1. Introduction
Three-dimensional (3D) printing and bioprinting is a rapidly growing field that aims to develop sophisticated constructs for tissue regeneration. These approaches hold the potential to achieve functional tissue constructs by repairing the complex architecture and organization of native tissues. As such, the preparation complex structure with a similar native tissue is important [
1]. Hydrogels are important materials for the preparation of 3D printed tissue-engineered scaffolds [
2]. The precursor solution act as 3D printing ink for preparing hydrogels. Its viscosity and transitions process of sol to gel determines the shape fidelity and structure of 3D printed hydrogels [
3]. Adding nanomaterial into 3D printing ink or preparing hydrogels with double networks are the regular methods [
4,
5,
6], but the biocompatibility and degradation properties of 3D printed hydrogels that are used as scaffolds for tissue engineering cannot be guaranteed [
7]. For the preparation of 3D printed, hydrogels act as scaffolds for tissue engineering, with a complex structure and high shape fidelity, however there are still many problems that need to be solved.
Sodium alginates, which are extracted from brown seaweed, are biocompatible polyanionic [
8]. As a result of the shear thinning properties of the alginate solution, it is often used as precursor solution for 3D printed tissue-engineered constructs [
9,
10]. When an alginate solution was used for preparing tissue-engineered scaffolds, the structure and shape fidelity of the 3D printed hydrogels is usually difficult to guarantee, because the viscosity of the maximum concentration of alginate solution is still insufficient, the deposited filaments are easily fused and collapsed. In addition, the alginate hydrogels that are crosslinked with Ca
2+ are mechanically weak [
4], and the deposited filaments are easily collapsed because of gravity, which also has influence on the structure and shape fidelity of 3D printed hydrogels.
Acting as a biocompatible polyanionic, the alginate can also be crosslinked with polycation to obtain polyion complex (PIC). The mechanical strength of PIC hydrogels, prepared with two oppositely charged polyelectrolytes, is controllable through changing reactive ion pairs [
11]. The mixing of bulk solutions of polycation and polyanion usually leads to inhomogeneous precipitation, where a strong PIC is formed at the interface of the two solutions, which quenches the further reaction [
11,
12]. As a result of the limited reactive ion pairs that are involved in the reaction, the crosslinking density of the biomaterial internal network is low. To solve this problem, Luo et al. [
13] polymerized one of the polyelectrolytes from its monomers solution in the presence of another oppositely charged polymer at 1:1 charge ratio. The cationic monomer 3-(methacryloylamino) propyl-trimethylammonium chloride (MPTC) was homopolymerized in the first step and was then mixed with the anionic monomer sodium p-styrenesulfonate (NaSS). After the well dispersion, the anionic monomer is polymerized in the second step, to form soft PIC hydrogel. Tensile and compression tests indicate that the hydrogels that are formed by the oppositely charged polyelectrolytes are tough, self-healing, and rebuildable.
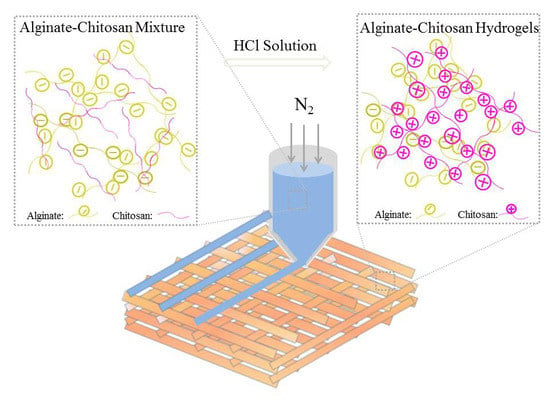
Chitosan is the only natural polycation polysaccharide, which is swelling, but it is insoluble in an aqueous solution. When the pH value of the aqueous solution is less than 4, chitosan is soluble and can react with anion or polyanion, such as alginate, to prepare the PIC hydrogels [
14]. In this work, chitosan powders were added into the alginate solution as the 3D printing ink, and chitosan is swelling but insoluble in the alginate solution, which can effectively improve the viscosity of the alginate solution. After being treated with a hydrochloric acid (HCl) solution, the chitosan powders that were dispersed in am alginate solution were soluble and reacted with alginate, thus, 3D printed of AlCh PIC hydrogels were obtained. A molecule of HCl is a small molecule that diffuses easily, thus, the further reaction between two oppositely charged polyelectrolytes will not be quenched. With the increase of the reactive ion pairs, the mechanical strength of the 3D printed AlCh PIC hydrogel can be improved, and the 3D printed hydrogels with complex structures could also be prepared correspondingly.
2. Materials and Methods
2.1. Materials
Sodium alginate (Al, viscosity: 180–220 mp·s) and chitosan (Ch, viscosity: 100–200 mp·s, and the degree of deacetylation >95%) were both obtained from Aladdin. HCl was obtained from Huachengda Chemical Co. Ltd. (Zhuhai, China). All off the reagents were used as they were received if there was no special explanation.
