Proteins as Nano-Carriers for Bioactive Compounds. The Case of 7S and 11S Soy Globulins and Folic Acid Complexation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. 7S and 11S Mixed Solution Preparation
2.3. SDS-PAGE Electrophoresis
2.4. Globulin-FA Interaction Studies: Fluorescence Measurements
2.5. UV–Vis Absorbance Spectra
2.6. Globulins Aggregation: Studies of Particle Size and Zeta Potential
2.7. Determination of FA in Samples
2.8. Microbiological Assays with Lactobacillus casei BL23
2.8.1. Calibration Curve Construction for L. casei BL23
2.8.2. FA Apparent Concentration [FAapp] in Nano-Complexes
2.9. Statistical Analysis
3. Results
3.1. 7S and 11S Globulin Isolation
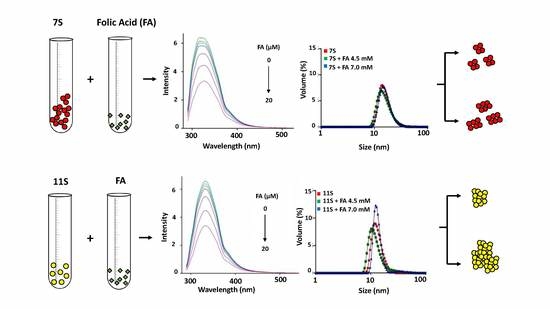
3.2. Globulins and FA Interactions
3.3. Absorption Spectrum of Fluorophore
3.4. Analysis of Protein Aggregation: Particle Size and ζ Potential Determination
3.5. Analysis of Protein Aggregation: Molecular Weight Estimation
3.6. Determination of FA Concentration by Microbiology Model
3.6.1. Calibration Curve for FA
3.6.2. 7S and 11S Loading Capacity
3.6.3. Biological Activity of FA in Nano-Complexes
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gupta, P.; Nayak, K.K. Characteristics of protein-based biopolymer and its application. Polym. Eng. Sci. 2015, 55, 485–498. [Google Scholar] [CrossRef]
- Chen, L.; Remondetto, G.E.; Subirade, M. Food protein-based materials as nutraceutical delivery systems. Trends Food Sci. Technol. 2006, 17, 272–283. [Google Scholar] [CrossRef]
- Perez, O.; Martinez, K.; Carrera Sanchez, C.; Patino, J.M.R. Strategy for Foaming Improvement of Whey Protein Concentrates by Addition of Hydroxypropylmethylcellulose and Heating: Relation with Interfacial Properties. Nutr. Food Technol. Open Access 2017, 3. [Google Scholar] [CrossRef]
- Yamauchi, F.; Yamagishi, T.; Iwabuchi, S. Molecular understanding of heat-induced phenomena of soybean protein. Food Rev. Int. 1991, 7, 283–322. [Google Scholar] [CrossRef]
- Utsumi, S.; Matsumura, Y.; Mori, T. Structure–Function relationships of soy proteins. In Food Proteins and Their Applications; Damodaran, S.P.A., Ed.; CRC Press: New York, NY, USA, 1997; pp. 257–291. [Google Scholar]
- Thanh, V.H.; Shibasaki, K. Mayor protein of soybean seeds. A straightforward fractionation and their characterization. J. Agric. Food Chem. 1976, 24, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Hermansson, A.M. Soy protein gelation. J. Am. Oil Chem. Soc. 1986, 63, 658–666. [Google Scholar] [CrossRef]
- Ruíz-Henestrosa, V.P.; Sánchez, C.C.; Pedroche, J.J.; Millán, F.; Rodríguez Patino, J.M. Improving the functional properties of soy glycinin by enzymatic treatment. Adsorption and foaming characteristics. Food Hydrocoll. 2009, 23, 377–386. [Google Scholar] [CrossRef]
- Martínez, K.; Carrera Sanchez, C. Ultrasound as a Method to Control Protein-Polysaccharide Foam Stability by Microscopic Parameter Alteration. J. Food Process. Preserv. 2015, 39, 1272–1277. [Google Scholar] [CrossRef]
- Ridner, S.H. Pretreatment lymphedema education and identified educational resources in breast cancer patients. Patient Educ. Couns. 2017, 61, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Wildman, R.E.C.; Wildman, R.; Wallace, T. Handbook of Nutraceuticals and Functional Foods, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Katouzian, I.; Jafari, S.M. Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends Food Sci. Technol. 2016, 53, 34–48. [Google Scholar] [CrossRef]
