Preparation and Characterization of Regenerated Cellulose Film from a Solution in Lithium Bromide Molten Salt Hydrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
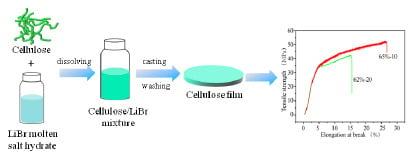
2.2. Cellulose Film Preparation in Aqueous Libr Solutions
2.3. Characterization
3. Results and Discussion
3.1. FT-IR Analysis
3.2. CP/MAS 13C NMR Analysis
3.3. XRD Analysis
3.4. SEM Analysis
3.5. UV-Vis Spectrophotometric Analysis
3.6. Mechanical Properties of Films
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tardy, A.; Nicolas, J.; Gigmes, D.; Lefay, C.; Guillaneuf, Y. Radical ring-opening polymerization: Scope, limitations, and application to (bio)degradable materials. Chem. Rev. 2017, 117, 1319–1406. [Google Scholar] [CrossRef] [PubMed]
- Sanla-Ead, N.; Jangchud, A.; Chonhenchob, V.; Suppakul, P. Antimicrobial activity of cinnamaldehyde and eugenol and their activity after incorporation into cellulose-based packaging films. Packag. Technol. Sci. 2012, 25, 7–17. [Google Scholar] [CrossRef]
- Ma, J.; Zhu, W.; Min, D.; Wang, Z.; Zhou, X. Preparation of antibacterial self-reinforced zinc oxide–cellulose composite by the synthesis of ZnO in partially dissolved cellulose. Cellulose 2016, 23, 3199–3208. [Google Scholar] [CrossRef]
- Cheng, D.; An, X.Y.; Zhang, J.H.; Tian, X.F.; He, Z.B.; Wen, Y.B.; Ni, Y.H. Facile preparation of regenerated cellulose film from cotton linter using organic electrolyte solution (OES). Cellulose 2017, 24, 1631–1639. [Google Scholar] [CrossRef]
- Hinner, L.P.; Wissner, J.L.; Beurer, A.; Nebel, B.A.; Hauer, B. Homogeneous vinyl ester-based synthesis of different cellulose derivatives in 1-ethyl-3-methyl-imidazolium acetate. Green Chem. 2016, 18, 6099–6107. [Google Scholar] [CrossRef] [Green Version]
- Guillou, J.; Lavadiya, D.N.; Munro, T.; Fronk, T.; Ban, H. From lignocellulose to biocomposite: Multi-level modelling and experimental investigation of the thermal properties of kenaf fiber reinforced composites based on constituent materials. Appl. Therm. Eng. 2018, 128, 1372–1381. [Google Scholar] [CrossRef]
- Dupont, A.L. Cellulose in lithium chloride/N,N-dimethylacetamide, optimisation of a dissolution method using paper substrates and stability of the solutions. Polymer 2003, 44, 4117–4126. [Google Scholar] [CrossRef]
- Stryuk, S.; Eckelt, J.; Wolf, B. Solutions of cellulose in DMAc + LiCl: Migration of the solute in an electrical field. Cellulose 2005, 12, 145–149. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocolloid. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Wang, H.H.; Liu, C.F.; Zhang, A.P.; Ren, J.L. Synthesis of thermoplastic xylan-lactide copolymer with amidine-mediated organocatalyst in ionic liquid. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rynkowska, E.; Fatyeyeva, k.; Kujawa, J.; Dzieszkowski, K.; Wolan, A.; Kujawski, W. The effect of reactive ionic liquid or plasticizer incorporation on the physicochemical and transport properties of cellulose acetate propionate-based membranes. Polymers 2018, 10, 86. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.G.; Li, Y.Q.; Lu, Z.X.; Cao, S.L.; Fan, M.Z.; Huang, L.L.; Chen, L.H. Facile cellulose dissolution and characterization in the newly synthesized 1,3-diallyl-2-ethylimidazolium acetate ionic liquid. Polymers 2017, 9, 526. [Google Scholar] [CrossRef]
