The Anomalies of Hyaluronan Structures in Presence of Surface Active Phospholipids—Molecular Mass Dependence
Abstract
1. Introduction
2. Materials and Methods
Hydrogen Bond Identification and Strength
3. Results
3.1. Influence of Hyaluronan Molecular Mass on Cross-Linking in Presence of PL
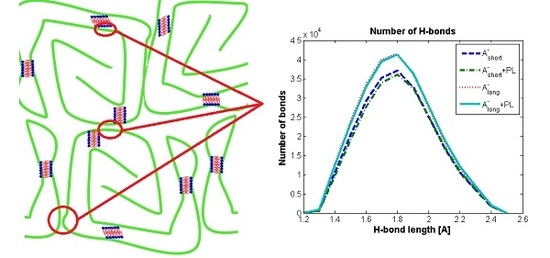
3.2. Hyaluronan-Phospholipd Bonding
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jung, S.; Petelska, A.; Bełdowski, P.; Augé, W.K.; Casey, T.; Walczak, D.; Lemke, K.; Gadomski, A. Hyaluronic acid and phospholipid interactions useful for repaired articular cartilage surfaces—A mini review toward tribological surgical adjuvants. Colloid Polym. Sci. 2017, 295, 403–4122. [Google Scholar] [CrossRef] [PubMed]
- Matej, D. Boundary cartilage lubrication: Review of current concepts. Wien. Med. Wochenschr. 2014, 164, 88–94. [Google Scholar]
- Hari, G.G.; Hales, C.A. Chemistry and Biology of Hyaluronan; Elsevier Science: Amsterdam, The Netherlands, 2008; ISBN 9780080472225. [Google Scholar]
- Pawlak, Z.; Urbaniak, W.; Hagner-Derengowska, M.; Hagner, W. The Probable Explanation for the low friction of natural joints. Cell Biochem. Biophys. 2015, 71, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, Z.; Urbaniak, W.; Afara, I.O.; Yusuf, A.Q.; Banaszak-Piechowska, A.; Oloyede, A. Tribological efficacy and stability of phospholipid-based membrane lubricants in varying pH chemical conditions. Biointerphases 2016, 11, 019002. [Google Scholar] [CrossRef] [PubMed]
- Snelling, S.; Rout, R.; Davidson, R.; Clark, I.; Carr, A.; Hulley, P.A.; Price, A.J. A gene expression study of normal and damaged cartilage in anteromedial gonarthrosis, a phenotype of osteoarthritis. Osteoarthr. Cartil. 2014, 22, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Augé, W.K. Conceptualization of surface-confined nano-assemblies as a biophysical battery circuit during tissue rescue: A bridge to accessing genomic control mechanisms. Int. J. Nanosyst. 2012, 5, 1–18. [Google Scholar]
- Banquy, X.; Lee, D.W.; Das, S.; Israelachvili, J. Shear-induced aggregation of mammalian synovial fluid components under boundary lubrication conditions. Adv. Funct. Mater. 2014, 24, 3152–3161. [Google Scholar] [CrossRef]
- Pawlak, Z.; Urbaniak, W.; Hagner-Derengowska, M.; Hagner, W. Lamellar slippage of bilayers—A hypothesis on low friction of natural joints. Biointerphases 2014, 9, 041004. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, Z.; Gadomski, A.; Sojka, M.; Urbaniak, W.; Bełdowski, P. The amphoteric effect on friction between the bovine cartilage/cartilage surfaces under slightly sheared hydration lubrication mode. Colloids Surf. B Biointerfaces 2016, 146, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Augé, W.K.; Ganguly, K.; Goodwin, P.M.; Gadomski, A.; Gehlert, R.J. Lipid distribution in human knee and hip articular cartilage correlated to tissue surface roughness and surface active phospholipid layer presence: Evidence of cooperative interfacial lipid delivery mechanisms. Osteoarthr. Cartil. 2014, 22, S312–S313. [Google Scholar] [CrossRef]
- Chen, Y.; Crawford, R.W.; Oloyede, A. Unsaturated phosphatidylcholines lining on the surface of cartilage and its possible physiological roles. J. Orthop. Surg. Res. 2007, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Wierzcholski, K. Joint cartilage lubrication with phospholipid bilayer. Tribologia 2016, 2, 145–157. [Google Scholar]