- Adank, C.; Green, T.J.; Skeaff, C.M.; Briars, B. Weekly High-Dose Folic Acid Supplementation Is Effective in Lowering Serum Homocysteine Concentrations in Women. Ann. Nutr. Metab. 2003, 47, 55–59. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, C.; Negri, E.; Pelucchi, C.; Franceschi, S. Dietary folate and colorectal cancer. Int. J. Cancer 2002, 102, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.L. The methionineehomocysteine cycle and its effects on cognitive deseases. Altern. Med. Rev. 2003, 8, 7–20. [Google Scholar] [PubMed]
- Berry, R.J.; Li, Z.; Erickson, J.D.; Li, S.; Moore, C.A.; Wang, H.; Mulinare, J.; Zhao, P.; Wong, L.-Y.C.; Gindler, J.; et al. Prevention of Neural-Tube Defects with Folic Acid in China. N. Engl. J. Med. 1999, 341, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Madziva, H.; Kailasapathy, K.Ã.; Phillips, M. Evaluation of alginate–pectin capsules in Cheddar cheese as a food carrier for the delivery of folic acid. LWT-Food Sci. Technol. 2006, 39, 146–151. [Google Scholar] [CrossRef]
- Motkar, G.; Lonare, M.; Patil, O.; Mohanty, S. Self-assembly of folic acid in aqueous media. AIChE J. 2013, 59, 1360–1368. [Google Scholar] [CrossRef]
- Langer, R.; Peppas, N.A. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003, 49, 2990–3006. [Google Scholar] [CrossRef]
- Liang, L.; Subirade, M.; La, V. β-Lactoglobulin/Folic Acid Complexes: Formation, Characterization, and Biological Implication. J. Phys. Chem. B 2010, 114, 6707–6712. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Tajmir-Riahi, H.A.; Subirade, M. Interaction of β-Lactoglobulin with Resveratrol and its Biological Implications. Biomacromolecules 2008, 9, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Subirade, M. Study of the acid and thermal stability of β-lactoglobulin-ligand complexes using fluorescence quenching. Food Chem. 2012, 132, 2023–2029. [Google Scholar] [CrossRef]
- Pérez, O.E.; David-Birman, T.; Kesselman, E.; Levi-Tal, S.; Lesmes, U. Food Hydrocolloids Milk protein e vitamin interactions: Formation of beta-lactoglobulin/folic acid nano-complexes and their impact on in vitro gastro-duodenal proteolysis. Food Hydrocoll. 2014, 38, 40–47. [Google Scholar] [CrossRef]
- Nagano, T.; Nishinarit, K.; Company, F.O. Dynamic Viscoelastic Study on the Gelation of 75 Globulin from Soybeans. J. Agric. Food Chem. 1992, 40, 941–944. [Google Scholar] [CrossRef]
- Ruíz-Henestrosa, V.P.; Sánchez, C.C.; Escobar, M.D.M.Y.; Jiménez, J.J.P.; Rodríguez, F.M.; Patino, J.M.R. Interfacial and foaming characteristics of soy globulins as a function of pH and ionic strength. Colloids Surf. A Physicochem. Eng. Asp. 2007, 309, 202–215. [Google Scholar] [CrossRef]
- Perez, O.E.; Pilosof, A.M.R. Pulsed electric fields effects on the molecular structure and gelation of β-lactoglobulin concentrate and egg white. Food Res. Int. 2004, 37, 102–110. [Google Scholar] [CrossRef]
- Lakowicz, J.R. (Ed.) Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; ISBN 0387312781. [Google Scholar]
- Silva, C.P.; Martínez, J.; Martínez, K.; Farías, M.; Leskow, F.C.; Pérez, O. Proposed molecular model for electrostatic interactions between insulin and chitosan. Nano-complexation and activity in cultured cells. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 425–434. [Google Scholar] [CrossRef]
- Stepánek, P. Dynamic Light Scattering: The Method and Some Applications; Brown, W., Ed.; Clarendon Press: Oxford, UK, 1993; ISBN 0198539428, 9780198539421. [Google Scholar]
