Preparation and Hydrogen Storage Characteristics of Surfactant-Modified Graphene
Abstract
:1. Introduction and Background
2. Methodology
2.1. Materials and Apparatus
2.2. Preparation of Surfactant Modified Graphene
2.2.1. Preparation of Graphite Oxide
2.2.2. Preparation of Graphene
2.2.3. Inspection of Physical Structure
2.2.4. Inspection on the Hydrogen Storage Capacity
3. Characterization of Graphite Oxide and Graphene Samples
3.1. XRD Characterization
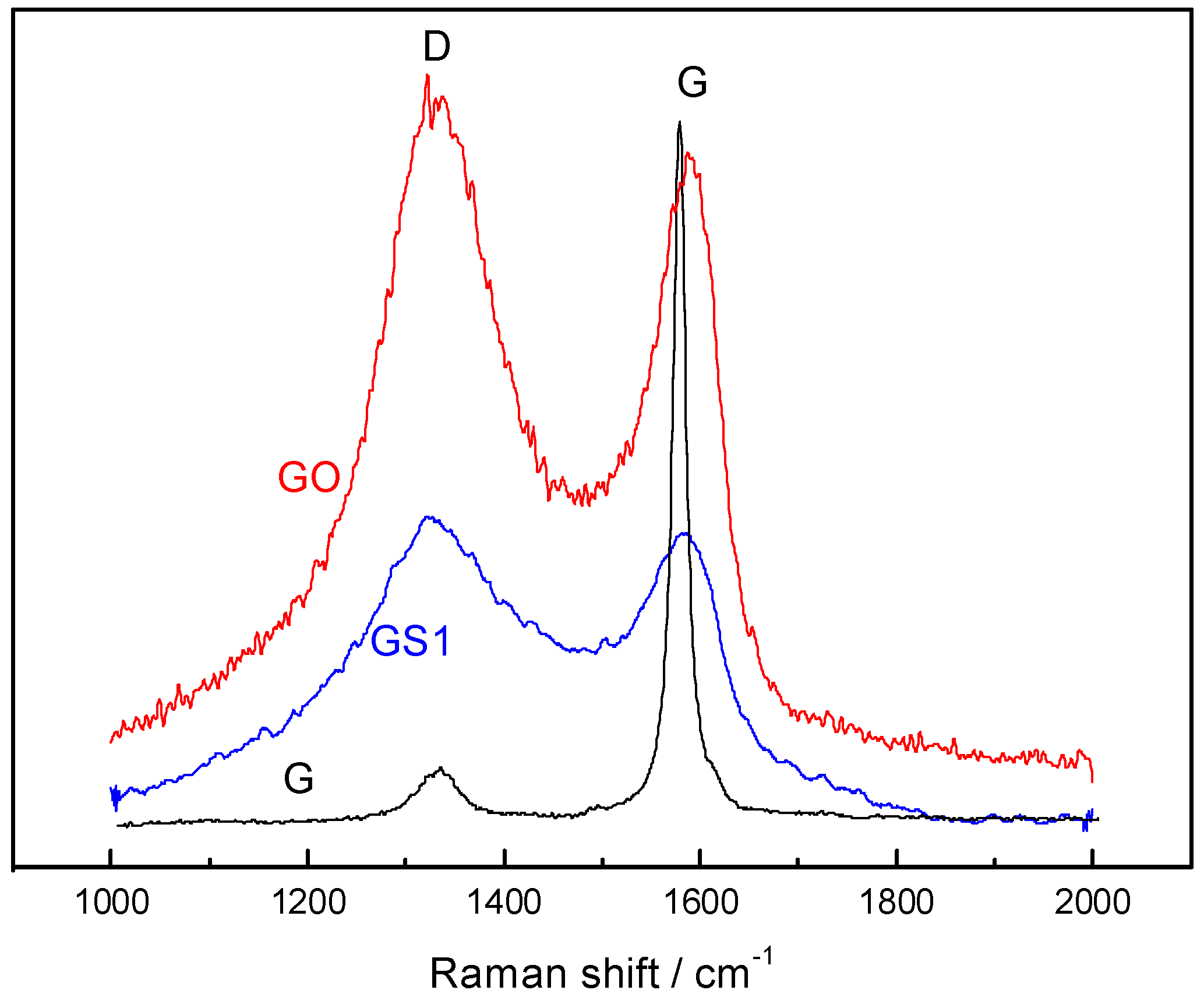
3.2. Raman Spectroscopy
3.3. FT-IR Characterization
3.4. XPS Characterization
3.5. TG Analysis
3.6. N2 Adsorption–Desorption Characterization
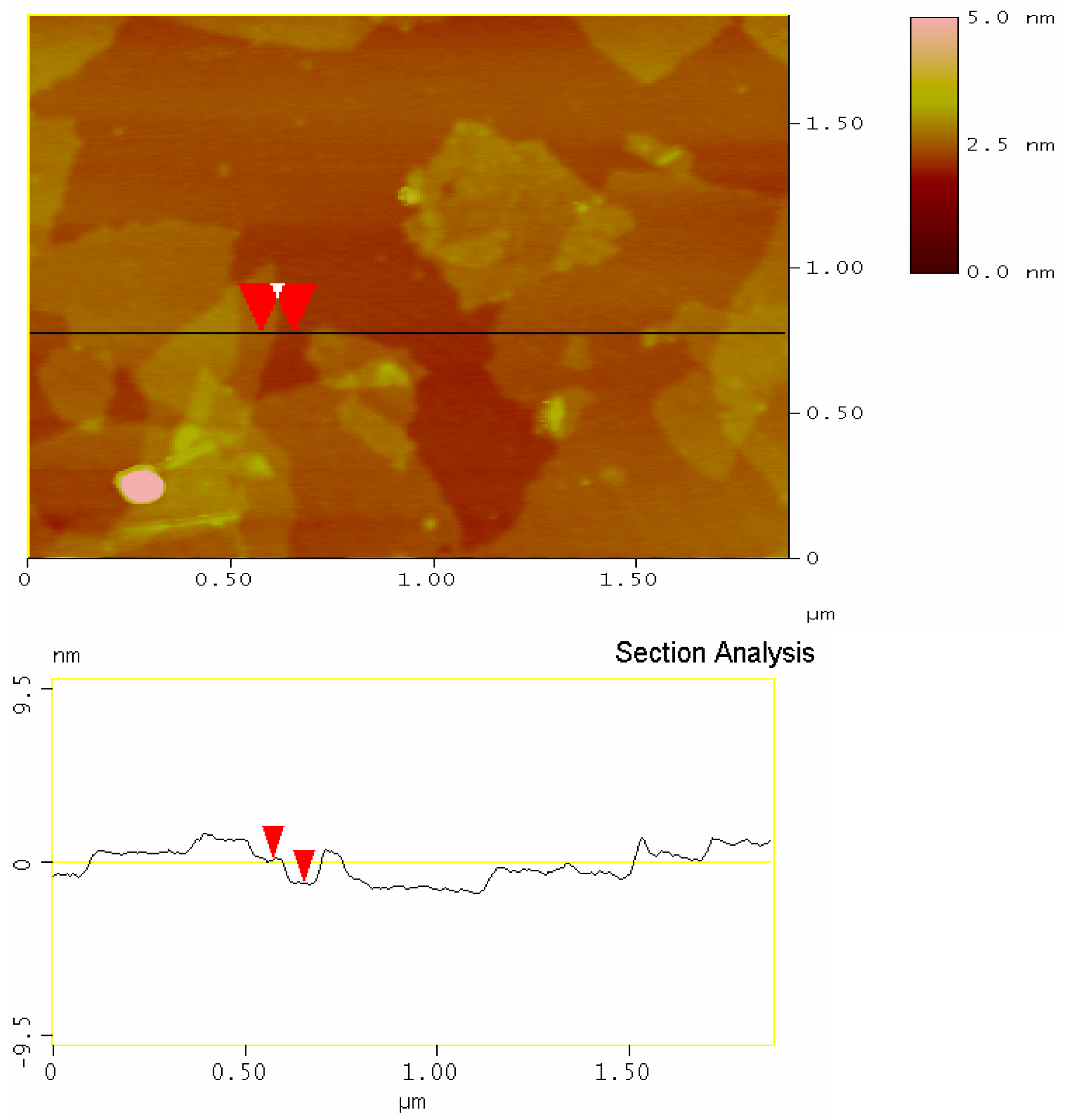
3.7. Morphological Representation
3.8. Dispersibility of Graphene Aqueous Solution
4. Validations of the Hydrogen Storage Capacity
4.1. Hydrogen Adsorption
4.2. Hydrogen Storage Capacity-Fitting
4.2.1. Langmuir Model
4.2.2. Freundlich Adsorption Model
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haryanto, A.; Fernando, S.; Murali, N.; Adhikari, S. Current Status of Hydrogen Production Techniques by Steam Reforming of Ethanol: A Review. Energy Fuels 2005, 19, 2098–2106. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Gallorini, F.; Servili, S. Hydrogen production through sorption-enhanced steam methane reforming and membrane technology: A review. Energy 2008, 33, 554–570. [Google Scholar] [CrossRef]
- Rosen, M.A. Advances in hydrogen production by thermochemical water decomposition: A review. Energy 2010, 35, 1068–1076. [Google Scholar] [CrossRef]
- Yang, J.; Wu, H.; Huang, G.; Liang, Y.; Liao, Y. Modeling and coupling effect evaluation of thermal conductivity of ternary opacifier/fiber/aerogel composites for super-thermal insulation. Mater. Des. 2017, 133, 224–236. [Google Scholar] [CrossRef]
- Yang, J.; Wu, H.; Wang, M.; Liang, Y. Prediction and optimization of radiative thermal properties of nano TiO2 assembled fibrous insulations. Int. J. Heat Mass Transf. 2018, 117, 729–739. [Google Scholar] [CrossRef]
- Khosravi, A.; Koury, R.N.N.; Machado, L.; Pabon, J.J.G. Energy, exergy and economic analysis of a hybrid renewable energy with hydrogen storage system. Energy 2018, 148, 1087–1102. [Google Scholar] [CrossRef]