- Kosińska, M.K.; Ludwig, T.E.; Liebisch, G.; Zhang, R.; Siebert, H.C.; Wilhelm, J.; Kaesser, U.; Dettmeyer, R.B.; Klein, H.; Ishaque, B.; et al. Articular joint lubricants during osteoarthritis and rheumatoid arthritis display altered levels and molecular species. PLoS ONE 2015, 10, e0125192. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Banquy, X.; Das, S.; Cadirov, N.; Jay, G.; Israelachvili, J. Effects of molecular weight of grafted hyaluronic acid on wear initiation. J. Acta Biomater. 2014, 10, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Kwieciński, J.J.; Dorosz, S.G.; Ludwig, T.E.; Abubacker, S.; Cowman, M.K.; Schmidt, T.A. The effect of molecular weight on hyaluronan’s cartilage boundary lubricating ability—Alone and in combination with proteoglycan 4. Osteoarthr. Cartil. 2011, 19, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, D.W.; Nitzan, U.; Dan, P.; Yedgar, S. The role of hyaluronic acid in protecting surface-active phospholipids from lysis by exogenous phospholipase A2. Rheumatology 2001, 40, 335–340. [Google Scholar] [CrossRef]
- Seror, J.; Zhu, L.; Goldberg, R.; Day, A.J.; Klein, J. Supramolecular synergy in the boundary lubrication of synovial joints. Nat. Commun. 2015, 6, 6497. [Google Scholar] [CrossRef] [PubMed]
- Gadomski, A.; Bełdowski, P.; Rubi, J.M.; Urbaniak, W.; Augé, W.K.; Santamaria-Holek, I.; Pawlak, Z. Some conceptual thoughts toward nanoscale oriented friction in a model of articular cartilage. Math. Biosci. 2013, 244, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Hills, B.A. Boundary lubrication in vivo. Proc. Inst. Mech. Eng. H 2000, 214, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Band, P.A.; Heeter, J.; Wiśniewski, H.G.; Liublinska, V.; Pattanayak, C.W.; Karia, R.J.; Stabler, T.; Balazs, E.A.; Kraus, V.B. Hyaluronan molecular weight distribution is associated with the risk of knee osteoarthritis progression. Osteoarthr. Cartil. 2015, 23, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kosińska, M.K.; Liebisch, G.; Lochnit, G.; Wilhelm, J.; Klein, H.; Kaesser, U.; Lasczkowski, G.; Rickert, M.; Schmitz, G.; Steinmeyer, J. A lipidomic study of phospholipid classes and species in human synovial fluid. Arthritis Rheum. 2013, 65, 2323–2333. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Hutadilok, N.; Adam, N. Interactions of hyaluronan (hyaluronic acid) with phospholipids as determined by gel permeation chromatography, multi-angle laser-light-scattering photometry and 1H-NMR spectroscopy. Int. J. Biol. Macromol. 1994, 16, 237–244. [Google Scholar] [CrossRef]
- Pasquali-Ronchetti, I.; Quaglino, D.; Mori, G.; Bacchelli, B.; Ghosh, P. Hyaluronan-phospholipid interaction. J. Struct. Biol. 1997, 120, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Seror, J.; Day, J.A.; Kampf, N.; Klein, J. Ultra-low friction between boundary layers of hyaluronanphosphatidylcholine complexes. Acta Biomater. 2017, 59, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Siódmiak, J.; Bełdowski, P.; Augé, W.K., II; Ledziński, D.; Smigiel, S.; Gadomski, A. Molecular Dynamic Analysis of Hyaluronic Acid and Phospholipid Interaction in Tribological Surgical Adjuvant Design for Osteoarthritis. Molecules 2017, 22, 1436. [Google Scholar] [CrossRef]