2.2. Preparation of 3D Printing Ink
The 3D printing ink was prepared as two steps. Firstly, a 10% (
w/
v) alginate solution was prepared by adding sodium alginate into deionized water (DI water) at room temperature. Secondly, a certain amount of chitosan (as seen in
Table 1) was added to the alginate solution and stirred sufficiently to ensure a good dispersion of chitosan in the alginate solution. To remove the air bubbles in the 3D printing ink, it was placed in vacuum drying oven at 30 °C for 30 min.
2.3. Preparation of 3D Printed Hydrogels
The 3D-BIOPLOTTER
TM (Envisionter Gmbh, Karlsruhe, Germany) with a resolution of 100 μm was used for the printing of all of the types of hydrogels. The 3D printing ink (Al1Ch0.2–Al1Ch1.2) was loaded into a dispensing unit consisting of a cartridge and a nozzle (400 μm), and was extruded out with applied nitrogen gas along the X–Y–Z target paths. The spacing between the two deposited fibers was set as 900 μm. After a layer of deposition was completed, the staying between the layers was 15 s. In the meantime, an HCl solution (0.5 mol/L) was sprayed on the deposited fibers with a single-use syringe, in order to protonate the NH
2 on the chitosan. As the amino group was converted to ammonium, the 3D printed alginate–chitosan polyion complex hydrogels were prepared because the electrostatic interaction between the two oppositely charged polyelectrolytes (as shown in
Figure 1). To verify the effects of the addition of chitosan on the morphology of, 3D printed alginate hydrogels were prepared in the same ways, except that the HCl solution was replaced with a CaCl
2 solution (0.1 mol/L). If it was not specifically stated, the angle of layers of all of the hydrogels that were used for the characterization was 90°.
2.4. Rheological Test of 3D Printing Ink
Rheological properties of 3D printing ink with a different molar ratio of alginate to chitosan were measured on a rotary rheometer (AR-G2, TA, Waters, Newcastle, DE, USA), with parallel circular plates of a 40 mm diameter. Steady rate sweeps were conducted by varying the shear rates from 0.01 to 1000 s−1 at 30 °C, and the viscosity was measured at different shear rates.
2.5. Fourier-Transform Infrared Spectroscopy
A Fourier-transform infrared (FTIR) spectroscopy (Nexus Por Euro, Bruker, Karlsruhe, Germany) was used to verify the electrostatic interaction between alginate and chitosan. Using the FTIR spectra of alginate, chitosan, and 3D printed, the AlCh PIC hydrogel was obtained under the following conditions: the average of 32 scans between 400 cm−1 and 4000 cm−1 at a resolution of 4 cm−1.
2.6. Scanning Electron Microscope (SEM) Analysis
The structure and architecture of the 3D printed hydrogels was observed using an SEM (Quanta 200, FEI, Eindhoven, The Netherlands). The 3D printed hydrogels were prefrozen at −20 °C in a refrigerator and then freeze-dried in a freeze-drying machine (VIRTIS Genesis, Warminster, PA, USA). Before being mounted on aluminum stubs, they were treated with gold sputtering. The front and side morphology of the freeze-dried hydrogels were examined under SEM at an accelerated voltage of 10 KV, and the work distance (WD) was about 10 mm, which was adjusted according to the sample height.
2.7. Mechanical Properties
Compression tests of the rectangular block hydrogels with a size 10 mm × 10 mm × 3.2 mm were conducted on a universal material testing machine (Instron 5967, Instron, Norwood, MA, USA). The compression stress–strain curves were obtained when hydrogels were uniaxially compressed at a displacement rate of 1 mm/min to 90% strain. The compression strength and toughness were calculated through stress–strain curves. Compression stress with 90% strain represented the compression strength, while the toughness was equal to the area under the stress–strain curves. The compression modulus was calculated from the slope of the linear elastic region of the stress–strain curves, which was between 0% and 30% strain for all of the samples. In order to get the mean and standard deviation calculations, five parallel samples of each group were used for testing.
2.8. Swelling Ratio and Water Absorption of Hydrogels
For the swelling ratio and water absorption, rectangular block hydrogels of different internal structures, in size of 10 mm × 10 mm × 3.2 mm, were measured according to the reported methods by the authors of [
15]. The 3D printed hydrogels were prefrozen at −20 °C in a refrigerator and then freeze-dried in a freeze-drying machine (VIRTIS Genesis, Warminster, PA, USA) to obtain freeze-dried hydrogels. The weight of the freeze-dried hydrogels was denoted as W
0, and after that, they were immersed in phosphate buffered saline (PBS, pH = 7.4) at the 37 °C constant temperature using a shake table. At a fixed time, the PBS on the scaffold surface was wiped off, and the weight of hydrogels at the fixed time was denoted as W. In order to get the mean and standard deviation calculations, five parallel samples of each group were used for testing. The swelling ratio and water absorption of the hydrogels in equilibrium were calculated as the following:
2.9. Degradation Properties of Hydrogels
Rectangular block hydrogels, with size 10 mm × 10 mm × 3.2 mm, were pre-weighted and immersed in PBS at 37 °C. At different time intervals, thee samples were taken out and rinsed with deionized water to remove the extra PBS (pH = 7.4) on the surface of the samples, and then, they were freeze dried and weighed again. The PBS solution was changed every 3 days. In order to get the mean and standard deviation calculations, five parallel samples of each group were used for testing. The degradation mass ratio was calculated as the following formula:
where W
1 is the initial weight of the hydrogel and W
2 is final weight of the hydrogel.