- Nagpal, M.; Kakkar, R. An evolving energy solution: Intermediate hydrogen storage. Int. J. Hydrog. Energy 2018, 43, 12168–12188. [Google Scholar] [CrossRef]
- Assaf, J.; Shabani, B. Experimental study of a novel hybrid solar-thermal/PV-hydrogen system: Towards 100% renewable heat and power supply to standalone applications. Energy 2018, 157, 862–876. [Google Scholar] [CrossRef]
- Khiareddine, A.; Ben Salah, C.; Rekioua, D.; Mimouni, M.F. Sizing methodology for hybrid photovoltaic/wind/hydrogen/battery integrated to energy management strategy for pumping system. Energy 2018, 153, 743–762. [Google Scholar] [CrossRef]
- Campíñez-Romero, S.; Colmenar-Santos, A.; Pérez-Molina, C.; Mur-Pérez, F. A hydrogen refuelling stations infrastructure deployment for cities supported on fuel cell taxi roll-out. Energy 2018, 148, 1018–1031. [Google Scholar] [CrossRef]
- Onishi, N.; Laurenczy, G.; Beller, M.; Himeda, Y. Recent progress for reversible homogeneous catalytic hydrogen storage in formic acid and in methanol. Coord. Chem. Rev. 2018, 373, 317–332. [Google Scholar] [CrossRef]
- Muir, S.S.; Yao, X. Progress in sodium borohydride as a hydrogen storage material: Development of hydrolysis catalysts and reaction systems. Int. J. Hydrog. Energy 2011, 36, 5983–5997. [Google Scholar] [CrossRef]
- Yanik, M.O.; Yigit, E.A.; Akansu, Y.E.; Sahmetlioglu, E. Magnetic conductive polymer-graphene nanocomposites based supercapacitors for energy storage. Energy 2017, 138, 883–889. [Google Scholar] [CrossRef]
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Dillon, A.C.; Jones, K.M.; Bekkedahl, T.A.; Kiang, C.H.; Bethune, D.S.; Heben, M.J. Storage of hydrogen in single-walled carbon nanotubes. Nature 1997, 386, 377. [Google Scholar] [CrossRef]
- Liu, C.; Fan, Y.Y.; Liu, M.; Cong, H.T.; Cheng, H.M.; Dresselhaus, M.S. Hydrogen Storage in Single-Walled Carbon Nanotubes at Room Temperature. Science 1999, 286, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhou, Y.; Sun, Y. A comparative study of hydrogen adsorption on superactivated carbon versus carbon nanotubes. Int. J. Hydrog. Energy 2004, 29, 475–479. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Liao, B.; Liu, M.; Wei, Y.-L.; Lu, M.-Q.; Cheng, H.-M. Hydrogen uptake in vapor-grown carbon nanofibers. Carbon N. Y. 1999, 37, 1649–1652. [Google Scholar] [CrossRef]
