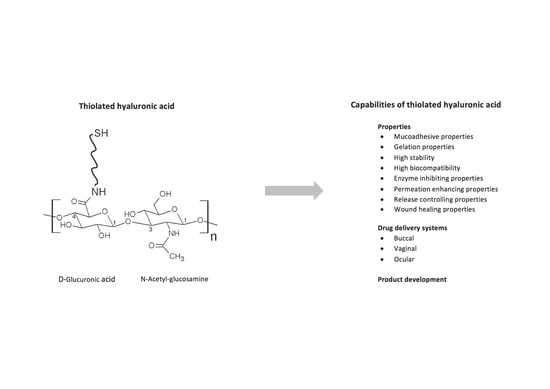
Thiolated Hyaluronic Acid as Versatile Mucoadhesive Polymer: From the Chemistry Behind to Product Developments—What Are the Capabilities?
Abstract
:1. Introduction
2. The Chemistry Behind
3. Properties of Thiolated Hyaluronic Acid
3.1. Mucoadhesive Properties
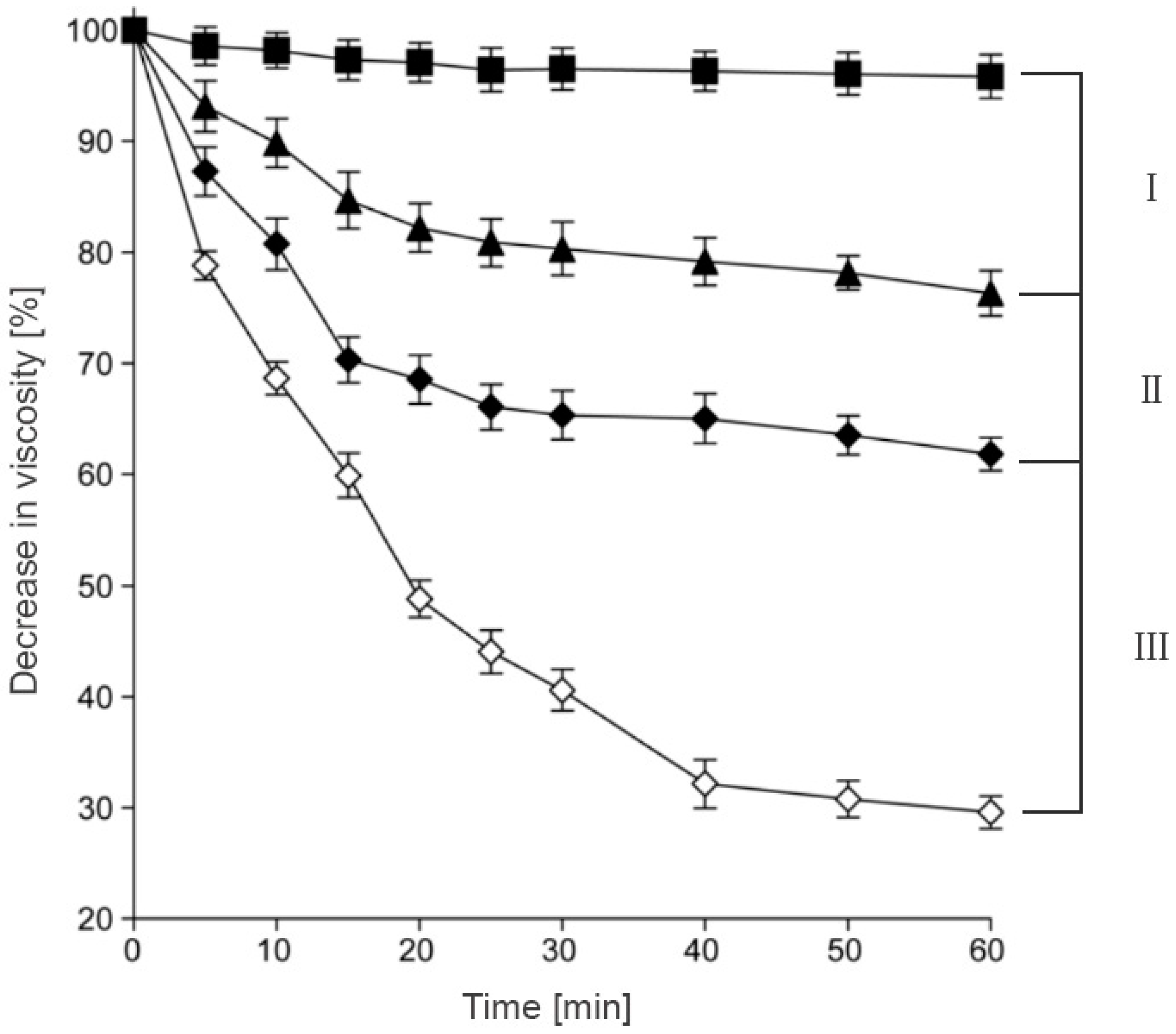
3.2. Cohesive Properties
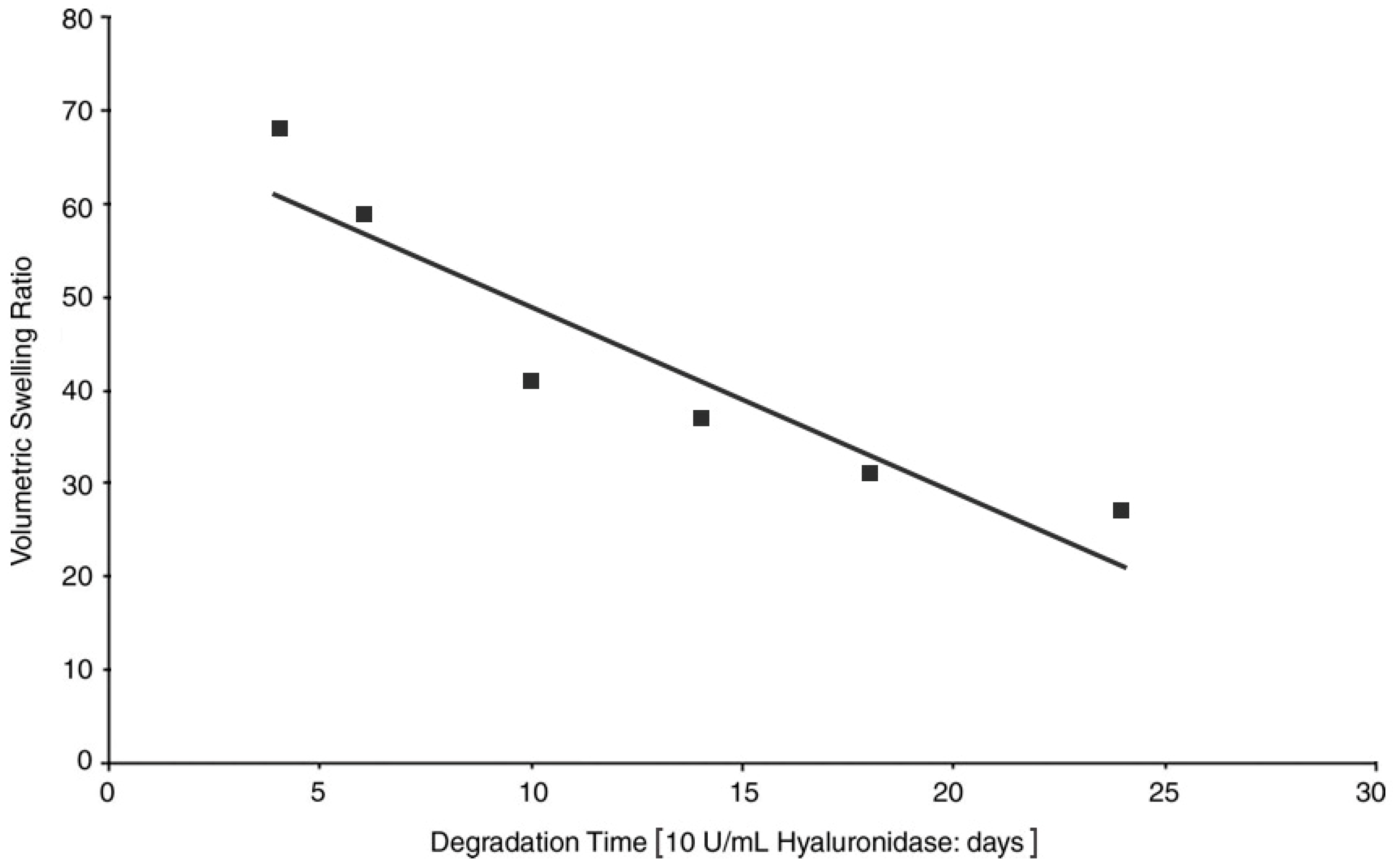
3.3. Stability against Degradation
3.4. Biocompatibility
3.5. Permeation Enhancing Properties
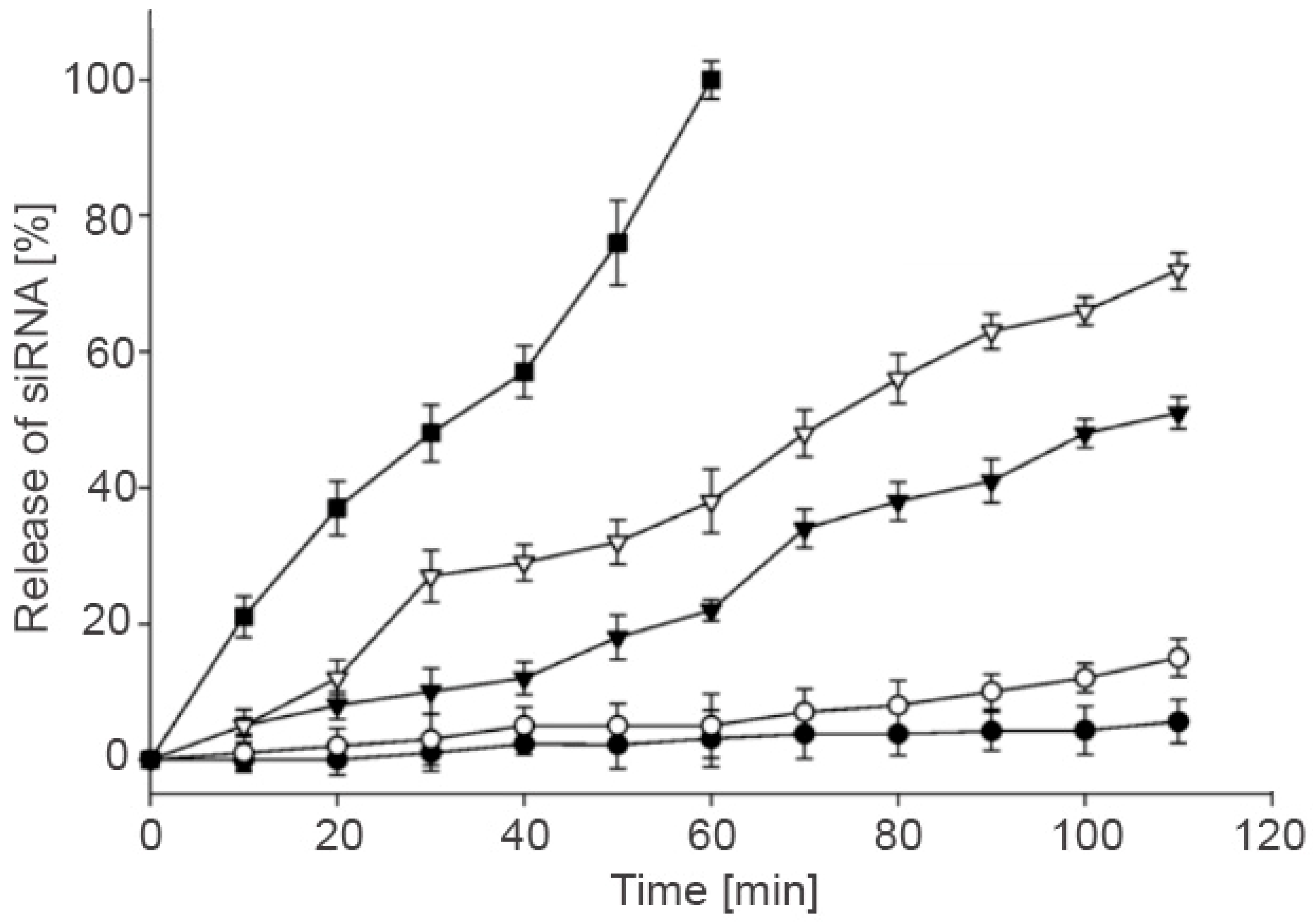
3.6. Controlled Release
3.7. Further Applications of Thiolated HA
4. Delivery Systems
4.1. Buccal Drug Delivery
4.2. Vaginal Drug Delivery
4.3. Ocular Drug Delivery
5. Product Development
6. Conclusions
Conflicts of Interest
References
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A. Muco-Adhesive Polymers, Use Thereof and Method For Producting the Same. Patent Family WO0025823, 11 May 2000. [Google Scholar]
- Prestwich, G.D. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J. Control. Release 2011, 155, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Shahnaz, G.; Dünnhaupt, S.; Müller, C.; Hintzen, F.; Bernkop-Schnürch, A. Preactivated thiomers as mucoadhesive polymers for drug delivery. Biomaterials 2012, 33, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Wirostko, B.; Mann, B.K.; Williams, D.L.; Prestwich, G.D. Ophthalmic Uses of a Thiol-Modified Hyaluronan-Based Hydrogel. Adv. Wound Care (New Rochelle) 2014, 3, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Nyström, B.; Kjøniksen, A.L.; Beheshti, N.; Maleki, A.; Zhu, K.; Knudsen, K.D.; Pamies, R.; Hernández Cifre, J.G.; García de la Torre, J. Characterization of polyelectrolyte features in polysaccharide systems and mucin. Adv. Colloid Interface Sci. 2010, 158, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Sheehan, J.K. Hyaluronan: Polysaccharide chaos to protein organisation. Curr. Opin. Struct. Biol. 2001, 11, 617–622. [Google Scholar] [CrossRef]
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Robert, L.; Robert, A.M.; Renard, G. Biological effects of hyaluronan in connective tissues, eye, skin, venous wall. Role in aging. Pathol. Biol. (Paris) 2010, 58, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Laurent, U.B.; Fraser, J.R. Turnover of hyaluronan in synovial joints: Elimination of labelled hyaluronan from the knee joint of the rabbit. Exp. Physiol. 1991, 76, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Aruffo, A.; Stamenkovic, I.; Melnick, M.; Underhill, C.B.; Seed, B. CD44 is the principal cell surface receptor for hyaluronate. Cell 1990, 61, 1303–1313. [Google Scholar] [CrossRef]
- Stern, R.; Kogan, G.; Jedrzejas, M.J.; Soltés, L. The many ways to cleave hyaluronan. Biotechnol. Adv. 2007, 25, 537–557. [Google Scholar] [CrossRef] [PubMed]
