Polysiloxane-Based Side Chain Liquid Crystal Polymers: From Synthesis to Structure–Phase Transition Behavior Relationships
Abstract
:1. Introduction
2. Synthetic Methods of Polysiloxane-Based Side Chain Liquid Crystal Polymers (PSCLCPs)
2.1. Hydrosilylation Reaction
2.2. Chlorination Reaction
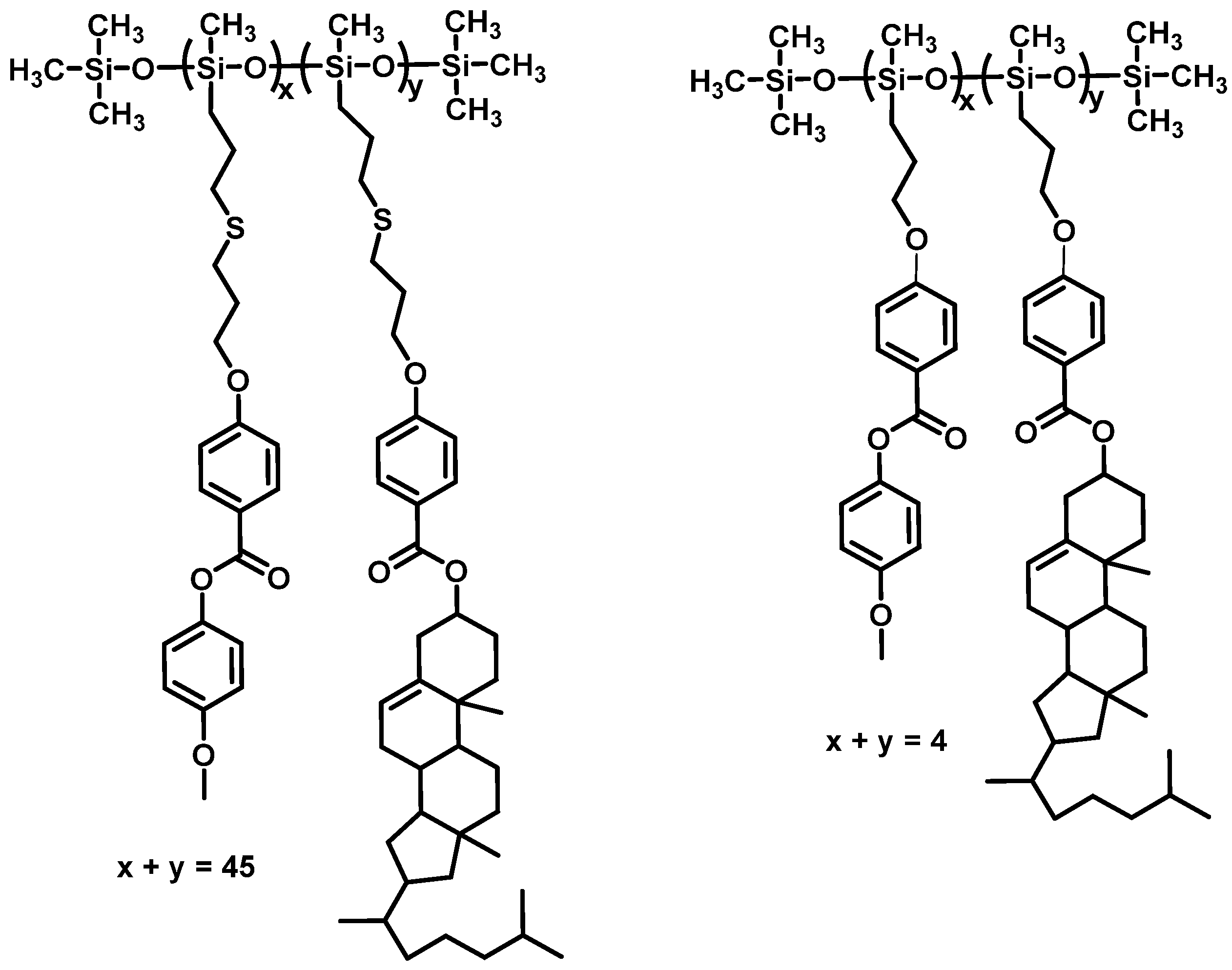
2.3. Thiol–Ene Click Chemistry
2.4. Atom Transfer Radical Polymerization (ATRP) Technique
2.5. Esterification Reaction
2.6. Williamson Nucleophilic Substitution
2.7. Supramolecular Interaction
3. The Relationship between the Molecular Structures and LC Phase Transitions & Structures of Polysiloxane-Based Side Chain Liquid Crystal Polymers (PSCLCPs)
3.1. Dependence of the Main Chain on Phase Transition Behaviors & Phase Structures of the PSCLCPs
3.1.1. Dependence of the Type and Nature of the Polysiloxanes Main Chain
3.1.2. Dependence of Polymerization Degree of the Linear Polysiloxanes and the Degree of Substitution of the Mesogenic Structural Unit
3.1.3. Dependence of the Ring Size of the Cyclic Polysiloxanes Main Chain
3.1.4. Dependence of the Shape of the Polysiloxanes Main Chain
3.2. Dependence of the Flexible Spacer on Phase Transition Behaviors & Phase Structures of the PSCLCPs
3.2.1. Dependence of the Shape of the Polysiloxanes Main Chain
3.2.2. Dependence of the Polyoxyethylenic Spacer on the Phase Transition Behaviors & Phase Structures of the PSCLCPs
3.2.3. Dependence of the Thioether Spacer on the Phase Transition Behaviors & Phase Structures of the PSCLCPs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Noll, W. Chemistry and Technology of Silicones; Academic Press: New York, NY, USA, 1968. [Google Scholar]
- Eaborn, C. Organosilicon Compounds; Butterworths Scientific Publications: London, UK, 1960. [Google Scholar]
- Dolgov, O.; Voronkov, M.G.; Grinblat, M. Organosilicon Liquid Rubbers; Rubber and Plastics Research Association of Great Britain: Shawbury, UK, 1975. [Google Scholar]
- Lynch, W. Handbook of Silicone Rubber Fabrication; Van Nostrand and Reinhold: New York, NY, USA, 1978. [Google Scholar]
