Therapeutic Effect and Metabolic Mechanism of A Selenium-Polysaccharide from Ziyang Green Tea on Chronic Fatigue Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Physicochemical Characteristic of Se-TP
2.3. Establishment of CFS Model
2.4. Analysis of Serum Metabolites
2.5. Urine Sample Preparation and Metabolites Extraction
2.6. GC-MS Detection
2.7. Statistics and Analysis of Data
3. Results
3.1. Composition and Morphology of Se-TP
3.2. Behavioral Test Results
3.3. Analysis of Related Serum Metabolites
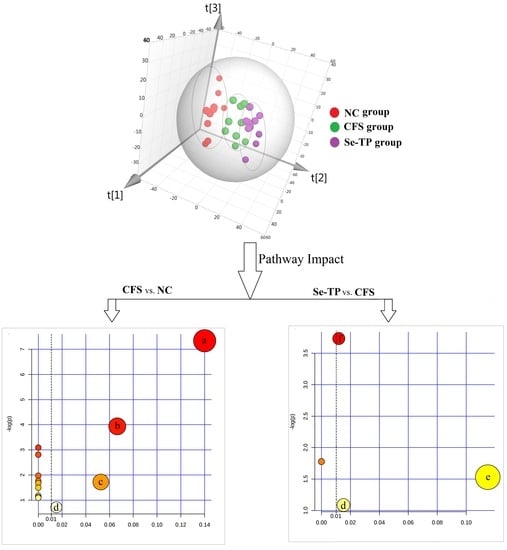
3.4. Analysis of Urine’s Metabolic Profiling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X.Q.; Takashi, T.; Zhu, S.; Junji, M.; Seiichiro, S.; Junichi, Y.; Kazuya, K.; Tohru, I.; Tsugiyasu, K. Effect of Hochu-ekki-to (TJ-41), a Japanese Herbal Medicine, on Daily Activity in a Murine Model of Chronic Fatigue Syndrome. Evid.-Based Complement. Altern. Med. 2004, 1, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Son, C.G. A case of chronic fatigue syndrome improved by traditional Korean medicine. Chin. J. Integr. Med. 2013, 2, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A.K. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Steele, L.; Dobbins, J.G.; Fukuda, K.; Reyes, M.; Randall, B.; Koppelman, M.; Reeves, W.C. The epidemiology of chronic fatigue in San Francisco. Am. J. Chin. Med. 1998, 105, 83S–90S. [Google Scholar] [CrossRef]
- Wessely, S.; Chalder, T.; Hirsch, S. The prevalence and morbidity of chronic fatigue and chronic fatigue syndrome: A prospective primary care study. Am. J. Public Health 1997, 87, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yu, H.; Wing, Y.; Lee, A.M.; Lee, D.T.; Chen, C.; Lin, K.; Weiss, M.G. Psychiatric morbidity and illness experience of primary care patients with chronic fatigue in Hong Kong. Am. J. Psychtatry 2000, 157, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Nojima, J.; Motoki, Y.; Yamaguti, K.; Nakatomi, Y.; Okawa, N.; Fujiwara, K.; Watanabe, Y. A potential biomarker for fatigue: Oxidative stress and anti-oxidative activity. Biol. Psychol. 2016, 118, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, A.S.; Cleare, A.J. Hypothalamic-pituitary-adrenal axis dysfunction in chronic fatigue syndrome. Nat. Rev. Endocrinol. 2012, 8, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, H.; Shoenfeld, Y.; Amital, H. The common immunogenic etiology of chronic fatigue syndrome: From infections to vaccines via adjuvants to the ASIA syndrome. Infect. Dis. Clin. 2011, 25, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Li, X.X.; Liu, J.P.; Luo, H.; Ma, L.X.; Alraek, T. Traditional Chinese medicine for chronic fatigue syndrome: A systematic review of randomized clinical trials. Complement. Ther. Med. 2014, 22, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.P.; He, X.R.; Yang, Y.; Li, M.X.; Hao, D.J.; Jia, Z.P. The genus Epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011, 34, 519–541. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Q.; Chen, B.C.L.; Dong, C.X.; Yang, Y.H.; Ko, C.H.; Gar-Lee Yue, G.; Chen, D.; Wong, C.K.; Bik-San Lau, C.; Tu, P.F.; et al. Isolation, structure characterization, and immunomodulating activity of a hyperbranched polysaccharide from the fruiting bodies of ganoderma sinense. J. Agric. Food Chem. 2012, 60, 4276–4281. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yang, J.; Lu, J.; Qiao, Q.; Wu, T.; Du, X.; Bao, G.; He, X. A polysaccharide from Salvia miltiorrhiza Bunge improves immune function in gastric cancer rats. Carbohydr. Polym. 2014, 111, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.P.; Chen, J.P.; Wang, Z.Z.; Xiong, Z.Y.; Li, Q.X. Morphological and structural characterization of a polysaccharide from Gynostemma pentaphyllum Makino and its anti-exercise fatigue activity. Carbohydr. Polym. 2008, 74, 868–874. [Google Scholar] [CrossRef]
- Chi, A.; Kang, C.; Zhang, Y.; Tang, L.; Guo, H.; Li, H.; Zhang, K. Immunomodulating and antioxidant effects of polysaccharide conjugates from the fruits of Ziziphus Jujube on Chronic Fatigue Syndrome rats. Carbohydr. Polym. 2015, 122, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.F.; Charlet, L. Selenium environmental cycling and bioavailability: A structural chemist point of view. Rev. Environ. Sci. Biol. 2009, 8, 81–110. [Google Scholar] [CrossRef]
- Sanmartín, C.; Plano, D.; Sharma, A.K.; Palop, J.A. Selenium Compounds, Apoptosis and Other Types of Cell Death: An Overview for Cancer Therapy. Int. J. Mol. Sci. 2012, 13, 9649–9672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Chen, J.; Zhang, D.; Zhang, Y.; Wen, Y.; Li, L.; Zheng, L. Tumoricidal effects of a selenium (Se)-polysaccharide from Ziyang green tea on human osteosarcoma U-2 OS cells. Carbohydr. Polym. 2013, 98, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhang, J.; Tang, Q.; Liu, Y.; Zhang, A.; Pan, Y. Structural elucidation of a neutral fuco galactan from the mycelium of Coprinus comatus. Carbohydr. Res. 2006, 341, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, B.; Wang, X.; Yao, J.; Zhang, J. Synthesis of selenium-containing polysaccharides and evaluation of antioxidant activity in vitroInt. Int. J. Biol. Macromol. 2012, 51, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, Y.; Sun, Y.; Yang, X. Protective effects of Ziyang tea polysaccharides on CCl4-induced oxidative liver damage in mice. Food Chem. 2014, 143, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Liu, Y.; Chen, X.; Wei, X. Extraction, characterization and antioxidant activities of Se-enriched tea polysaccharides. Int. J. Biol. Macromol. 