- Price, R.D.; Berry, M.G.; Navsaria, H.A. Hyaluronic acid: The scientific and clinical evidence. J. Plast. Reconstr. Aesthet. Surg. 2007, 60, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Kafedjiiski, K.; Jetti, R.K.; Föger, F.; Hoyer, H.; Werle, M.; Hoffer, M.; Bernkop-Schnürch, A. Synthesis and in vitro evaluation of thiolated hyaluronic acid for mucoadhesive drug delivery. Int. J. Pharm. 2007, 343, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, G.; Jin, K.; Yin, Z. Hyaluronic acid l-cysteine conjugate exhibits controlled-release potential for mucoadhesive drug delivery. Pharmazie 2012, 67, 224–228. [Google Scholar] [PubMed]
- Pereira de Sousa, I.; Suchaoin, W.; Zupančič, O.; Leichner, C.; Bernkop-Schnürch, A. Totally S-protected hyaluronic acid: Evaluation of stability and mucoadhesive properties as liquid dosage form. Carbohydr. Polym. 2016, 152, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Sahdev, P.; Ochyl, L.J.; Akerberg, J.J.; Moon, J.J. Cationic liposome-hyaluronic acid hybrid nanoparticles for intranasal vaccination with subunit antigens. J. Control. Release 2015, 208, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Fu, F.; Shi, X.; Yin, Z. Controlled release of a model protein drug ovalbumin from thiolated hyaluronic acid matrix. J. Drug Deliv. Sci. Technol. 2015, 30, 74–81. [Google Scholar] [CrossRef]
- Laffleur, F.; Röggla, J.; Idrees, M.A.; Griessinger, J. Chemical modification of hyaluronic acid for intraoral application. J. Pharm. Sci. 2014, 103, 2414–2423. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.; Laffleur, F.; Bernkop-Schnürch, A. Preactivated hyaluronic acid: A potential mucoadhesive polymer for vaginal delivery. Int. J. Pharm. 2015, 478, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F.; Wagner, J.; Mahmood, A. In vitro and ex vivo evaluation of biomaterials’ distinctive properties as a result of thiolation. Future Med. Chem. 2015, 7, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F.; Psenner, J.; Suchaoin, W. Permeation enhancement via thiolation: In vitro and ex vivo evaluation of hyaluronic acid-cysteine ethyl ester. J. Pharm. Sci. 2015, 104, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F.; Wagner, J.; Barthelmes, J. A potential tailor-made hyaluronic acid buccal delivery system comprising rotigotine for Parkinson’s disease? Future Med. Chem. 2015, 7, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F.; Schmelzle, F.; Ganner, A.; Vanicek, S. In Vitro and Ex Vivo Evaluation of Novel Curcumin-Loaded Excipient for Buccal Delivery. AAPS PharmSciTech 2017, 18, 2102–2109. [Google Scholar] [CrossRef] [PubMed]