- Shibaev, V.P.; Yakovlev, I.V.; Kostromin, S.G. Features of optical-recording of information on oriented films of liquid-crystalline comb-shaped polymer under the action of selective optical excitement. Vysokomol. Soedin. Ser. A 1990, 32, 1552–1559. [Google Scholar]
- Shibaev, V.P.; Kostromin, S.G.; Ivanov, S.A. Polymers as Electroactive and Photooptical Active Media; Springer: Berlin, Germany, 1996; p. 37. [Google Scholar]
- Hsu, C.S. The application of side-chain liquid-crystalline polymers. Prog. Polym. Sci. 1997, 22, 829–871. [Google Scholar] [CrossRef]
- Shibaev, V.P. Liquid-crystalline polymer systems: From the past to the present. Vysokomol. Soedin. Ser. A 2014, 56, 593–630. [Google Scholar] [CrossRef]
- West, R.; Barton, T.J. Organosilicon Chem., Parts I and II. J. Chem. Educ. 1980, 57, 334. [Google Scholar] [CrossRef]
- Swinburne, M.L.; Willmot, D.; Patrick, D. The use of debonding microspheres in electrothermal debonding. Eur. J. Orthod. 2011, 33, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, F.; Galehassadi, M.; Mahkam, M. Synthesis and characterization of new silyl cross-linking agent for drug delivery system. J. Appl. Polym. Sci. 2011, 122, 2368–2373. [Google Scholar] [CrossRef]
- Mojsiewicz-Pienkowska, K.; Jamrogiewicz, M.; Zebrowska, M.; Sznitowska, M.; Centkowska, K. Technology of an adhesive silicone film as drug carrier in transdermal therapy. I: Analytical methods used for characterization and design of the universal elastomer layers. J. Pharm. Biomed. Anal. 2011, 56, 131–138. [Google Scholar] [PubMed]
- Finkelmann, H.; Rehage, G. Investigations on liquid crystalline polysiloxanes, 2 optical properties of cholesteric phases and influence of the flexible spacer on the mobility of the mesogenic groups. Makromol. Chem. Rapid Commun. 1980, 1, 733–740. [Google Scholar] [CrossRef]
- Finkelmann, H.; Ringsdorf, H.; Wendorff, J.H. Model consideration and examples of enantiotropic liquid crystalline polymers. Macromol. Chem. 1978, 179, 273–276. [Google Scholar] [CrossRef]
- Wang, L.Z.; Jiang, Y.Y. An active and stable hydrosilylation catalyst: A silica-supported poly-γ-mercaptopropylsiloxane-platinum complex. J. Organomet. Chem. 1983, 251, 39–44. [Google Scholar] [CrossRef]