2015, 77, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.; Li, H.; Kang, C.; Guo, H.; Wang, Y.; Guo, F.; Tang, L. Anti-fatigue activity of a novel polysaccharide conjugates from Ziyang green tea. Int. J. Biol. Macromol. 2015, 80, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Yang, D.; Wang, X.; Dai, J.; Wang, M.; Lei, Y. Metabonomic study of chronic heart failure and effects of chinese herbal decoction in rats. J. Chromatogr. A 2014, 1362, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Ren, Y.; Wang, Z.; Kang, C.; Jiang, H.; Chi, A. Detection of urine metabolites in a rat model of chronic fatigue syndrome before and after exercise. BioMed Res. Int. 2017, 2017, 8182020. [Google Scholar] [CrossRef] [PubMed]
- Lalremruta, V.; Prasanna, G.S. Evaluation of protective effect of Aegle marmelos Corr. In an animal model of chronic fatigue syndrome. Indian J. Pharmacol. 2012, 44, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, A.K.; Kuhad, A.; Chopra, K. Epigallocatechin gallate ameliorates behavioral and biochemical deficits in rat model of load-induced chronic fatigue syndrome. Brain Res. Bull. 2011, 86, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Takashi, T.; Yu, F.; Zhu, S.J.; Moriya, J.; Sumino, H.; Morimoto, S.; Yamaguchi, N.; Kanda, T. Beneficial effect of brewers’ yeast extract on daily activity in a murine model of chronic fatigue syndrome. Evid.-Based. Complement. Altern. Med. 2016, 3, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Salvador, C.; Martins, M.R.; Caldeira, A.T. Microanalysis characterization of bioactive protein-bound polysaccharides produced by amanita ponderosa cultures. Microsc. Microanal. 2015, 21, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.; Shen, Z.; Zhu, W.; Sun, Y.; Kang, Y.; Fei, G. Characterization of a protein-bound polysaccharide from herba epimedii and its metabolic mechanism in chronic fatigue syndrome. J. Ethnopharmacol. 2017, 203, 241. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.; Zhang, Y.; Kang, Y.; Shen, Z. Metabolic mechanism of a polysaccharide from schisandra chinensis to relieve chronic fatigue syndrome. Int. J. Biol. Macromol. 2016, 93, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Lyle, N.; Gomes, A.; Sur, T.; Munshi, S.; Paul, S.; Chatterjee, S.; Bhattacharyya, D. The role of antioxidant properties of Nardostachys jatamansi in alleviation of the symptoms of the chronic fatigue syndrome. Behav. Brain Res. 2009, 202, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Shevchuk, N.A. Possible use of repeated cold stress for reducing fatigue in chronic fatigue syndrome: A hypothesis. Behav. Brain Funct. 2007, 3, 55. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Yuan, J.; Lv, S.; Tu, J. Effects of exercise on behavior and peripheral blood lymphocyte apoptosis in a rat model of chronic fatigue syndromn. J. Huazhong. Univ. Sci. Technol. Med. Sci. 2010, 30, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Ottenweller, J.E.; Natelson, B.H.; Gause, W.C.; Carroll, K.K.; Beldowicz, D.; Zhou, X.D.; LaManca, J.J. Mouse running activity is lowered by Brucella abortus treatment: A potential model to study chronic fatigue. Physiol. Behav. 1998, 63, 795–801. [Google Scholar] [CrossRef]
