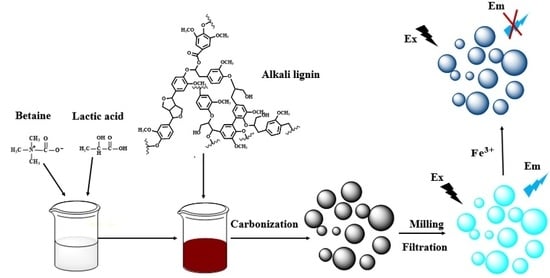
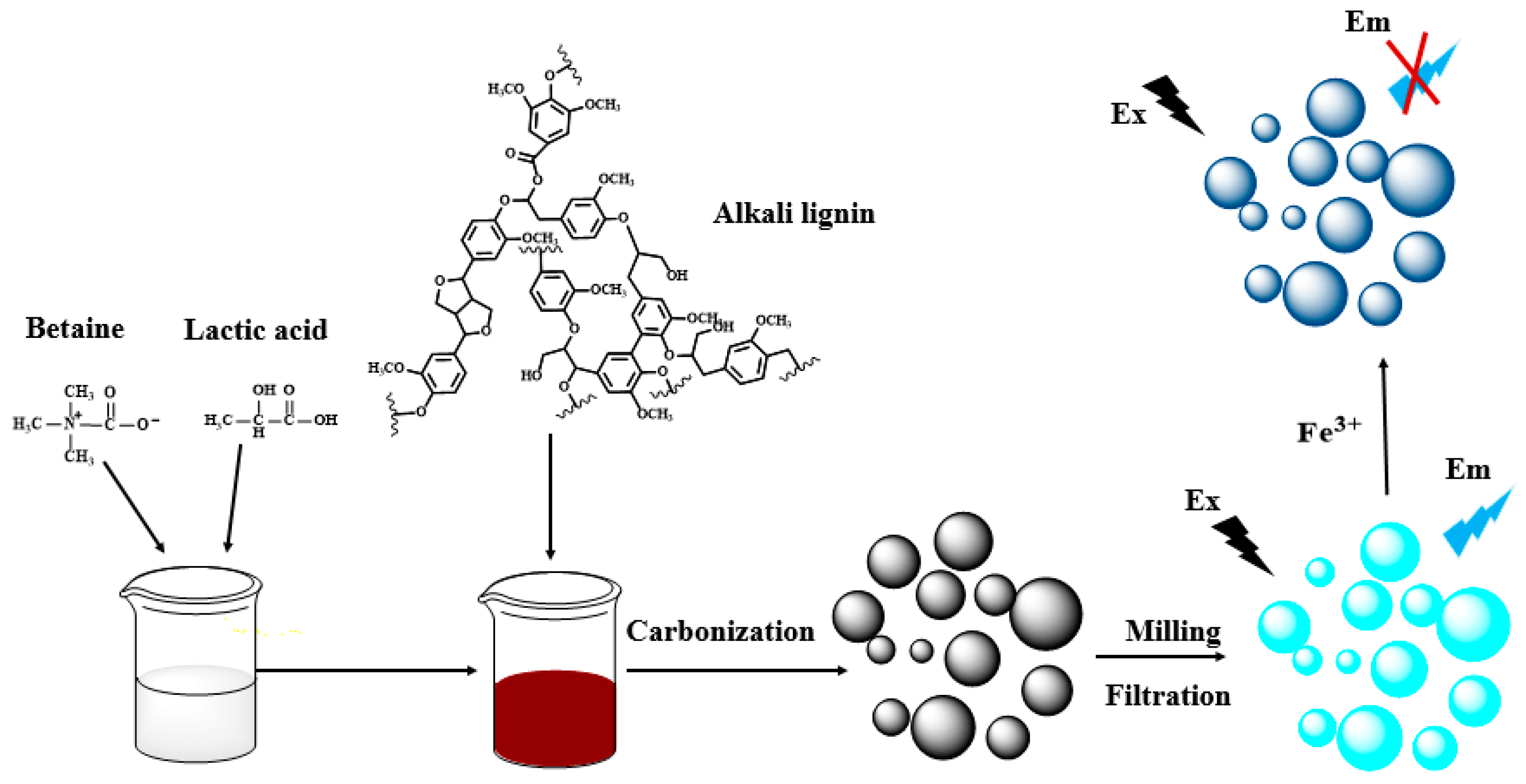
Synthesis of Nitrogen-Doped Lignin/DES Carbon Quantum Dots as a Fluorescent Probe for the Detection of Fe3+ Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of DES and Dissolution of Alkali Lignin
2.3. Synthesis of Nitrogen-Doped Lignin Carbon Quantum Dots (N-L-CQDs)
2.4. Characterizations
2.4.1. The Measurement of Quantum Yield of N-L-CQDs
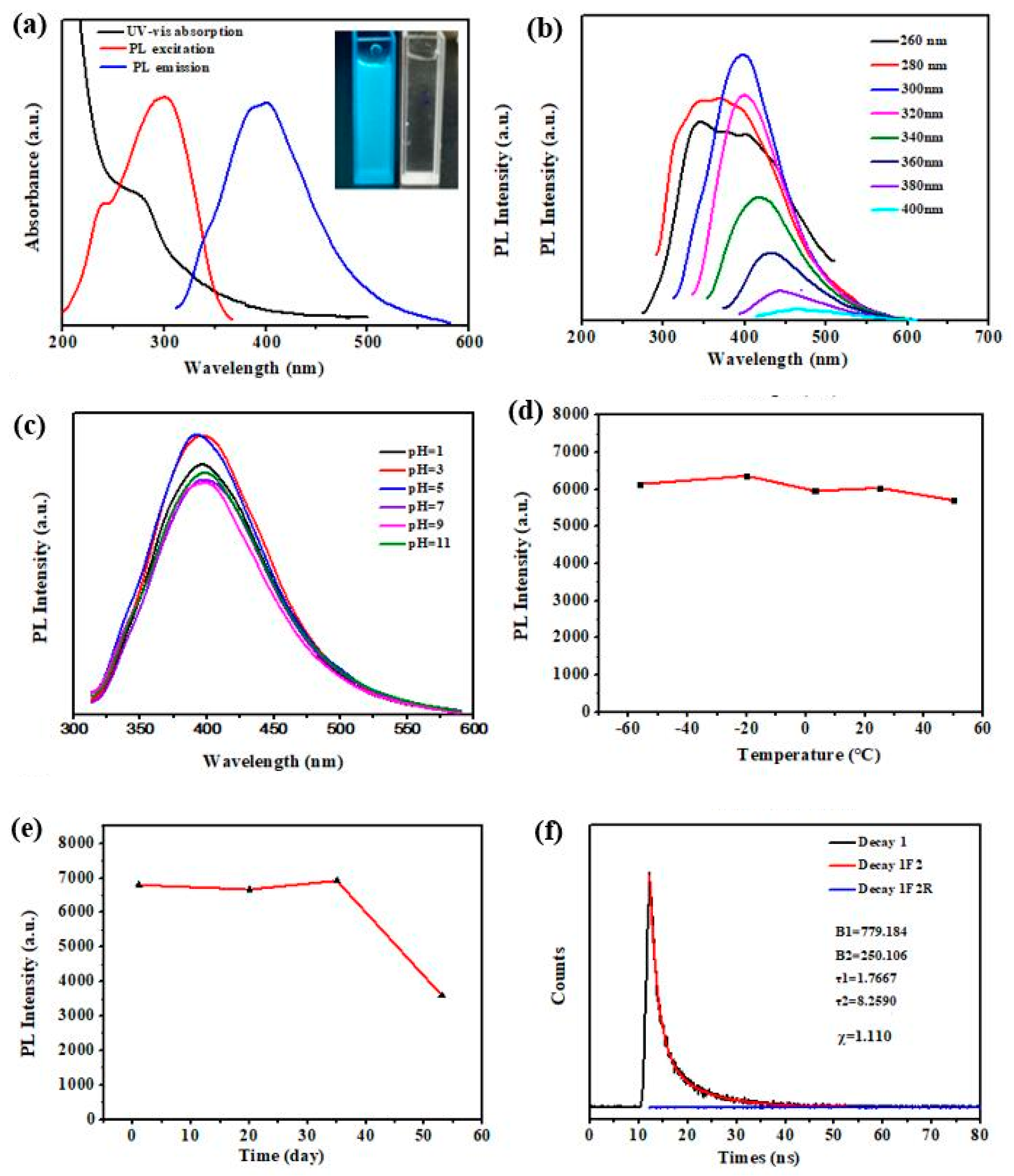
2.4.2. Effect of pH, Temperature, and Time on the Fluorescence Sensing of N-L-CQDs Aqueous Solutions
2.4.3. Cytotoxicity Assay
2.4.4. Fluorescence Detection of Fe3+
2.4.5. Selective Detection of Metal Cations
2.4.6. Detection of Fe3+ in Real Water Samples
3. Results and Discussion
3.1. Physiochemical Characterizations
3.2. Optical Properties of N-L-CQDs
3.3. Detection of Fe3+ Ions
3.4. Cytotoxicity Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Guo, X. Chemically Doped Fluorescent Carbon and Graphene Quantum Dots for Bioimaging, Sensor, Catalytic and Photoelectronic Applications. Nanoscale 2016, 8, 2532–2543. [Google Scholar] [CrossRef] [PubMed]
- Zong, J.; Yang, X.; Trinchi, A.; Hardin, S.; Cole, I.; Zhu, Y.; Li, C.; Muster, T.; Wei, G. Carbon dots as fluorescent probes for “off-on” detection of Cu2+ and L-cysteine in aqueous solution. Biosens. Bioelectron. 2014, 51, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.J.; Li, Y.; Zhu, R.H.; Shan, D.; Fan, Y.R.; Zhang, X.J. Ethylenediamine-assisted hydrothermal synthesis of nitrogen-doped carbon quantum dots as fluorescent probes for sensitive biosensing and bioimaging. Sens. Actuators B 2015, 218, 229–236. [Google Scholar] [CrossRef]
- Hou, J.; Cheng, H.; Yang, C.; Takeda, O.; Zhu, H. Hierarchical carbon quantum dots/hydrogenated-γ-TaON heterojunctions for broad spectrum photocatalytic performance. Nano Energy 2015, 18, 143–153. [Google Scholar] [CrossRef]
- Posthuma-Trumpie, G.A.; Wichers, J.H.; Koets, M.; Berendsen, L.B.; van Amerongen, A. Amorphous carbon nanoparticles: A versatile label for rapid diagnostic (immuno) assays. Anal. Bioanal. Chem. 2012, 402, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Su, H.; Wang, K.; Wong, W.K.; Zhu, X. Facile synthesis of N-rich carbon quantum dots from porphyrins as efficient probes for bioimaging and biosensing in living cells. Int. J. Nanomed. 