- Ouasti, S.; Donno, R.; Cellesi, F.; Sherratt, M.J.; Terenghi, G.; Tirelli, N. Network connectivity, mechanical properties and cell adhesion for hyaluronic acid/PEG hydrogels. Biomaterials 2011, 32, 6456–6470. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; He, M.; Sui, J.; Cai, H.; Sun, Y.; Liang, J.; Fan, Y.; Zhang, X. The self-crosslinking smart hyaluronic acid hydrogels as injectable three-dimensional scaffolds for cells culture. Colloids Surf. B Biointerfaces 2016, 140, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; He, R.; Zhou, G.; Tang, C.; Yin, C. Multilayered mucoadhesive hydrogel films based on thiolated hyaluronic acid and polyvinylalcohol for insulin delivery. Acta Biomater. 2012, 8, 3643–3651. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, F.; Ru, Y. Hyperbranched phosphoramidate-hyaluronan hybrid: A reduction-sensitive injectable hydrogel for controlled protein release. Carbohydr. Polym. 2015, 117, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Mok, H.; Lee, S.; Oh, Y.K.; Park, T.G. Target-specific intracellular delivery of siRNA using degradable hyaluronic acid nanogels. J. Control. Release 2007, 119, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Liu, J.; Zhao, Z.; Dong, L.; Cai, H.; Yin, L.; Zhou, J.; Huo, M. Smart nanoparticles with a detachable outer shell for maximized synergistic antitumor efficacy of therapeutics with varying physicochemical properties. J. Control. Release 2016, 243, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bae, K.H.; Yamashita, A.; Chung, J.E.; Kurisawa, M. Thiol-Mediated Synthesis of Hyaluronic Acid-Epigallocatechin-3-O-Gallate Conjugates for the Formation of Injectable Hydrogels with Free Radical Scavenging Property and Degradation Resistance. Biomacromolecules 2017, 18, 3143–3155. [Google Scholar] [CrossRef] [PubMed]
- Korogiannaki, M.; Zhang, J.; Sheardown, H. Surface modification of model hydrogel contact lenses with hyaluronic acid via thiol-ene “click” chemistry for enhancing surface characteristics. J. Biomater. Appl. 2017, 32, 446–462. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.K.; Park, J.K.; Tomimatsu, T.; Shimoboji, T. Synthesis and degradation test of hyaluronic acid hydrogels. Int. J. Biol. Macromol. 2007, 40, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.K.; Kim, J.S.; Shimobouji, T. Injectable hyaluronic acid microhydrogels for controlled release formulation of erythropoietin. J. Biomed. Mater. Res. A 2007, 80, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Prestwich, G.D.; Mann, B.K. Thiolated carboxymethyl-hyaluronic-acid-based biomaterials enhance wound healing in rats, dogs, and horses. ISRN Vet. Sci. 2011, 2011, 851593. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Mann, B.K. A Crosslinked HA-Based Hydrogel Ameliorates Dry Eye Symptoms in Dogs. Int. J. Biomater. 2013, 2013, 460437. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Mann, B.K. Efficacy of a crosslinked hyaluronic acid-based hydrogel as a tear film supplement: A masked controlled study. PLoS ONE 2014, 9, e99766. [Google Scholar] [CrossRef] [PubMed]