- Koontz, J.L.; Phillips, K.M.; Wunderlich, K.M.; Exler, J.; Holden, J.M.; Gebhardt, S.E.; Haytowitz, D.B. Comparison of total folate concentrations in foods determined by microbiological assay at several experienced U.S. commercial laboratories. J. AOAC Int. 2005, 88, 805–813. [Google Scholar] [PubMed]
- Arzeni, C.; Pérez, O.E.; Guy, J. Egg albumin-folic acid nanocomplexes: Performance as a functional ingredient. J. Funct. Foods 2015, 18, 379–386. [Google Scholar] [CrossRef]
- Madziva, H.; Kailasapathy, K.; Phillips, M. Alginate–pectin microcapsules as a potential for folic acid delivery in foods. J. Microencapsul. 2005, 22, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G.O. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Cui, Y.; Guo, J.; Xu, B.; Chen, Z. Binding of chlorpyrifos and cypermethrin to blood proteins. Pestic. Biochem. Physiol. 2006, 85, 110–114. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Weber, G. Quenching of Fluorescence by Oxygen. A Probe for Structural Fluctuations in Macromoleculest. Biochemistry 1973, 12, 4161–4170. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, Y.; Zhang, H.; Liu, Z.; Fei, Z. Probing deep into the binding mechanisms of folic acid with α-amylase, pepsin and trypsin: An experimental and computational study. Food Chem. 2017, 226, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Bechet, J.; Greenson, M.; Wiame, J.M. Mutations affecting the repressibility of arginine biosynthetic enzymes in Saccharomyces cerevisiae. Eur. J. Biochem. 1970, 12, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.Z.; Lian, Y.J.; Ryu, J.H.; Sung, M.K.; Park, J.S.; Park, H.J.; Park, B.K.; Shin, J.S.; Lee, M.S.; Cheon, C.I. Expression and purification of His-tagged flavonol synthase of Camellia sinensis from Escherichia coli. Protein Expr. Purif. 2007, 55, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Ding, L.; Tian, Y.; Song, D.; Zhou, X.; Liu, X.; Zhang, H. Investigation of the interaction between flavonoids and human serum albumin. J. Mol. Struct. 2004, 703, 37–45. [Google Scholar] [CrossRef]
- Wei, X.L.; Xiao, J.B.; Wang, Y.; Bai, Y. Which model based on fluorescence quenching is suitable to study the interaction between trans-resveratrol and BSA? Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Koppel, D.E. Analysis of Macromolecular Polydispersity in Intensity Correlation Spectroscopy: The Method of Cumulants. J. Chem. Phys. 1972, 57, 4814–4820. [Google Scholar] [CrossRef]
- Cheema, M.; Mohan, M.S.; Campagna, S.R.; Jurat-Fuentes, J.L.; Harte, F.M. The association of low-molecular-weight hydrophobic compounds with native casein micelles in bovine milk. J. Dairy Sci. 2015, 98, 5155–5163. [Google Scholar] [CrossRef] [PubMed]
- Vinod, S.; Philip, J. Role of field-induced nanostructures, zippering and size polydispersity on effective thermal transport in magnetic fluids without significant viscosity enhancement. J. Magn. Magn. Mater. 2017, 444, 29–42. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. Food and Drug Administration Food Standards: Amendment of Standards of Identity for Enriched Grain Products to Require Addition of Folic Acid. Fed. Regist. 1996, 61, 8781–8807. [Google Scholar]