2017, 12, 7375–7391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wang, H.; Shimizu, Y.; Pyatenko, A.; Kawaguchi, K.; Koshizaki, N. Preparation of carbon quantum dots with tunable photoluminescence by rapid laser passivation in ordinary organic solvents. Chem. Commun. 2011, 47, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Wu, C.; Liu, D.; Wang, H.; Su, W.; Li, H.; Zhang, Y.; Yao, S. A facile and simple method for synthesis of graphene oxide quantum dots from black carbon. Green Chem. 2017, 19, 900–904. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2015, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.; Yang, X.; Lee, S.T. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem. 2010, 49, 4430–4434. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Booker, C.; Li, R.; Zhou, X.; Sham, T.K.; Sun, X.; Ding, Z. An electrochemical avenue to blue luminescent nanocrystals from multiwalled carbon nanotubes (MWCNTs). J. Am. Chem. Soc. 2007, 129, 744–745. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ye, T.; Mao, C. Fluorescent carbon nanoparticles derived from candle soot. Angew. Chem. 2007, 46, 6473–6475. [Google Scholar] [CrossRef] [PubMed]
- De, B.; Karak, N. A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana juice. RSC Adv. 2013, 3, 8286–8290. [Google Scholar] [CrossRef]
- Zhu, C.; Zhai, J.; Dong, S. Bifunctional fluorescent carbon nanodots: Green synthesis via soy milk and application as metal-free electrocatalysts for oxygen reduction. Chem. Commun. 2012, 48, 9367–9369. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, X.; Liu, Y.; Yu, H.; Kang, Z.; Lee, S.-T. Synthesis of fluorescent carbon nanoparticles directly from active carbon via a one-step ultrasonic treatment. Mater. Res. Bull. 2011, 46, 147–151. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, X.; Li, Y.; Wang, Z.; Yang, F.; Yang, X. Microwave synthesis of fluorescent carbon nanoparticles with electrochemiluminescence properties. Chem. Commun. 2009, 5118–5120. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; He, Z.; Jiang, L.P.; Zhu, J.J. Microwave-assisted synthesis of wavelength-tunable photoluminescent carbon nanodots and their potential applications. ACS Appl. Mater. Interfaces 2015, 7, 4913–4920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shen, W.; Pan, D.; Zhang, Z.; Fang, Y.; Wu, M. Controlled synthesis of green and blue luminescent carbon nanoparticles with high yields by the carbonization of sucrose. New J. Chem. 2010, 34, 591–593. [Google Scholar] [CrossRef]