- Mendes, F.R.S.; Bastos, M.S.R.; Mendes, L.G.; Silva, A.R.A.; Sousa, F.D.; Monteiro-Moreira, A.C.O.; Cheng, H.N.; Biswas, A.; Moreira, R.A. Preparation and evaluation of hemicellulose films and their blends. Food Hydrocolloid. 2017, 70, 181–190. [Google Scholar] [CrossRef]
- Chinsirikul, W.; Rojsatean, J.; Hararak, B.; Kerddonfag, N.; Aontee, A.; Jaieau, K.; Kumsang, P.; Sripethdee, C. Flexible and tough poly(lactic acid) films for packaging applications: Property and processability improvement by effective reactive blending. Packag. Technol. Sci. 2015, 28, 741–759. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geter, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Yudianti, R.; Syampurwadi, A.; Onggo, H.; Karina, M.; Uyama, H.; Azuma, J. Properties of bacterial cellulose transparent film regenerated from dimethylacetamide–LiCl solution. Polym. Advan. Technol. 2016, 27, 1102–1107. [Google Scholar] [CrossRef]
- Quanling, Y.; Tsuguyuki, S.; Akira, I. Transparent, flexible, and high-strength regenerated cellulose/saponite nanocomposite films with high gas barrier properties. J. Appl. Polym. Sci. 2013, 130, 3168–3174. [Google Scholar]
- Ashok, B.; Reddy, K.O.; Madhukar, K.; Cai, J.; Zhang, L.; Rajulu, A.V. Properties of cellulose/thespesia lampas short fibers bio-composite films. Carbohydr. Polym. 2015, 127, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Huber, T.; Müssig, J.; Curnow, O.; Pang, S.; Bickerton, S.; Staiger, M.P. A critical review of all-cellulose composites. J. Mater. Sci. 2012, 47, 1171–1186. [Google Scholar] [CrossRef]
- Zhang, J.M.; Luo, N.; Zhang, X.Y.; Xu, L.L.; Wu, L.; Yu, J.; He, J.S.; Zhang, J. All-cellulose nanocomposites reinforced with in situ retained cellulose nanocrystals during selective dissolution of cellulose in an ionic liquid. ACS Sustain. Chem. Eng. 2016, 4, 4417–4423. [Google Scholar] [CrossRef]
- Yan, C.; Huiquan, L.; Yi, Z.; Jun, Z.; Jiasong, H. Structure and properties of novel regenerated cellulose films prepared from cornhusk cellulose in room temperature ionic liquids. J. Appl. Polym. Sci. 2010, 116, 547–554. [Google Scholar]
- Reddy, K.O.; Zhang, J.; Zhang, J.; Rajulu, A.V. Preparation and properties of self-reinforced cellulose composite films from agave microfibrils using an ionic liquid. Carbohydr. Polym. 2014, 114, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.H.; Liu, X.; Wu, M.; Wu, Y.Y.; Zhang, X.M.; Sun, R.C. Fabrication and characterization of regenerated cellulose films using different ionic liquids. J. Spectrosc. 2014, 2014, 8. [Google Scholar] [CrossRef]
- Duchemin, B.J.C.; Mathew, A.P.; Oksman, K. All-cellulose composites by partial dissolution in the ionic liquid 1-butyl-3-methylimidazolium chloride. Compos. Part. A: Appl. Sci. Manuf. 2009, 40, 2031–2037. [Google Scholar] [CrossRef]
- Yang, Y.J.; Shin, J.M.; Kang, T.H.; Kimura, S.; Wada, M.; Kim, U.J. Cellulose dissolution in aqueous lithium bromide solutions. Cellulose 2014, 21, 1175–1181. [Google Scholar] [CrossRef]
- Deng, W.H.; Kennedy, J.R.; Tsilomelekis, G.; Zheng, W.Q.; Nikolakis, V. Cellulose hydrolysis in acidified libr molten salt hydrate media. Ind. Eng. Chem. Res. 2015, 54, 5226–5236. [Google Scholar] [CrossRef]
- Montero, C.; Clair, B.; Alméras, T.; Lee, A.v.d.; Gril, J. Relationship between wood elastic strain under bending and cellulose crystal strain. Compos. Sci. Technol. 2012, 72, 175–181. [Google Scholar] [CrossRef]
- Popescu, M.C.; Popescu, C.M.; Lisa, G.; Sakata, Y. Evaluation of morphological and chemical aspects of different wood species by spectroscopy and thermal methods. J. Mol. Struct. 2011, 988, 65–72. [Google Scholar] [CrossRef]