2.10. Cell Viability Assay and Cell Morphology
Human adipose-derived stem cells (hASCs) were purchased from Cyagen Biosciences (Guangzhou, China) and were incubated to passage 5–10 in a culture medium (DMEM (dulbecco’s modified eagle medium) consisting of 10% FBS, (fetal bovine serum) 1% penicillin/streptomycin) at 37 °C in a constant temperature incubator. To seed the cells on the 3D printed AlCh PIC hydrogels, there were three steps. Firstly, the Al1Ch1.0 hydrogels, with size 10 mm × 10 mm × 2 mm, were freeze-dried with a freeze-dring machine and were placed in the 24-well plates to be sterilized by 15 KGy γ radiation. Secondly, the cell culture medium was added into the pores to immerse the dried hydrogels for 24 h in a super clean table. Thirdly, the cell culture medium was taken out, the material was air-dried in a super clean table for 30 min, and the cultured cells were dissociated with 0.25% trypsin-EDTA and centrifuged, and then, hASCs in 100 μL complete medium were seeded on each sample and incubated at 37 °C in a constant temperature incubator for 1 h. After this, 900 μL of the complete medium was added to each sample so as to give a cell density of 2 × 105 cells per mL. The complete medium was changed every 2 days.
The cell viability of the constructs was examined by a Live–Dead viability Kit after the cells were cultured for 3 days. For the Live–Dead assay, firstly, the cell-laden hydrogels were washed with sterilized PBS 3 times; secondly, 100 μL of the live–dying stain was added to the pores; thirdly, 24-well plates were incubated at the 37 °C in a constant temperature incubator for 30 min; and finally, they were washed with sterilized PBS (pH = 7.4) 3 times. With the aid of a fluorescence inverted microscope, the live and dead cells that were distributed on the hydrogels were observed.
The morphology and adhesive properties of hASCs in the 3D printed hydrogels were observed by SEM (Quanta 200, FEI, Eindhoven, The Netherlands) after the cells were cultured for 3 days. The cells were fixed by glutaraldehyde (2.5%, v/v) overnight, after being washed with PBS, and they were dehydrated with an isocratic gradient ethanol solution. Then, they were dried in an air oven at 37 °C for 3 h and the morphology and adhesive properties of the hASCs were observed.
2.11. Proliferation of hASCs Cultured on Materials
The hASCs were incubated to passage 5–10 in a culture medium (DMEM consisting of 10% FBS, 1% penicillin/streptomycin) at 37 °C in a constant temperature incubator. Then, 2 × 105/mL of hASCs in 100 μL were seeded on the sterilized Al1Ch1.0 hydrogels, with size 10 mm × 10 mm × 2 mm, and 1 h later, 900 μL of a culture medium was added to each sample, in order to give a cell density of 2 × 105 cells per mL. Samples were taken out to place in another 24-well plate at fixed time, the proliferation of the hASCs were evaluated with a Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan), according to the manufacturer’s instruction. There was 1 mL of CCK-8 solution added to pores after hhe cells were incubated for 1 h at 37 °C in a constant temperature incubator. Finally, the optical density of the CCK-8 solution was measured at 450 nm using a microplate reader (Therm 3001, Ithaca, NY, USA). In order to get the mean and standard deviation calculations, five pores were used for testing.
2.12. Statistical Analysis
The data were expressed as mean ± standard deviation (SD). The statistical analysis was performed using the one way ANOVA (analysis of variance) test to determine significant differences. A p-value < 0.05 was considered as statistically significant.
4. Conclusions
In this study, we prepared 3D printed hydrogels by changing the pH so as to induce the poly-complexation of the alginate and chitosan after the 3D printing ink was extruded out. The addition of chitosan into the alginate solution ws useful for improving the viscosity of 3D printing ink. Moreover, the molecule of HCl was a small molecule that diffused easily, and the gelation process, through the change of pH to induce poly-complexation between the alginate and chitosan, was fast. Thus, the deposited filaments were not easily collapsed or fused, and complex constructs could be prepared through this method. The physicochemical properties of the obtained hydrogels could be controlled by controlling the content of chitosan. By printing the nose with the alginate–chitosan mixture, we proved that the prepared 3D printing ink could be used to print tissues or organs with complex structures. It was possible to fabricate other tissues or organs with this alginate–chitosan mixture.
Finally, hASCs were pluripotent cells. We verified that the hASCs that were seeded on the 3D printed AlCh PIC hydrogels adhered and proliferated, so the methods in this work could prepare hydrogels with a complex structure, to provide cells with appropriate growing environment. A further induced differentiation of hASCs in vitro could obtain neo tissue to repair or even replicate damaged tissues, thus the 3D printed AlCh PIC hydrogels had potential to be used for tissue engineering.