- Singh, A.; Naidu, P.S.; Gupta, S.; Kulkarni, S.K. Effect of natural and synthetic antioxidants in a mouse model of chronic fatigue syndrome. J. Med. Food 2002, 5, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.B.; Lange, G.; DeLuca, J. Relationship of brain MRI abnormalities and physical functional status in chronic fatigue syndrome. Int. J. Neurosci. 2001, 107, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Heins, M.J.; Knoop, H.; Bleijenberg, G. The role of the therapeutic relationship in cognitive behaviour therapy for chronic fatigue syndrome. Behav. Res. Ther. 2013, 51, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Heins, M.J.; Knoop, H.; Lobbestael, J.; Bleijenberg, G. Childhood maltreatment and the response to cognitive behavior therapy for chronic fatigue syndrome. J. Psychosom. Res. 2011, 71, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, C.C.; Kelly, R.M.; Wiley, R.G.; Walsh, T.J. Impaired acquisition of a Morris water maze task following selective destruction of cerebellar purkinje cells with OX7-saporin. Behav. Brain Res. 2000, 109, 37–47. [Google Scholar] [CrossRef]
- Wheatland, R. Chronic ACTH auto antibodies are a significant pathological factor in the disruption of the hypothalamic pituitary-adrenal axis in chronic fatigue syndrome, anorexia nervosa and major depression. Med. Hypotheses 2005, 65, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Saccenti, E.; Hoefsloot, H.C.J.; Smilde, A.K.; Westerhuis, J.A.; Hendriks, M.M.W.B. Reflections on univariate and multivariate analysis of metabolomics data. Metabolomics 2014, 10, 361–374. [Google Scholar] [CrossRef]
- Eriksson, L.; Antti, H.; Gottfries, J.; Holmes, E.; Johansson, E.; Lindgren, F.; Long, I.; Lundstedt, T.; Trygg, J.; Wold, S. Using chemometrics for navigating in the large data sets of genomics, proteomics, and metabonomics (gpm). Anal. Bioanal. Chem. 2004, 380, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Nagyszakal, D.; Barupal, D.K.; Lee, B.; Che, X.; Williams, B.L.; Kahn, E.J.R.; Ukaigwe, J.E.; Bateman, L.; Klimas, N.G.; Komaroff, A.L.; et al. Insights into myalgic encephalomyelitis/chronic fatigue syndrome phenotypes through comprehensive metabolomics. Sci. Rep. 2018, 8, 10056. [Google Scholar] [CrossRef] [PubMed]
- Castro-Marrero, J.; Serrano-Pertierra, E.; Oliveira-Rodríguez, M.; Zaragozá, M.C.; Martínez-Martínez, A.; Blanco-López, M.; Alegre, J. Circulating extracellular vesicles as potential biomarkers in chronic fatigue syndrome/myalgic encephalomyelitis: An exploratory pilot study. J. Extracell. Vesicles 2018, 7, 1453730. [Google Scholar] [CrossRef] [PubMed]