- Colter, J.; Wirostko, B.; Coats, B. Finite Element Design Optimization of a Hyaluronic Acid-Based Hydrogel Drug Delivery Device for Improved Retention. Ann. Biomed. Eng. 2018, 46, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.Z.; Liu, Y.; Luo, Y.; Roberts, M.C.; Prestwich, G.D. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules 2002, 3, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shu, X.Z.; Gray, S.D.; Prestwich, G.D. Disulfide-crosslinked hyaluronan-gelatin sponge: Growth of fibrous tissue in vivo. J. Biomed. Mater. Res. A 2004, 68, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.Z.; Ghosh, K.; Liu, Y.; Palumbo, F.S.; Luo, Y.; Clark, R.A.; Prestwich, G.D. Attachment and spreading of fibroblasts on an RGD peptide-modified injectable hyaluronan hydrogel. J. Biomed. Mater. Res. A 2004, 68, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.Z.; Ahmad, S.; Liu, Y.; Prestwich, G.D. Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices for tissue engineering. J. Biomed. Mater. Res. A 2006, 79, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Ghosh, K.; Shu, X.Z.; Li, B.; Sokolov, J.C.; Prestwich, G.D.; Clark, R.A.; Rafailovich, M.H. Electrospun three-dimensional hyaluronic acid nanofibrous scaffolds. Biomaterials 2006, 27, 3782–3792. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Ghosh, K.; Li, B.; Sokolov, J.C.; Clark, R.A.; Rafailovich, M.H. Dual-syringe reactive electrospinning of cross-linked hyaluronic acid hydrogel nanofibers for tissue engineering applications. Macromol. Biosci. 2006, 6, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Horn, E.M.; Beaumont, M.; Shu, X.Z.; Harvey, A.; Prestwich, G.D.; Horn, K.M.; Gibson, A.R.; Preul, M.C.; Panitch, A. Influence of cross-linked hyaluronic acid hydrogels on neurite outgrowth and recovery from spinal cord injury. J. Neurosurg. Spine 2007, 6, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, R.R.; Shu, X.Z.; McGill, L.; Petersen, E.; Prestwich, G.D. Structural variations in a single hyaluronan derivative significantly alter wound-healing effects in the rabbit maxillary sinus. Laryngoscope 2007, 117, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Vanderhooft, J.L.; Alcoutlabi, M.; Magda, J.J.; Prestwich, G.D. Rheological Properties of Cross-Linked Hyaluronan–Gelatin Hydrogels for Tissue Engineering. Macromol. Biosci. 2009, 9, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Espandar, L.; Mamalis, N.; Prestwich, G.D. A cross-linked hyaluronan gel accelerates healing of corneal epithelial abrasion and alkali burn injuries in rabbits. Vet. Ophthalmol. 2010, 13, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Censi, R.; Fieten, P.J.; di Martino, P.; Hennink, W.E.; Vermonden, T. In situ forming hydrogels by tandem thermal gelling and Michael addition reaction between thermosensitive triblock copolymers and thiolated hyaluronan. Macromolecules 2010, 43, 5771–5778. [Google Scholar] [CrossRef]
