc. 1990, 112, 587–592. [Google Scholar] [CrossRef]
- Winter, H.H.; Chambon, F. Analysis of linear viscoelasticity of a crosslinking polymer at the gel point. J. Rheol. 1986, 30, 367–382. [Google Scholar] [CrossRef]
- Winter, H.H.; Mours, M. Rheology of polymers near liquid-solid transitions. In Advances in Polymer Science; Springer-Verlag: Berlin, Germany, 1997; Volume 134, pp. 165–234. [Google Scholar]
- Winter, H.H. Glass transition as the Rheological Inverse of Gelation. Macromolecules 2013, 46, 2425–2432. [Google Scholar] [CrossRef]
- Barnes, H.A. A review of the slip (wall depletion) of polymer solutions, emulsions and particle suspensions in viscometers: Its cause, character, and cure. J. Non-Newton. Fluid Mech. 1995, 56, 221–251. [Google Scholar] [CrossRef]
- Barnes, H.A. The yield stress—A review or ‘παντα ρει’—Everything flows? J. Non-Newton. Fluid Mech. 1999, 81, 133–178. [Google Scholar] [CrossRef]
- Auffret, Y.; Roux, D.C.D.; El Kissi, N.; Dunstan, D.E.; Pignot-Paintrand, I. Stress and strain controlled rheometry on a concentrated lyotropic lamellar phase of AOT/Water/Iso-octane. Rheol. Acta 2009, 48, 423–432. [Google Scholar] [CrossRef]
- Saramito, P. A new constitutive equation for elastoviscoplastic fluid flows. J. Non-Newton. Fluid Mech. 2007, 145, 1–14. [Google Scholar] [CrossRef]
- Cheddadi, I.; Saramito, P.; Graner, F. Steady Couette flows of elastoviscoplastic fluids are nonunique. J. Rheol. 2012, 56, 213–239. [Google Scholar] [CrossRef]
- Meeker, S.P.; Bonnecaze, R.T.; Cloitre, M. Slip and flow in pastes of soft particles: Direct observation and rheology. J. Rheol. 2004, 48, 1295–1320. [Google Scholar] [CrossRef]


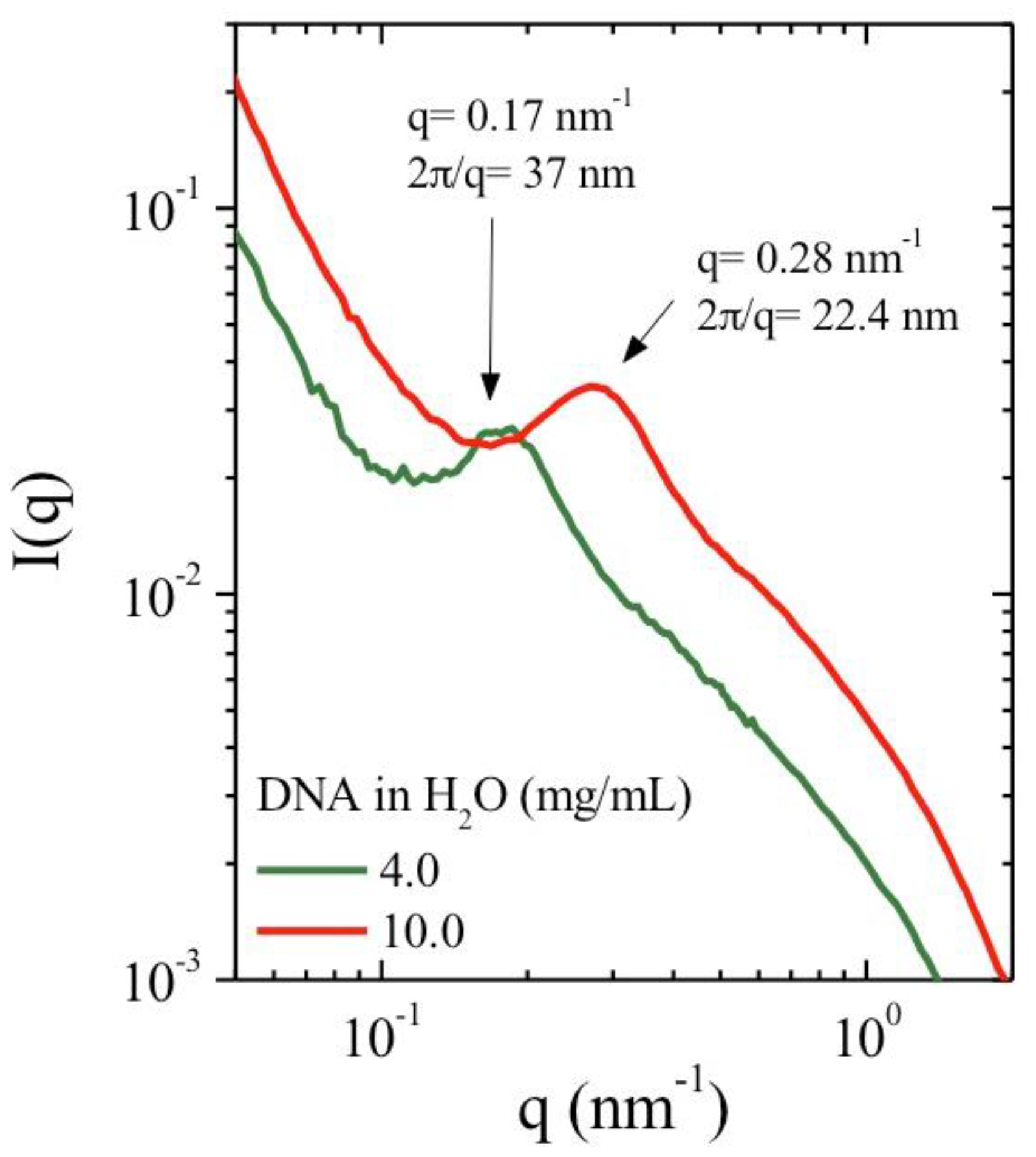