- Yamano, E.; Kataoka, Y. New diagnostic biomarkers for chronic fatigue syndrome. Brain Nerve= Shinkei Kenkyū no Shinpo 2018, 70, 27–34. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Shishkanova, N.V.; Morgunov, I.G.; Finogenova, T.V. Oxygen requirements for growth and citric acid production of yarrowia lipolytica. FEMS. Yeast Res. 2010, 3, 217–222. [Google Scholar] [CrossRef]
- Newsholme, P.; Bender, K.; Kiely, A.; Brennan, L. Amino acid metabolism, insulin secretion and diabetes. Biochem. Soc. Trans. 2007, 35, 1180–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bode, C.; Gräle, M.H. Immune Regulation by Sphingosine 1-Phosphate and Its Receptors. Arch. Immunol. Ther. Exp. 2012, 60, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Melendez, A.J. Sphingosine kinase signalling in immune cells: Potential as novel therapeutic targets. Biochim. Biophys. Acta 2008, 1784, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Gonzálezusano, A.; Cauli, O.; Agusti, A.; Felipo, V. Pregnenolone sulfaterestores the Glutamate-nitric-oxide-c GMP pathway and Extracellular GABA in cerebellum and learning and motor coordination in hy-perammonemic rats. ACS Chem. Neurosci. 2014, 5, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Kreinin, A.; Bawakny, N.; Ritsner, M.S. Adjunctive pregnenolone ameliorates the cognitive deficits in recent-onset schizophrenia. Clin. Schizophr. Relat. Psychoses 2017, 10, 201–210. [Google Scholar] [CrossRef] [PubMed]








| Groups (n = 10) | Body Weight (g) | Diet | Morris Water Maze Test | Open-Field Test | Tail-Suspension Test | ||||
|---|---|---|---|---|---|---|---|---|---|
| Initial Weight | Last Weight | Fodder (g/d) | Water (mL/d) | Time of Searching for Platform (s) | Number of Times | Number of Times | Number of Standing Events | Motionless Time (s) | |
| NC | 149.25 ± 10.63 | 201.37 ± 9.09 | 20.84 ± 4.45 | 54.65 ± 8.97 | 31.22 ± 14.53 | 1.38 ± 1.30 | 46.25 ± 5.23 | 15.88 ± 4.12 | 78.75 ± 15.37 |
| CFS | 151.41 ± 10.27 | 177.90 ± 11.13 aa | 16.61 ± 3.15 aa | 45.89 ± 12.45 | 50.09 ± 18.09 a | 0.75 ± 0.71 | 33.69 ± 5.19 aa | 11.50 ± 2.48 a | 100.50 ± 11.94 aa |
| Se-TP (100 mg/kg) | 148.98 ± 9.56 | 181.38 ± 14.27 aa | 17.65 ± 4.17 | 47.64 ± 13.27 | 45.66 ± 10.97 a | 1.12 ± 0.67 | 36.58 ± 7.21 aa | 14.26 ± 3.85 | 89.55 ± 9.13 b |
| Se-TP (200 mg/kg) | 149.17 ± 9.81 | 188.79 ± 9.02 aab | 19.11 ± 2.98 | 43.88 ± 7.09 aa | 37.53 ± 5.09 b | 1.34 ± 0.97 | 39.43 ± 6.31 ab | 14.28 ± 3.67 b | 84.36 ± 11.71 bb |
| Groups (n = 10) | BUN (mmol/L) | BLA (mmol/L) | CORT (ng/mL) | ALD (pg/mL) |
|---|---|---|---|---|
| NC | 6.33 ± 1.82 | 3.65 ± 0.76 | 152.49 ± 12.45 | 72.36 ± 5.26 |
| CFS | 8.24 ± 2.05 a | 3.77 ± 1.62 | 141.84 ± 9.37 a | 68.48 ± 5.71 |
| Se-TP (100 mg/kg) | 6.54 ± 2.26 | 2.94 ± 0.86 | 149.73 ± 8.92 | 73.12 ± 6.53 |