- Puvvada, N.; Kumar, B.N.; Konar, S.; Kalita, H.; Mandal, M.; Pathak, A. Synthesis of biocompatible multicolor luminescent carbon dots for bioimaging applications. Sci. Technol. Adv. Mater. 2012, 13, 045008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Cui, J.; Zheng, M.; Hu, C.; Tan, S.; Xiao, Y.; Yang, Q.; Liu, Y. One-step synthesis of amino-functionalized fluorescent carbon nanoparticles by hydrothermal carbonization of chitosan. Chem. Commun. 2012, 48, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Luderer, M.; Yang, X.; Swain, C.; Zhang, H.; Nelson, K.; Stacy, A.J.; Shen, B.; Lanza, G.M.; Pan, D. Surface passivation of carbon nanoparticles with branched macromolecules influences near infrared bioimaging. Theranostics 2013, 3, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yin, Y.; Wang, C.-F.; Chen, S. Plant leaf-derived fluorescent carbon dots for sensing, patterning and coding. J. Mater. Chem. C 2013, 1, 4925–4932. [Google Scholar] [CrossRef]
- Varisco, M.; Zufferey, D.; Ruggi, A.; Zhang, Y.; Erni, R.; Mamula, O. Synthesis of hydrophilic and hydrophobic carbon quantum dots from waste of wine fermentation. R. Soc. Open Sci. 2017, 4, 170900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; Zhang, X.; Sheng, Y.; Shen, J.; Huang, P.; Guo, S.; Pan, J.; Liu, B.; Feng, B. Simple one-step synthesis of water-soluble fluorescent carbon dots from waste paper. New J. Chem. 2014, 38, 906–909. [Google Scholar] [CrossRef]
- Mancera, C.; Ferrando, F.; Salvadó, J.; El Mansouri, N.E. Kraft lignin behavior during reaction in an alkaline medium. Biomass Bioenergy 2011, 35, 2072–2079. [Google Scholar] [CrossRef]
- Chatterjee, S.; Saito, T. Lignin-Derived Advanced Carbon Materials. ChemSusChem 2015, 8, 3941–3958. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Mao, W.; Huang, J.; Li, S.; Huang, K.; Li, M.; Xia, J.; Chen, Q. Economical, green route to highly fluorescence intensity carbon materials based on ligninsulfonate/graphene quantum dots composites: Application as excellent fluorescent sensing platform for detection of Fe3+ ions. Sens. Actuators B Chem. 2016, 230, 54–60. [Google Scholar] [CrossRef]