- Xiao, J.B.; Chen, X.Q.; Jiang, X.Y.; Hilczer, M.; Tachiya, M. Probing the Interaction of Trans-resveratrol with Bovine Serum Albumin: A Fluorescence Quenching Study with Tachiya Model. J. Fluoresc. 2008, 18, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Cao, H.; Wang, Y.; Zhao, J.; Wei, X. Glycosylation of Dietary Flavonoids Decreases the Affinities for Plasma Protein. J. Agric. Food Chem. 2009, 57, 6642–6648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Liu, X.; Li, Y.; Yin, X.; Subirade, M.; Zhou, P.; Liang, L. The folic acid/β-casein complex: Characteristics and physicochemical implications. Food Res. Int. 2014, 57, 162–167. [Google Scholar] [CrossRef]
- Liang, L.; Zhang, J.; Zhou, P.; Subirade, M. Protective effect of ligand-binding proteins against folic acid loss due to photodecomposition. Food Chem. 2013, 141, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Berezhkovskiy, L.M. On the Calculation of the Concentration Dependence of Drug Binding to Plasma Proteins with Multiple Binding Sites of Different Affinities: Determination of the Possible Variation of the Unbound Drug Fraction and Calculation of the Number of Binding Sites. J. Pharm. Sci. 2017, 96, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.H.; Lorente, C.; Capparelli, A.L.; Pokhrel, M.R.; Braun, A.M.; Oliveros, E. Fluorescence of pterin, 6-formylpterin, 6-carboxypterin and folic acid in aqueous solution: PH effects. Photochem. Photobiol. Sci. 2002, 1, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, P.; Hasni, I.; Tajmir-Riahi, H.A. Folic acid complexes with human and bovine serum albumins. Food Chem. 2011, 129, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, P.; Tajmir-Riahi, H.A. Locating the Binding Sites of Folic Acid with Milk α- and β-Caseins. J. Phys. Chem. B 2012, 116, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Ghai, R.; Falconer, R.J.; Collins, B.M. Applications of isothermal titration calorimetry in pure and applied research-survey of the literature from 2010. J. Mol. Recognit. 2012, 25, 32–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Tan, C.-Y.; Zhuang, S.-L.; Zhai, P.-Z.; Cui, Y.; Zhou, Q.-H.; Zhang, H.-M.; Fei, Z. In vitro and in silico investigations of the binding interactions between chlorophenols and trypsin. J. Hazard. Mater. 2014, 278, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Penalva, R.; Esparza, I.; Agüeros, M.; Gonzalez-Navarro, C.J.; Gonzalez-Ferrero, C.; Irache, J.M. Food Hydrocolloids Casein nanoparticles as carriers for the oral delivery of folic acid. Food Hydrocoll. 2015, 44, 399–406. [Google Scholar] [CrossRef]
- Le Brun, V.; Friess, W.; Bassarab, S.; Mühlau, S.; Garidel, P. A critical evaluation of self-interaction chromatography as a predictive tool for the assessment of protein–protein interactions in protein formulation development: A case study of a therapeutic monoclonal antibody. Eur. J. Pharm. Biopharm. 2010, 75, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Peñalva, R.; Esparza, I.; González-Navarro, C.J.; Quincoces, G.; Peñuelas, I.; Irache, J.M. Zein nanoparticles for oral folic acid delivery. J. Drug Deliv. Sci. Technol. 2015, 30, 450–457. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Khan, J.M.; Khan, M.S.; Ali, M.S.; Al-Senaidy, A.M.; Alsenaidy, M.A.; Husain, F.M.; Al-Lohedan, H.A. Synthetic food additive dye “Tartrazine” triggers amorphous aggregation in cationic myoglobin. Int. J. Biol. Macromol. 2017, 98, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Stirpe, A.; Pantusa, M.; Rizzuti, B.; De Santo, M.P.; Sportelli, L.; Bartucci, R.; Guzzi, R. Resveratrol induces thermal stabilization of human serum albumin and modulates the early aggregation stage. Int. J. Biol. Macromol. 2016, 92, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Von Staszewski, M.; Jagus, R.J.; Pilosof, A.M.R. Influence of green tea polyphenols on the colloidal stability and gelation of WPC. Food Hydrocoll. 2011, 25, 1077–1084. [Google Scholar] [CrossRef]