- Skardal, A.; Zhang, J.; Prestwich, G.D. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials 2010, 31, 6173–6181. [Google Scholar] [CrossRef] [PubMed]
- Horkay, F.; Magda, J.; Alcoutlabi, M.; Atzet, S.; Zarembinski, T. Structural, mechanical and osmotic properties of injectable hyaluronan-based composite hydrogels. Polymer 2010, 51, 4424–4430. [Google Scholar] [CrossRef] [PubMed]
- Dubbini, A.; Censi, R.; Butini, M.E.; Sabbieti, M.G.; Agas, D.; Vermonden, T.; Di Martino, P. Injectable hyaluronic acid/PEG-p(HPMAm-lac)-based hydrogels dually cross-linked by thermal gelling and Michael addition. Eur. Polym. J. 2015, 72, 423–437. [Google Scholar] [CrossRef]
- Köwitsch, A.; Niepel, M.S.; Michanetzis, G.P.; Missirlis, Y.F.; Groth, T. Effect of Immobilized Thiolated Glycosaminoglycans on Fibronectin Adsorption and Behavior of Fibroblasts. Macromol. Biosci. 2016, 16, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Sabbieti, M.G.; Dubbini, A.; Laus, F.; Paggi, E.; Marchegiani, A.; Capitani, M.; Marchetti, L.; Dini, F.; Vermonden, T.; Di Martino, P.; et al. In vivo biocompatibility of p(HPMAm-lac)-PEG hydrogels hybridized with hyaluronan. J. Tissue Eng. Regen. Med. 2016, 11, 3056–3067. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, X.; Shu, G.D. Prestwich, Biocompatibility and stability of disulfide-crosslinked hyaluronan films. Biomaterials 2005, 26, 4737–4746. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Li, H.; Li, N.; Miao, X.; Xie, M.; Du, W.; Zhang, L.M. Conjugating an anticancer drug onto thiolated hyaluronic acid by acid liable hydrazone linkage for its gelation and dual stimuli-response release. Carbohydr. Polym. 2015, 128, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Thibeault, S.L.; Klemuk, S.A.; Chen, X.; Quinchia Johnson, B.H. In Vivo engineering of the vocal fold ECM with injectable HA hydrogels-late effects on tissue repair and biomechanics in a rabbit model. J. Voice 2011, 25, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Young, J.L.; Engler, A.J. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials 2011, 32, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Serban, M.A.; Yang, G.; Prestwich, G.D. Synthesis, characterization and chondroprotective properties of a hyaluronan thioethyl ether derivative. Biomaterials 2008, 29, 1388–1399. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Meng, J.; Ezoulin, M.J.; Youm, I.; Dim, D.C.; Molteni, A.; Hung, W.T.; Christenson, L.K.; Youan, B.C. Stimuli-sensitive thiolated hyaluronic acid based nanofibers: Synthesis, preclinical safety and in vitro anti-HIV activity. Nanomedicine 2016, 11, 2935–2958. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chau, Y. Formulation of in situ chemically cross-linked hydrogel depots for protein release: From the blob model perspective. Biomacromolecules 2015, 16, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lau, L.C.; Lo, A.C.; Chau, Y. Injectable Chemically Crosslinked Hydrogel for the Controlled Release of Bevacizumab in Vitreous: A 6-Month In Vivo Study. Transl. Vis. Sci. Technol. 2015, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Wilcox, M.; Pearson, J.P.; Borrós, S. Optimal design for studying mucoadhesive polymers interaction with gastric mucin using a quartz crystal microbalance with dissipation (QCM-D): Comparison of two different mucin origins. Eur. J. Pharm. Biopharm. 2015, 96, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Hornof, M.; Zoidl, T. Thiolated polymers—Thiomers: Synthesis and in vitro evaluation of chitosan-2-iminothiolane conjugates. Int. J. Pharm. 2003, 260, 229–237. [Google Scholar] [CrossRef]
- Salamat-Miller, N.; Chittchang, M.; Johnston, T.P. The use of mucoadhesive polymers in buccal drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1666–1691. [Google Scholar] [CrossRef] [PubMed]
- Shinkar, D.M.; Dhake, A.S.; Setty, C.M. Drug delivery from the oral cavity: A focus on mucoadhesive buccal drug delivery systems. PDA J. Pharm. Sci. Technol. 2012, 66, 466–500. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, S.S.; Pereira, P.; Correia, A.; Moreira, S.; Rocha, H.; Gama, F.M. Biocompatibility of a Self-Assembled Crosslinkable Hyaluronic Acid Nanogel. Macromol. Biosci. 2016, 16, 1610–1620. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Parisi, L.; Piergianni, M.; Smerieri, A.; Passeri, G.; Guizzardi, S.; Costa, F.; Lumetti, S.; Manfredi, E.; Macaluso, G.M. Improved scaffold biocompatibility through anti-Fibronectin aptamer functionalization. Acta Biomater. 2016, 42, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Clausen, A.E.; Kast, C.E.; Bernkop-Schnürch, A. The role of glutathione in the permeation enhancing effect of thiolated polymers. Pharm. Res. 2002, 19, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Choi, K.Y.; Ko, H.; Jeon, J.; Saravanakumar, G.; Suh, Y.D.; Lee, D.S.; Park, J.H. Bioreducible core-crosslinked hyaluronic acid micelle for targeted cancer therapy. J. Control. Release 2015, 200, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Campani, V.; Giarra, S.; de Rosa, G. Lipid-based core-shell nanoparticles: Evolution and potentialities in drug delivery. OpenNano 2018, 3, 5–17. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Wade, R.J.; Bassin, E.J.; Gramlich, W.M.; Burdick, J.A. Nanofibrous hydrogels with spatially patterned biochemical signals to control cell behavior. Adv. Mater. 2015, 27, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: http://www.who.int/features/factfiles/vision/01_en.html (accessed on 15 September 2017).