- Wei, L.; Tong, G.; Wang, M.; Tian, T.; Zeng, Y.; Wang, S. Preparation of the alkaline lignin pyrolytic based carbon quantum dots/TiO2 composite photocatalyst. J. For. Eng. 2016. [Google Scholar] [CrossRef]
- Ding, Z.; Li, F.; Wen, J.; Wang, X.; Sun, R. Gram-scale synthesis of single-crystalline graphene quantum dots derived from lignin biomass. Green Chem. 2018, 20, 1383–1390. [Google Scholar] [CrossRef]
- Liu, W.J.; Hong, J.; Yu, H.Q.; Bruijnincx, P.C.A.; Rinaldi, R.; Weckhuysen, B. Thermochemical conversion of lignin to functional materials: A review and future directions. Green Chem. 2015, 17, 4888–4907. [Google Scholar] [CrossRef]
- Kim, J.Y.; Shin, E.J.; Eom, I.Y.; Won, K.; Kim, Y.H.; Choi, D.; Choi, I.G.; Choi, J.W. Structural features of lignin macromolecules extracted with ionic liquid from poplar wood. Bioresour. Technol. 2011, 102, 9020–9025. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.-T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, J.; Liu, Y.; Pu, P.; Wang, X.; Chen, Y.; Gao, C.; Chen, J.; Zhou, H. Enhancing the luminescence of carbon dots by doping nitrogen element and its application in the detection of Fe(III). J. Mater. Sci. 2015, 50, 2571–2576. [Google Scholar] [CrossRef]
- Qian, Z.; Shan, X.; Chai, L.; Ma, J.; Chen, J.; Feng, H. Si-doped carbon quantum dots: A facile and general preparation strategy, bioimaging application, and multifunctional sensor. ACS Appl. Mater. Interfaces 2014, 6, 6797–6805. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Zhang, L.; Zou, M.; Lan, C.; Zhan, Z.; Zhao, S. Nitrogen and sulfur co-doped carbon dots: A facile and green fluorescence probe for free chlorine. Sens. Actuators B Chem. 2015, 219, 50–56. [Google Scholar] [CrossRef]
- Xu, G.Y.; Han, J.P.; Bing, D.; Ping, N.; Jin, P.; Hui, D.; Li, H.S.; Zhang, X.G. Biomass-derived porous carbon materials with sulfur and nitrogen dual-doping for energy storage. Green Chem. 2015, 17, 1668–1674. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, S.; Xiao, L.; Wang, H.; Li, S.; Yuan, Q. Fabrication of a nitrogen-doped graphene quantum dot from MOF-derived porous carbon and its application for highly selective fluorescence detection of Fe3+. J. Mater. Chem. C 2015, 3, 291–297. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Visanko, M.; Liimatainen, H. Deep eutectic solvent system based on choline chloride-urea as a pre-treatment for nanofibrillation of wood cellulose. Green Chem. 2015, 17, 3401–3406. [Google Scholar] [CrossRef]