| Polymer Concentration (mg/mL) | κ−1water (nm) | κ−10.01 M NaCl (nm) | κ−10.1 M NaCl (nm) |
|---|---|---|---|
| 4 | 8.42 | 2.83 | 0.94 |
| 10 | 5.36 | 2.61 | 0.93 |
| Solvent Properties | H2O | 0.01 M NaCl | 0.1 M NaCl |
|---|---|---|---|
| 4 mg/mL Birefringence | σ = 5 Pa | σ = 4.6 Pa | σ = 4.5 Pa |
| 4 mg/mL Texture | NO | NO | NO |
| 10 mg/mL Birefringence | > 1 s−1 σ = 8 Pa | > 1 s−1 σ = 9 Pa | ~100 s−1 σ = 10 Pa |
| 10 mg/mL Texture | ~1–10 s−1 σ = 10 Pa | ~10 s−1 σ = 15 Pa | Heterogeneous birefringent domains σ = 8 Pa |
| Characteristic Parameters | H2O | 0.01 M NaCl | 0.1 M NaCl |
|---|---|---|---|
| G0 (Pa) | 0.84 | 0.68 | 0.78 |
| ω0 (rad/s) | 0.31 | 0.82 | 0.59 |
| η0 (Pa.s) for | 18 at = 10−3s−1 | 4.1 | 7.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo-Anaya, L.M.; Roux, D.C.D.; Soltero Martínez, J.F.A.; Carvajal Ramos, F.; Pignon, F.; Mannix, O.; Rinaudo, M. Role of Electrostatic Interactions on Supramolecular Organization in Calf-Thymus DNA Solutions under Flow. Polymers 2018, 10, 1204. https://doi.org/10.3390/polym10111204
Bravo-Anaya LM, Roux DCD, Soltero Martínez JFA, Carvajal Ramos F, Pignon F, Mannix O, Rinaudo M. Role of Electrostatic Interactions on Supramolecular Organization in Calf-Thymus DNA Solutions under Flow. Polymers. 2018; 10(11):1204. https://doi.org/10.3390/polym10111204
Chicago/Turabian StyleBravo-Anaya, L. Mónica, Denis C. D. Roux, J. Félix Armando Soltero Martínez, Francisco Carvajal Ramos, Frédéric Pignon, Oonagh Mannix, and Marguerite Rinaudo. 2018. "Role of Electrostatic Interactions on Supramolecular Organization in Calf-Thymus DNA Solutions under Flow" Polymers 10, no. 11: 1204. https://doi.org/10.3390/polym10111204