- Espandar, L.; Bunnell, B.; Wang, G.Y.; Gregory, P.; McBride, C.; Moshirfar, M. Adipose-derived stem cells on hyaluronic acid-derived scaffold: A new horizon in bioengineered cornea. Arch. Ophthalmol. 2012, 130, 202–208. [Google Scholar] [CrossRef] [PubMed]
- ESIBIO Stem Cell Solutions. Available online: http://www.esibio.com/index.php/products/product-category/hydrogels-kits/hystem-hydrogels/ (accessed on 2 January 2018).
- Prestwich, G.D.; Erickson, I.E.; Zarembinski, T.I.; West, M.; Tew, W.P. The translational imperative: Making cell therapy simple and effective. Acta Biomater. 2012, 8, 4200–4207. [Google Scholar] [CrossRef] [PubMed]
- Gwon, K.; Kim, E.; Tae, G. Heparin-hyaluronic acid hydrogel in support of cellular activities of 3D encapsulated adipose derived stem cells. Acta Biomater. 2017, 49, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, R.; Zarembinski, T.I.; Doty, N.; Jiang, C.; Regatieri, C.; Zhang, X.; Young, M.J. The application of hyaluronic acid hydrogels to retinal progenitor cell transplantation. Tissue Eng. Part A 2013, 19, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Zarembinski, T.I.; Doty, N.J.; Erickson, I.E.; Srinivas, R.; Wirostko, B.M.; Tew, W.P. Thiolated hyaluronan-based hydrogels crosslinked using oxidized glutathione: An injectable matrix designed for ophthalmic applications. Acta Biomater. 2014, 10, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Dong, Y.; Wang, W. Encapsulation and 3D culture of human adipose-derived stem cells in an in-situ crosslinked hybrid hydrogel composed of PEG-based hyperbranched copolymer and hyaluronic acid. Stem Cell Res. Ther. 2013, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Walimbe, T.; Panitch, A.; Sivasankar, P.M. A Review of Hyaluronic Acid and Hyaluronic Acid-based Hydrogels for Vocal Fold Tissue Engineering. J. Voice 2017, 31, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Hauptstein, S.; Hintzen, F.; Müller, C.; Ohm, M.; Bernkop-Schnürch, A. Development and in vitro evaluation of a buccal drug delivery system based on preactivated thiolated pectin. Drug Dev. Ind. Pharm. 2014, 40, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F.; Fischer, A.; Schmutzler, M.; Hintzen, F.; Bernkop-Schnürch, A. Evaluation of functional characteristics of preactivated thiolated chitosan as potential therapeutic agent for dry mouth syndrome. Acta Biomater. 2015, 21, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F.; Shahnaz, G.; Islambulchilar, Z.; Bernkop-Schnürch, A. Design and in vitro evaluation of a novel polymeric excipient for buccal applications. Future Med. Chem. 2013, 5, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Hombach, J.; Palmberger, T.F.; Bernkop-Schnürch, A. Development and in vitro evaluation of a mucoadhesive vaginal delivery system for nystatin. J. Pharm. Sci. 2009, 98, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Friedl, H.E.; Dünnhaupt, S.; Waldner, C.; Bernkop-Schnürch, A. Preactivated thiomers for vaginal drug delivery vehicles. Biomaterials 2013, 34, 7811–7818. [Google Scholar] [CrossRef] [PubMed]
- Baloglu, E.; Ay Senyıgıt, Z.; Karavana, S.Y.; Vetter, A.; Metın, D.Y.; Hilmioglu Polat, S.; Guneri, T.; Bernkop-Schnurch, A. In vitro evaluation of mucoadhesive vaginal tablets of antifungal drugs prepared with thiolated polymer and development of a new dissolution technique for vaginal formulations. Chem. Pharm. Bull. (Tokyo) 2011, 59, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Baloglu, E.; Senyigit, Z.A.; Karavana, S.Y.; Bernkop-Schnürch, A. Strategies to prolong the intravaginal residence time of drug delivery systems. J. Pharm. Pharm. Sci. 2009, 12, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Schnichels, S.; Schneider, N.; Hohenadl, C.; Hurst, J.; Schatz, A.; Januschowski, K.; Spitzer, M.S. Efficacy of two different thiol-modified crosslinked hyaluronate formulations as vitreous replacement compared to silicone oil in a model of retinal detachment. PLoS ONE 2017, 12, e0172895. [Google Scholar] [CrossRef] [PubMed]
- ESIBIO Stem Cell Solutions. Available online: http://www.esibio.com/index.php/products/popular-brands/glycosil/glycosil-hyaluronic-acid/ (accessed on 2 January 2018).