- Kumar, A.K.; Parikh, B.S.; Pravakar, M. Natural deep eutectic solvent mediated pretreatment of rice straw: Bioanalytical characterization of lignin extract and enzymatic hydrolysis of pretreated biomass residue. Environ. Sci. Pollut. Res. 2016, 23, 9265–9275. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Peng, X.; Zhong, L.; Jing, S.; Cao, X.; Lu, F.; Sun, R. Choline chloride/urea as an effective plasticizer for production of cellulose films. Carbohydr. Polym. 2015, 117, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.D.; Feng, G.J.; Ye, M.; Huang, C.M.; Zhang, Y. Significantly enhanced enzymatic hydrolysis of rice straw via a high-performance two-stage deep eutectic solvents synergistic pretreatment. Bioresour. Technol. 2017, 238, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.-Y.; Zhang, Z.-Y. Graphitized carbon dots emitting strong green photoluminescence. J. Mater. Chem. C 2013, 1, 4902–4907. [Google Scholar] [CrossRef]
- Zhang, G.; Zhu, J.; Zeng, W.; Hou, S.; Gong, F.; Li, F.; Li, C.C.; Duan, H. Tin quantum dots embedded in nitrogen-doped carbon nanofibers as excellent anode for lithium-ion batteries. Nano Energy 2014, 9, 61–70. [Google Scholar] [CrossRef]
- Park, Y.; Yoo, J.; Lim, B.; Kwon, W.; Rhee, S.W. Improving the functionality of carbon nanodots: Doping and surface functionalization. J. Mater. Chem. A 2016, 4, 11582–11603. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar]
- Liu, R.; Wu, D.; Feng, X.; Mullen, K. Bottom-up fabrication of photoluminescent graphene quantum dots with uniform morphology. J. Mater. Sci. 2011, 133, 15221–15223. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Ji, R.; Cao, X.; Lin, J.; Jiang, H.; Li, X.; Teng, K.S.; Chi, M.L.; Zeng, S.; Hao, J. Deep Ultraviolet Photoluminescence of Water-Soluble Self-Passivated Graphene Quantum Dots. Acs Nano 2012, 6, 5102–5110. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.-J.; Sun, Q.; Zhang, L.; Zhang, Y.; Lu, A.-H. Photoluminescent carbon dots directly derived from polyethylene glycol and their application for cellular imaging. Carbon 2014, 71, 87–93. [Google Scholar] [CrossRef]