- Bełdowski, P.; Weber, P.; Andrysiak, T.; Augé, W.K., II; Ledziński, D.; De Leon, T.; Gadomski, A. Anomalous Behavior of Hyaluronan Crosslinking Due to the Presence of Excess Phospholipids in the Articular Cartilage System of Osteoarthritis. Int. J. Mol. Sci. 2017, 18, 2779. [Google Scholar] [CrossRef]
- Connolly, M.L. Analytical molecular surface calculation. J. Appl. Cryst. 1983, 16, 548–558. [Google Scholar] [CrossRef]
- Richmond, T.J. Solvent accessible surface area and excluded volume in proteins. Analytical equations for overlapping spheres and implications for the hydrophobic effect. J. Mol. Biol. 1984, 178, 63–89. [Google Scholar] [CrossRef]
- Das, S.; Banquy, X.; Zappone, B.; Israelachvili, J. Synergistic interactions between grafted hyaluronic acid and lubricin provide enhanced wear protection and lubrication. Biomacromolecules 2013, 14, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Temple-Wong, M.M.; Ren, S.; Quach, P.; Hansen, B.C.; Chen, A.C.; Hasegawa, A.; D’Lima, D.D.; Koziol, J.; Masuda, K.; Lotz, M.K.; et al. Hyaluronan concentration and size distribution in human knee synovial fluid: Variations with age and cartilage degeneration. Arthritis Res. Ther. 2016, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Wieland, D.C.F.; Degen, P.; Zander, T.; Gayer, S.; Raj, A.; An, J.; Dédinaité, A.; Claesson, P.; Willumeit-Römer, R. Structure of DPPC-hyaluronan interfacial layers—Effects of molecular weight and ion composition. Soft Matter 2016, 12, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.; Wang, M.; Zander, T.; Wieland, D.C.F.; Liu, X.; An, J.; Garamus, V.M.; Willumeit-Römer, R.; Fielden, M.; Claesson, P.M.; et al. Lubrication synergy: Mixture of hyaluronan and dipalmitoylphosphatidylcholine (DPPC) vesicles. J. Colloid Interface Sci. 2017, 488, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, C.; Thormann, E.; Dédinaité, A. Hyaluronan and phospholipid association in biolubrication. Biomacromolecules 2013, 14, 4198–4206. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, Z. Articular Cartilage: Lamellar-Repulsive Lubrication of Natural Joints; Kindle Direct Publishing: Seattle, WA, USA, 2018; 171p. [Google Scholar]
- Oates, K.M.N.; Krause, W.E.; Jones, R.L.; Colby, R.H. Rheopexy of synovial fluid and protein aggregation. J. R. Soc. Interface 2006, 3, 167–174. [Google Scholar] [CrossRef] [PubMed]









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bełdowski, P.; Andrysiak, T.; Mreła, A.; Pawlak, Z.; Augé, W.K., II; Gadomski, A. The Anomalies of Hyaluronan Structures in Presence of Surface Active Phospholipids—Molecular Mass Dependence. Polymers 2018, 10, 273. https://doi.org/10.3390/polym10030273
Bełdowski P, Andrysiak T, Mreła A, Pawlak Z, Augé WK II, Gadomski A. The Anomalies of Hyaluronan Structures in Presence of Surface Active Phospholipids—Molecular Mass Dependence. Polymers. 2018; 10(3):273. https://doi.org/10.3390/polym10030273
Chicago/Turabian StyleBełdowski, Piotr, Tomasz Andrysiak, Aleksandra Mreła, Zenon Pawlak, Wayne K. Augé, II, and Adam Gadomski. 2018. "The Anomalies of Hyaluronan Structures in Presence of Surface Active Phospholipids—Molecular Mass Dependence" Polymers 10, no. 3: 273. https://doi.org/10.3390/polym10030273
APA StyleBełdowski, P., Andrysiak, T., Mreła, A., Pawlak, Z., Augé, W. K., II, & Gadomski, A. (2018). The Anomalies of Hyaluronan Structures in Presence of Surface Active Phospholipids—Molecular Mass Dependence. Polymers, 10(3), 273. https://doi.org/10.3390/polym10030273