- Bi, X.; Liang, A.; Tan, Y.; Maturavongsadit, P.; Higginbothem, A.; Gado, T.; Gramling, A.; Bahn, H.; Wang, Q. Thiol-ene crosslinking polyamidoamine dendrimer-hyaluronic acid hydrogel system for biomedical applications. J. Biomater. Sci. Polym. Ed. 2016, 27, 743–757. [Google Scholar] [CrossRef] [PubMed]
- VORNIA Biomaterials. Available online: http://www.vornia.com/products-page/functionalized-biopolymers/thiol-modified-hyaluronic-acid/ (accessed on 2 January 2018).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01887873?term=Croma+pharma&draw=2&rank=8 (accessed on 2 January 2018).
- Eyegate Pharmaceuticals. Available online: http://www.eyegatepharma.com/pipeline/ocular-bandage-gel/ (accessed on 2 January 2018).



| Type of thiolated HA | Targeted chemical group of HA | Ligand | Reaction type | Reference |
|---|---|---|---|---|
| HA-SH | Carboxylic | l-Cysteine | Amidation | [15,16,17,18] |
| HA-SH | Carboxylic | l-Cysteine ethyl ester | Amidation | [14,19,20,21,22,23,24] |
| HA-SH | Carboxylic | Cysteamine | Amidation | [25,26,27,28] |
| HA-SH | Carboxylic | Cysteamine | Amidation | [29,30,31,32] |
| HA(-ADH)-SH | Carboxylic | 1. Adipic acid dihydrazide (ADH) 2. 2-Immoinothiolane | 1. Amidation 2. Thiolation with Traut’s reagent | [33,34] |
| Thiolated carboxymethyl HA | Carboxylic | 5,5′-Dithiobis (2-nitrobenzoic acid) | Amidation | [35,36,37,38] |
| HA-DTPH | Carboxylic | Dithiobis (propanoic dihydrazide) | Amidation | [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57] |
| HA-DTBH | Carboxylic | Dithiobis (butyric dihydrazide) | Amidation | [39] |
| HA-PDPH | Carboxylic | 3-(2-pyridyldithio) propionyl hydrazide | Amidation | [58] |
| HA-SH | Hydroxyl | Ethylene sulfide | Ether formation | [59,60] |
| HA-SH | Hydroxyl | 1. Divinyl sulfone 2. Dithiothreitol | 1. Ether formation 2. Amidation | [61,62] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griesser, J.; Hetényi, G.; Bernkop-Schnürch, A. Thiolated Hyaluronic Acid as Versatile Mucoadhesive Polymer: From the Chemistry Behind to Product Developments—What Are the Capabilities? Polymers 2018, 10, 243. https://doi.org/10.3390/polym10030243
Griesser J, Hetényi G, Bernkop-Schnürch A. Thiolated Hyaluronic Acid as Versatile Mucoadhesive Polymer: From the Chemistry Behind to Product Developments—What Are the Capabilities? Polymers. 2018; 10(3):243. https://doi.org/10.3390/polym10030243
Chicago/Turabian StyleGriesser, Janine, Gergely Hetényi, and Andreas Bernkop-Schnürch. 2018. "Thiolated Hyaluronic Acid as Versatile Mucoadhesive Polymer: From the Chemistry Behind to Product Developments—What Are the Capabilities?" Polymers 10, no. 3: 243. https://doi.org/10.3390/polym10030243
APA StyleGriesser, J., Hetényi, G., & Bernkop-Schnürch, A. (2018). Thiolated Hyaluronic Acid as Versatile Mucoadhesive Polymer: From the Chemistry Behind to Product Developments—What Are the Capabilities? Polymers, 10(3), 243. https://doi.org/10.3390/polym10030243