| Se-TP (200 mg/kg) | 6.29 ± 1.47 b | 3.76 ± 1.41 | 156.12 ± 16.07 b | 67.57 ± 4.85 a |
| No. | RT (min) a | m/zb | KEGG c | VIP | p-Value | Metabolite | Trend | |
|---|---|---|---|---|---|---|---|---|
| CFS vs. NC | 1 | 8.87 | 188 | C00383 | 2.34 | 0.008 | Malonic acid | ↓ |
| 2 | 11.00 | 245 | C00122 | 1.87 | 0.013 | Fumaric acid | ↑ | |
| 3 | 11.65 | 184 | C01732 | 1.81 | 0.020 | 2-methylfumarate | ↑ | |
| 4 | 12.73 | 233 | C00149 | 2.05 | 0.020 | l-malic acid | ↑ | |
| 5 | 12.93 | 122 | C02946 | 1.67 | 0.044 | 4-acetamidobutyric acid | ↓ | |
| 6 | 13.83 | 304 | C00026 | 1.03 | 0.034 | α-ketoglutaric acid | ↓ | |
| 7 | 14.48 | 292 | C00898 | 2.390 | 0.002 | (R,R)-tartaric acid | ↓ | |
| 8 | 18.27 | 333 | C00257 | 2.018 | 0.018 | d-gluconic acid | ↓ | |
| 9 | 18.77 | 117 | C00249 | 1.92 | 0.013 | Palmitic acid | ↑ | |
| 10 | 22.16 | 204 | C00319 | 2.16 | 0.021 | Sphingosine | ↓ | |
| 11 | 26.57 | 73 | C05485 | 2.82 | 0.000 | 21-hydroxypregnenolone | ↓ | |
| Se-TP vs. CFS | 1 | 9.74 | 179 | C00180 | 1.33 | 0.045 | Benzoic acid | ↓ |
| 2 | 10.88 | 215 | C00490 | 1.63 | 0.015 | Itaconic acid | ↑ | |
| 3 | 11.71 | 97 | C00489 | 1.71 | 0.026 | Glutaric acid | ↑ | |
| 4 | 12.93 | 122 | C02946 | 2.02 | 0.027 | 4-acetamidobutyric acid | ↑ | |
| 5 | 13.55 | 115 | C00300 | 1.49 | 0.043 | Creatine | ↑ | |
| 6 | 13.77 | 247 | C04411 | 1.78 | 0.041 | 2-hydroxy-3-isopropylbutanedioic acid | ↑ | |
| 7 | 18.90 | 268 | C00355 | 1.80 | 0.018 | l-dopa | ↑ | |
| 8 | 26.57 | 73 | C05485 | 1.72 | 0.035 | 21-hydroxypregnenolone | ↑ |
| R.T. | Metabolites | NC Group | CFS Group | Se-TP Group |
|---|---|---|---|---|
| 8.87 | Malonic acid | 0.0029 ± 0.0022 | 0.0005 ± 0.0001 aa | 0.0007 ± 0.0003 aa |
| 9.74 | Benzoic acid | 0.0412 ± 0.0169 | 0.0433 ± 0.0056 | 0.0327 ± 0.0003 bb |
| 10.88 | Itaconic acid | 0.0078 ± 0.0027 | 0.0059 ± 0.0045 | 0.0098 ± 0.0036 b |
| 11.00 | Fumaric acid | 0.0412 ± 0.0189 | 0.0757 ± 0.0387 a | 0.1059 ± 0.1363 |
| 11.65 | 2-methylfumarate | 0.0059 ± 0.0015 | 0.0075 ± 0.0015 a | 0.0094 ± 0.0032 aa |
| 11.71 | Glutaric acid | 0.0105 ± 0.0067 | 0.0103 ± 0.0057 | 0.0311 ± 0.0229 a,b |
| 12.73 | l-malic acid | 0.1060 ± 0.0473 | 0.2084 ± 0.1295 a | 0.2213 ± 0.1680 |
| 12.93 | 4-acetamidobutyric acid | 0.0016 ± 0.0007 | 0.0011 ± 0.0001 a | 0.0015 ± 0.0014 b |
| 13.55 | Creatine | 0.5819 ± 0.5568 | 0.8729 ± 0.4223 | 1.3787 ± 0.4483 aa,b |
| 13.77 | 2-hydroxy-3-isopropylbutanedioic acid | 0.0227 ± 0.0124 | 0.0306 ± 0.0189 | 0.0735 ± 0.0472 aa,b |
| 13.83 | α-ketoglutaric acid | 0.0009 ± 0.0004 | 0.0005 ± 0.0002 a | 0.0007 ± 0.0003 |
| 14.48 | Tartaric acid | 0.0035 ± 0.0022 | 0.0013 ± 0.0002 aa | 0.0017 ± 0.0008 a |
| 18.27 | Gluconic acid | 0.0026 ± 0.0012 | 0.0017 ± 0.0002 a | 0.0018 ± 0.0003 |
| 18.77 | Palmitic acid | 0.0273 ± 0.0076 | 0.0393 ± 0.0160 a | 0.0340 ± 0.0084 |
| 18.90 | l-dopa | 0.0057 ± 0.0021 | 0.0042 ± 0.0017 | 0.0062 ± 0.0023 b |