- Xie, Z.; Sun, X.; Jiao, J.; Xin, X. Ionic liquid-functionalized carbon quantum dots as fluorescent probes for sensitive and selective detection of iron ion and ascorbic acid. Colloids Surf. A 2017, 529, 38–44. [Google Scholar] [CrossRef]
- Xing, X.; Wang, D.; Chen, Z.; Zheng, B.; Li, B.; Wu, D. ZnTe quantum dots as fluorescence sensors for the detection of iron ions. J. Mater. Sci. Mater. Electron. 2018, 29, 14192–14199. [Google Scholar] [CrossRef]
- Deng, X.; Feng, Y.; Li, H.; Du, Z.; Teng, Q.; Wang, H. N-doped carbon quantum dots as fluorescent probes for highly selective and sensitive detection of Fe 3+ ions. Particuology 2018. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Shang, X.; Chao, D.; Zhou, L.; Zhang, H. Highly sensitive and selective detection of Fe 3+ by utilizing carbon quantum dots as fluorescent probes. Chem. Phys. Lett. 2018, 705, 1–6. [Google Scholar] [CrossRef]
- Yu, J.; Liu, C.; Yuan, K.; Lu, Z.; Cheng, Y.; Li, L.; Zhang, X.; Jin, P.; Meng, F.; Liu, H. Luminescence Mechanism of Carbon Dots by Tailoring Functional Groups for Sensing Fe(3+) Ions. Nanomate 2018, 8, 233. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Shi, Y.; Liu, X.; Wang, M.; Song, P.; Xu, F.; Zhang, X. Synthesis of Nitrogen-Doped Lignin/DES Carbon Quantum Dots as a Fluorescent Probe for the Detection of Fe3+ Ions. Polymers 2018, 10, 1282. https://doi.org/10.3390/polym10111282
Jiang X, Shi Y, Liu X, Wang M, Song P, Xu F, Zhang X. Synthesis of Nitrogen-Doped Lignin/DES Carbon Quantum Dots as a Fluorescent Probe for the Detection of Fe3+ Ions. Polymers. 2018; 10(11):1282. https://doi.org/10.3390/polym10111282
Chicago/Turabian StyleJiang, Xueqin, Yixin Shi, Xin Liu, Meng Wang, Pingping Song, Feng Xu, and Xueming Zhang. 2018. "Synthesis of Nitrogen-Doped Lignin/DES Carbon Quantum Dots as a Fluorescent Probe for the Detection of Fe3+ Ions" Polymers 10, no. 11: 1282. https://doi.org/10.3390/polym10111282
APA StyleJiang, X., Shi, Y., Liu, X., Wang, M., Song, P., Xu, F., & Zhang, X. (2018). Synthesis of Nitrogen-Doped Lignin/DES Carbon Quantum Dots as a Fluorescent Probe for the Detection of Fe3+ Ions. Polymers, 10(11), 1282. https://doi.org/10.3390/polym10111282