- Semple, K.T.; Doick, K.J.; Jones, K.C.; Burauel, P.; Craven, A.; Harms, H. Peer Reviewed: Defining Bioavailability and Bioaccessibility of Contaminated Soil and Sediment is Complicated. Environ. Sci. Technol. 2004, 38, 228A–231A. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.B.; Zevely, E.M.; Huennekens, F.M. Coupling of Energy to Folate Transport in Lactobacillus casei. J. Bacteriol. 1979, 139, 552–559. [Google Scholar] [PubMed]
- Eudes, A.; Erkens, G.B.; Slotboom, D.J.; Rodionov, D.A.; Naponelli, V.; Hanson, A.D. Identification of Genes Encoding the Folate- and Thiamine-Binding Membrane Proteins in Firmicutes. J. Bacteriol. 2008, 190, 7591–7594. [Google Scholar] [CrossRef] [PubMed]
- Kunji, E.R.S.; Mierau, I.; Hagting, A.; Poolman, B.; Konings, W.N. The proteotytic systems of lactic acid bacteria. Antonie Leeuwenhoek 1996, 70, 187–221. [Google Scholar] [CrossRef] [PubMed]
- Piuri, M.; Ruzal, S.M. Adaptation to high salt in Lactobacillus: Role of peptides and proteolytic enzymes. J. Appl. Microbiol. 2003, 372–379. [Google Scholar] [CrossRef]









| Parameters derived from stern-volmer Model | Parameters derived from modified double logarithm model | |||||
|---|---|---|---|---|---|---|
| n | Ks [M−1] | R2 | n | Ka [M−1] | R2 | |
| 7S-FA | 0.95 | 2.4 × 104 | 0.9982 | 0.8397 | 4.00 × 104 | 0.9909 |
| 11S-FA | 1.13 | 1.87 × 105 | 0.9693 | 0.9332 | 4.36 × 104 | 0.9946 |
| System (mM) * | Zave (d.nm) ** | ζ potential (mV) ** | PDI ** |
|---|---|---|---|
| 7S (5.5) | 19.57 ± 1.2 | −8 ± 2.0 | 0.404 ± 0.06 |
| 7S-FA (5.5–4.5) | 22.31 ± 1.2 | −12 ± 2.0 | 0.375 ± 0.09 |
| 7S-FA (5.5–7.0) | 33.86 ± 1.4 | −10 ± 2.0 | 0.498 ± 0.36 |
| 11S (3.0) | 21.52 ± 1.5 | −11 ± 2.0 | 0.535 ± 0.80 |
| 11S-FA (3.0–4.5) | 38.16 ± 1.6 | −12 ± 2.0 | 0.581 ± 0.09 |
| 11S-FA (3.0–7.0) | 51.33 ± 1.3 | −14 ± 2.0 | 0.851 ± 0.40 |
| [FA] µg/mL | 11S [FA]app | 7S [FA]app |
|---|---|---|
| 3.2 × 10−4 | 3.04 × 10−3 | 1.99 × 10−3 |
| 6.4 × 10−4 | 2.24 × 10−3 | 2.01 × 10−3 |
| 9.6 × 10−4 | 2.87 × 10−3 | 2.47 × 10−3 |
| 1.6 × 10−3 | 2.25 × 10−3 | 2.83 × 10−3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochnio, M.E.; Martínez, J.H.; Allievi, M.C.; Palavecino, M.; Martínez, K.D.; Pérez, O.E. Proteins as Nano-Carriers for Bioactive Compounds. The Case of 7S and 11S Soy Globulins and Folic Acid Complexation. Polymers 2018, 10, 149. https://doi.org/10.3390/polym10020149
Ochnio ME, Martínez JH, Allievi MC, Palavecino M, Martínez KD, Pérez OE. Proteins as Nano-Carriers for Bioactive Compounds. The Case of 7S and 11S Soy Globulins and Folic Acid Complexation. Polymers. 2018; 10(2):149. https://doi.org/10.3390/polym10020149
Chicago/Turabian StyleOchnio, María Emilia, Jimena H. Martínez, Mariana C. Allievi, Marcos Palavecino, Karina D. Martínez, and Oscar E. Pérez. 2018. "Proteins as Nano-Carriers for Bioactive Compounds. The Case of 7S and 11S Soy Globulins and Folic Acid Complexation" Polymers 10, no. 2: 149. https://doi.org/10.3390/polym10020149
APA StyleOchnio, M. E., Martínez, J. H., Allievi, M. C., Palavecino, M., Martínez, K. D., & Pérez, O. E. (2018). Proteins as Nano-Carriers for Bioactive Compounds. The Case of 7S and 11S Soy Globulins and Folic Acid Complexation. Polymers, 10(2), 149. https://doi.org/10.3390/polym10020149