| 22.16 | Sphingosine | 0.0023 ± 0.0004 | 0.0014 ± 0.0012 a | 0.0021 ± 0.0020 |
| 26.57 | 21-hydroxypregnenolone | 0.0022 ± 0.0003 | 0.0016 ± 0.0008 a | 0.0027 ± 0.0011 b |
| Pathway Name | Total a | Hits b | pc | −log(p) | Holm p d | FDR e | Impact Score f | |
|---|---|---|---|---|---|---|---|---|
| CFS vs. NC | Citrate cycle | 20 | 3 | 0.001 | 7.340 | 0.053 | 0.053 | 0.140 |
| Alanine-aspartate-glutamate metabolism | 24 | 2 | 0.019 | 3.936 | 1.0 | 0.791 | 0.066 | |
| d-Glutamine and d-glutamate metabolism | 5 | 1 | 0.046 | 3.088 | 1.0 | 1.0 | 0.0 | |
| Arginine-proline metabolism | 44 | 2 | 0.060 | 2.808 | 1.0 | 1.0 | 0.0 | |
| Glyoxylate-dicarboxylate metabolism | 16 | 1 | 0.139 | 1.972 | 1.0 | 1.0 | 0.0 | |
| Butanoate metabolism | 20 | 1 | 0.171 | 1.766 | 1.0 | 1.0 | 0.0 | |
| Sphingolipid metabolism | 21 | 1 | 0.179 | 1.721 | 1.0 | 1.0 | 0.053 | |
| Pyruvate metabolism | 22 | 1 | 0.187 | 1.679 | 1.0 | 1.0 | 0.0 | |
| Fatty acid elongation in mitochondria | 27 | 1 | 0.224 | 1.495 | 1.0 | 1.0 | 0.0 | |
| Fatty acid metabolism | 39 | 1 | 0.308 | 1.177 | 1.0 | 1.0 | 0.0 | |
| Tyrosine metabolism | 42 | 1 | 0.328 | 1.116 | 1.0 | 1.0 | 0.0 | |
| Biosynthesis of unsaturated fatty acids | 42 | 1 | 0.328 | 1.116 | 1.0 | 1.0 | 0.0 | |
| Fatty acid biosynthesis | 43 | 1 | 0.334 | 1.096 | 1.0 | 1.0 | 0.0 | |
| Steroid hormone biosynthesis | 70 | 1 | 0.488 | 0.718 | 1.0 | 1.0 | 0.015 | |
| Se-TP vs. CFS | Arginine-proline metabolism | 44 | 2 | 0.024 | 3.734 | 1.0 | 1.0 | 0.012 |
| Glycine-serine-threonine metabolism | 32 | 1 | 0.169 | 1.778 | 1.0 | 1.0 | 0.0 | |
| Tyrosine metabolism | 42 | 1 | 0.216 | 1.530 | 1.0 | 1.0 | 0.115 | |
| Steroid hormone biosynthesis | 70 | 1 | 0.337 | 1.088 | 1.0 | 1.0 | 0.015 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, C.; Song, J.; Zhao, S.; Jiang, H.; Wang, B.; Chi, A. Therapeutic Effect and Metabolic Mechanism of A Selenium-Polysaccharide from Ziyang Green Tea on Chronic Fatigue Syndrome. Polymers 2018, 10, 1269. https://doi.org/10.3390/polym10111269
Shao C, Song J, Zhao S, Jiang H, Wang B, Chi A. Therapeutic Effect and Metabolic Mechanism of A Selenium-Polysaccharide from Ziyang Green Tea on Chronic Fatigue Syndrome. Polymers. 2018; 10(11):1269. https://doi.org/10.3390/polym10111269
Chicago/Turabian StyleShao, Changzhuan, Jing Song, Shanguang Zhao, Hongke Jiang, Baoping Wang, and Aiping Chi. 2018. "Therapeutic Effect and Metabolic Mechanism of A Selenium-Polysaccharide from Ziyang Green Tea on Chronic Fatigue Syndrome" Polymers 10, no. 11: 1269. https://doi.org/10.3390/polym10111269
APA StyleShao, C., Song, J., Zhao, S., Jiang, H., Wang, B., & Chi, A. (2018). Therapeutic Effect and Metabolic Mechanism of A Selenium-Polysaccharide from Ziyang Green Tea on Chronic Fatigue Syndrome. Polymers, 10(11), 1269. https://doi.org/10.3390/polym10111269