- Gupta, B.K.; Srivastava, O.N. Further studies on microstructural characterization and hydrogenation behaviour of graphitic nanofibres. Int. J. Hydrog. Energy 2001, 26, 857–862. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, Y.; Sun, Y. Enhanced storage of hydrogen at the temperature of liquid nitrogen. Int. J. Hydrog. Energy 2004, 29, 319–322. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhary, A.; Malakkal, L.; Siripurapu, R.K.; Szpunar, B.; Szpunar, J. First principles calculations of hydrogen storage on Cu and Pd-decorated graphene. Int. J. Hydrog. Energy 2016, 41, 17652–17656. [Google Scholar] [CrossRef]
- Faye, O.; Eduok, U.; Szpunar, J.; Szpunar, B.; Samoura, A.; Beye, A. Hydrogen storage on bare Cu atom and Cu-functionalized boron-doped graphene: A first principles study. Int. J. Hydrog. Energy 2017, 42, 4233–4243. [Google Scholar] [CrossRef]
- Luo, Z.; Fan, X.; Pan, R.; An, Y. A first-principles study of Sc-decorated graphene with pyridinic-N defects for hydrogen storage. Int. J. Hydrog. Energy 2017, 42, 3106–3113. [Google Scholar] [CrossRef]
- Srinivas, G.; Zhu, Y.; Piner, R.; Skipper, N.; Ellerby, M.; Ruoff, R. Synthesis of graphene-like nanosheets and their hydrogen adsorption capacity. Carbon N. Y. 2010, 48, 630–635. [Google Scholar] [CrossRef]
- Ghosh, A.; Subrahmanyam, K.S.; Krishna, K.S.; Datta, S.; Govindaraj, A.; Pati, S.K.; Rao, C.N.R. Uptake of H2 and CO2 by Graphene. J. Phys. Chem. C 2008, 112, 15704–15707. [Google Scholar] [CrossRef]
- Ma, L.-P.; Wu, Z.-S.; Li, J.; Wu, E.-D.; Ren, W.-C.; Cheng, H.-M. Hydrogen adsorption behavior of graphene above critical temperature. Int. J. Hydrog. Energy 2009, 34, 2329–2332. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Gournis, D.; Petridis, D.; Szabó, T.; Szeri, A.; Dékány, I. Graphite Oxide: Chemical Reduction to Graphite and Surface Modification with Primary Aliphatic Amines and Amino Acids. Langmuir 2003, 19, 6050–6055. [Google Scholar] [CrossRef]