- Davidson, T.C.; Newman, R.H.; Ryan, M.J. Variations in the fibre repeat between samples of cellulose I from different sources. Carbohydr. Res. 2004, 339, 2889–2893. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.; Creely, J.J.; Jr, A.E.M.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the x-ray diffractometer. Text. Res. J. 2016, 29, 786–794. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, M.J.; Zhang, X.Q.; Liu, C.F.; Sun, R.C. Per-O-acetylation of cellulose in dimethyl sulfoxide with catalyzed transesterification. J. Agric. Food Chem. 2014, 62, 3446–3452. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Kubicki, J.D.; Fan, B.; Zhong, L.; Jarvis, M.C.; Kim, S.H. Hydrogen-bonding network and OH stretch vibration of cellulose: Comparison of computational modeling with polarized IR and SFG spectra. J. Phys. Chem. B 2015, 119, 15138–15149. [Google Scholar] [CrossRef] [PubMed]
- Maunu, S.; Liitiä, T.; Kauliomäki, S.; Hortling, B.; Sundquist, J. 13C CP/MAS NMR investigations of cellulose polymorphs in different pulps. Cellulose 2000, 7, 147–159. [Google Scholar] [CrossRef]
- Idström, A.; Schantz, S.; Sundberg, J.; Chmelka, B.F.; Gatenholm, P.; Nordstierna, L. 13C NMR assignments of regenerated cellulose from solid-state 2D NMR spectroscopy. Carbohydr. Polym. 2016, 151, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Luo, N.; Zhang, X.Y.; Xu, L.L.; Wu, J.; Yu, J.; He, J.S.; Zhang, J. All-cellulose nanocomposites reinforced with in situ retained cellulose nanocrystals during selective dissolution of cellulose in an ionic liquid. ACS Sustain. Chem. Eng. 2016, 4, 4417–4423. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Zhong, L.X.; Peng, X.W.; Yang, D.; Cao, X.F.; Sun, R.C. Long-chain anhydride modification: A new strategy for preparing xylan films. J. Agric. Food Chem. 2013, 61, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.H.; Wu, M.; Zhang, Q.H.; Tan, X.; Xu, F.; Zhang, X.M.; Sun, R.C. Comparison of physical properties of regenerated cellulose films fabricated with different cellulose feedstocks in ionic liquid. Carbohydr. Polym. 2015, 121, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.O.; Maheswari, C.U.; Dhlamini, M.S.; Mothudi, B.M.; Zhang, J.; Zhang, J.; Nagarajan, R.; Rajulu, A.V. Preparation and characterization of regenerated cellulose films using borassus fruit fibers and an ionic liquid. Carbohydr. Polym. 2017, 160, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.P.; Zhang, X.F.; Chen, Y.W.; Duan, Y.X.; Zhang, J.M. Green fabrication of regenerated cellulose/graphene films with simultaneous improvement of strength and toughness by tailoring the nanofiber diameter. ACS Sustain. Chem. Eng. 2018, 6, 1271–1278. [Google Scholar] [CrossRef]
- Tanvir, A.; Al-Maadeed, M.A.; Hassan, M.K. Secondary chain motion and mechanical properties of irradiated regenerated cellulose films. Starch-Starke 2017, 69, 1–8. [Google Scholar] [CrossRef]
- Ding, H. Handbook of plastic industry; Chemical Industry Press: Beijing, China, 1995. [Google Scholar]






| Sample | d-Spacing (nm) | Crystalline Size (nm) | ||||||
|---|---|---|---|---|---|---|---|---|
| 10 | 110 | 200 | 004 | 10 | 110 | 200 | 004 | |
| Cellulose | 0.595 | 0.534 | 0.392 | 0.258 | 7.35 | 7.84 | 8.79 | 7.47 |
| 60%-5 | 0.738 | 0.433 | 0.385 | 0.258 | 6.57 | 4.59 | 5.58 | 6.52 |