- Niyogi, S.; Bekyarova, E.; Itkis, M.E.; McWilliams, J.L.; Hamon, M.A.; Haddon, R.C. Solution Properties of Graphite and Graphene. J. Am. Chem. Soc. 2006, 128, 7720–7721. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Jafari-Asl, M.; Nabiyan, A.; Rezaei, B.; Dinari, M. Hydrogen storage in hybrid of layered double hydroxides/reduced graphene oxide using spillover mechanism. Energy 2016, 99, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Li, J.; Wang, X. Kinetic and thermodynamic study of 1-naphthol adsorption from aqueous solution to sulfonated graphene nanosheets. Chem. Eng. J. 2011, 173, 185–190. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, D.; Feng, X.; Müllen, K. Dispersion of Graphene Sheets in Organic Solvent Supported by Ionic Interactions. Adv. Mater. 2009, 21, 1679–1683. [Google Scholar] [CrossRef]
- Lu, X.; Xiao, Y.; Lei, Z.; Chen, J.; Zhang, H.; Ni, Y.; Zhang, Q. A promising electrochemical biosensing platform based on graphitized ordered mesoporous carbon. J. Mater. Chem. 2009, 19, 4707–4714. [Google Scholar] [CrossRef]
- Lin, Y.S.; Haynes, C.L. Synthesis and Characterization of Biocompatible and Size-Tunable Multifunctional Porous Silica Nanoparticles. Chem. Mater. 2009, 21, 3979–3986. [Google Scholar] [CrossRef]
- Pfaffeneder-Kmen, M.; Casas, I.F.; Naghilou, A.; Trettenhahn, G.; Kautek, W. A Multivariate Curve Resolution evaluation of an in-situ ATR-FTIR spectroscopy investigation of the electrochemical reduction of graphene oxide. Electrochim. Acta 2017, 255, 160–167. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon N. Y. 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon N. Y. 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Jhi, S.H.; Kwon, Y.K.; Bradley, K.; Gabrief, J.C.P. Hydrogen storage by physisorption: Beyond carbon. Solid State Commun. 2004, 129, 769–773. [Google Scholar] [CrossRef]












| Name | Chemical Formula | Molecular Weight | Level | Producer |
|---|---|---|---|---|
| Flake Graphite | C | 12.01 | 325 mesh, 99.8% | Alfa Aesar Tianjin Chemical Co., Ltd., Tianjin, China |
| Potassium Permanganate | KMnO4 | 158.04 | Analytically Pure | China Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| Strong Sulfuric | H2SO4 | 98.01 | 95.0–98.0% | China Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| Hydrogen Peroxide | H2O2 | 34.01 | 30% | China Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| Potassium Perchlorate | KClO4 | 138.56 | Analytically Pure | China Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| Muriatic Acid | HCl | 36.56 | 36–38% | China Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| Sodium Borohydride | NaBH4 | 37.85 | Analytically Pure | China Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| Sodium Nitrate | NaNO3 | 84.99 | Analytically Pure | China Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| Acetone | CH3COCH3 | 58.08 | Analytically Pure | China Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| SDBS | C18H29NaO3S | 348.48 | Analytically Pure | China Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| Equipment | Producer |
|---|---|
| Sartorius Electronic Scales | Beijing Sartorius Instrumentation System Co., Ltd., Beijing, China |
| DHG-9070A Electro-thermostatic Blast Oven | China Shanghai Yiheng Science and Technology Ltd., Shanghai, China |
| SHZ-D Circulating Vacuum Pump | China Gongyi Yuhua Instrumentation Co., Ltd., Gongyi, Henan, China |
| KQ-500VDF Ultrasonic Generator | China Kunshan Ultrasound Instrumentation Co., Ltd., Suzhou, Jiangsu, China |
| IKL RCT Basic Magnetic Stirrers | China Shanghai Yiheng Science and Technology Ltd., Shanghai, China |
| HC-3518 Centrifuge | China USTC Chuangxin Co., Ltd., Hefei, Anhui, China |
| T (K) | Langmuir Equation | Freundlich Equation | ||||
|---|---|---|---|---|---|---|
| b (mg/g) | R2 | K | n | R2 | ||
| 298 | 0.00123 | 0.2374 | 0.946 | 0.00414 | 0.486 | 0.997 |
| 328 | 0.00162 | 0.1300 | 0.963 | 0.00384 | 0.427 | 0.999 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Chen, J.; Yuan, W.; Li, B.; Li, L.; Wu, H.; Zhou, X. Preparation and Hydrogen Storage Characteristics of Surfactant-Modified Graphene. Polymers 2018, 10, 1220. https://doi.org/10.3390/polym10111220
Xu T, Chen J, Yuan W, Li B, Li L, Wu H, Zhou X. Preparation and Hydrogen Storage Characteristics of Surfactant-Modified Graphene. Polymers. 2018; 10(11):1220. https://doi.org/10.3390/polym10111220
Chicago/Turabian StyleXu, Tao, Jiayu Chen, Wenhui Yuan, Baoqing Li, Li Li, Huijun Wu, and Xiaoqing Zhou. 2018. "Preparation and Hydrogen Storage Characteristics of Surfactant-Modified Graphene" Polymers 10, no. 11: 1220. https://doi.org/10.3390/polym10111220
APA StyleXu, T., Chen, J., Yuan, W., Li, B., Li, L., Wu, H., & Zhou, X. (2018). Preparation and Hydrogen Storage Characteristics of Surfactant-Modified Graphene. Polymers, 10(11), 1220. https://doi.org/10.3390/polym10111220