| 60%-15 | 0.725 | 0.439 | 0.411 | 0.252 | 4.80 | 4.49 | 4.94 | 4.57 |
| 60%-25 | 0.731 | 0.437 | 0.409 | 0.254 | 4.64 | 4.45 | 4.52 | 4.47 |
| 60%-35 | 0.720 | 0.435 | 0.400 | 0.252 | 3.54 | 4.28 | 3.94 | 3.79 |
| 62%-5 | 0.720 | 0.440 | 0.410 | 0.253 | 6.44 | 4.54 | 5.42 | 6.47 |
| 62%-15 | 0.725 | 0.442 | 0.406 | 0.254 | 4.69 | 4.42 | 4.85 | 4.47 |
| 62%-25 | 0.725 | 0.437 | 0.404 | 0.251 | 4.31 | 4.37 | 4.38 | 4.30 |
| 62%-35 | 0.714 | 0.440 | 0.406 | 0.250 | 3.45 | 4.20 | 3.82 | 3.75 |
| 65%-5 | 0.720 | 0.439 | 0.408 | 0.255 | 6.38 | 4.47 | 5.36 | 6.31 |
| 65%-15 | 0.708 | 0.435 | 0.406 | 0.254 | 4.60 | 4.33 | 4.67 | 4.35 |
| 65%-25 | 0.714 | 0.435 | 0.409 | 0.254 | 4.28 | 4.29 | 4.29 | 4.17 |
| 65%-35 | 0.714 | 0.435 | 0.402 | 0.251 | 3.28 | 4.12 | 3.57 | 3.37 |
| Sample | Proportion of Crystalline Interior Chains (nm) | CrI | |||
|---|---|---|---|---|---|
| 10 | 110 | 200 | 004 | (%) | |
| Cellulose | 0.713 | 0.76 | 0.768 | 0.718 | 82.7 |
| 60%-5 | 0.683 | 0.565 | 0.628 | 0.657 | 75.8 |
| 60%-15 | 0.587 | 0.558 | 0.573 | 0.569 | 70.3 |
| 60%-25 | 0.576 | 0.554 | 0.527 | 0.548 | 63.6 |
| 60%-35 | 0.562 | 0.543 | 0.490 | 0.499 | 60.7 |
| 62%-5 | 0.671 | 0.558 | 0.613 | 0.527 | 67.9 |
| 62%-15 | 0.582 | 0.548 | 0.591 | 0.427 | 64.8 |
| 62%-25 | 0.567 | 0.540 | 0.546 | 0.408 | 62.1 |
| 62%-35 | 0.531 | 0.528 | 0.482 | 0.391 | 58.4 |
| 65%-5 | 0.628 | 0.529 | 0.599 | 0.497 | 61.3 |
| 65%-15 | 0.566 | 0.541 | 0.559 | 0.420 | 59.8 |
| 65%-25 | 0.540 | 0.538 | 0.527 | 0.399 | 55.8 |
| 65%-35 | 0.519 | 0.508 | 0.436 | 0.383 | 50.5 |
| Sample | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) |
|---|---|---|---|
| 60%-5 | 4.19 | 343.02 | 6.68 |
| 60%-10 | 6.74 | 398.71 | 8.75 |
| 60%-15 | 25.71 | 394.33 | 7.26 |
| 60%-20 | 33.79 | 727.40 | 11.60 |
| 60%-25 | 36.80 | 568.36 | 8.86 |
| 60%-30 | 37.51 | 396.06 | 6.87 |
| 60%-35 | 15.07 | 355.28 | 7.16 |
| 60%-40 | 13.93 | 493.76 | 5.17 |
| 62%-5 | 20.93 | 720.58 | 7.31 |
| 62%-10 | 20.30 | 573.22 | 9.23 |
| 62%-15 | 41.22 | 1210.99 | 16.09 |
| 62%-20 | 38.32 | 897.46 | 11.57 |
| 62%-25 | 37.91 | 741.68 | 7.49 |
| 62%-30 | 52.60 | 964.86 | 7.27 |
| 62%-35 | 45.77 | 1277.90 | 5.71 |
| 62%-40 | 22.79 | 886.71 | 6.35 |
| 65%-5 | 20.69 | 771.60 | 9.23 |
| 65%-10 | 50.67 | 771.65 | 26.03 |
| 65%-15 | 66.80 | 1506.63 | 22.96 |
| 65%-20 | 57.59 | 1362.12 | 10.43 |
| 65%-25 | 53.75 | 835.02 | 9.97 |
| 65%-30 | 49.38 | 1433.00 | 5.69 |
| 65%-35 | 42.92 | 1403.95 | 5.58 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Xiao, N.; Wang, H.; Liu, C.; Pan, X. Preparation and Characterization of Regenerated Cellulose Film from a Solution in Lithium Bromide Molten Salt Hydrate. Polymers 2018, 10, 614. https://doi.org/10.3390/polym10060614
Zhang X, Xiao N, Wang H, Liu C, Pan X. Preparation and Characterization of Regenerated Cellulose Film from a Solution in Lithium Bromide Molten Salt Hydrate. Polymers. 2018; 10(6):614. https://doi.org/10.3390/polym10060614
Chicago/Turabian StyleZhang, Xueqin, Naiyu Xiao, Huihui Wang, Chuanfu Liu, and Xuejun Pan. 2018. "Preparation and Characterization of Regenerated Cellulose Film from a Solution in Lithium Bromide Molten Salt Hydrate" Polymers 10, no. 6: 614. https://doi.org/10.3390/polym10060614
APA StyleZhang, X., Xiao, N., Wang, H., Liu, C., & Pan, X. (2018). Preparation and Characterization of Regenerated Cellulose Film from a Solution in Lithium Bromide Molten Salt Hydrate. Polymers, 10(6), 614. https://doi.org/10.3390/polym10060614