- Lewis, L.N.; Lewis, N. Platinum-catalyzed hydrosilylation-colloid formation as the essential step. J. Am. Chem. Soc. 1986, 108, 7228–7231. [Google Scholar] [CrossRef]
- Chalk, A.J.; Harrod, J.F. Homogeneous Catalysis. II. The Mechanism of the Hydrosilation of Olefins Catalyzed by Group VIII Metal Complexes. J. Am. Chem. Soc. 1965, 87, 16–21. [Google Scholar] [CrossRef]
- Takei, I.; Nishibayashi, Y.; Ishii, Y.; Mizobe, Y.; Uemura, S.; Hidai, M. Ruthenium-catalysed asymmetric hydrosilylation of ketoximes using chiral oxazolinylferrocenylphosphines. Chem. Commun. 2001, 22, 2360–2361. [Google Scholar] [CrossRef]
- Wu, W.; Li, C.J. A highly regio- and stereoselective transition metal-catalyzed hydrosilylation of terminal alkynes under ambient conditions of air, water, and room temperature. Chem. Commun. 2003, 14, 1668–1669. [Google Scholar] [CrossRef]
- Chauhan, M.; Hauck, B.J.; Keller, L.P.; Boudjouk, P. Hydrosilylation of alkynes catalyzed by platinum on carbon. J. Organomet. Chem. 2002, 645, 1–13. [Google Scholar] [CrossRef]
- Cooray, N.F.; Kakimoto, M.; Imai, Y.; Suzuki, Y. Novel fluorine-containing ferroelectric side chain liquid-crystalline polysiloxanes showing bistable fast switching. Macromolecules 1994, 27, 1592–1596. [Google Scholar] [CrossRef]
- Posner, T. Information on unsaturated compounds II. The addition of mercaptan to unsaturated hydrocarbon. Ber. Dtsch. Chem. Ges. 1905, 38, 646–657. [Google Scholar] [CrossRef]
- Khire, V.S.; Benoit, D.S.W.; Anseth, K.S.; Bowman, C.N. Ultrathin gradient films using thiol-ene polymerizations. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 7027–7039. [Google Scholar] [CrossRef]
- Khire, V.S.; Yi, Y.; Clark, N.A.; Bowman, C.N. Formation and surface modification of nanopatterned thiol-ene substrates using step and flash imprint lithography. Adv. Mater. 2008, 20, 3308–3313. [Google Scholar] [CrossRef]
- Khire, V.S.; Kloxin, C.C.; Clouch, C.C.; Anseth, K.S.; Brownman, C.N. Sythesis, characterization and cleavage of linear polymers attched to silica nanoparticles formed using thiolacrylate conjugate addition reaction. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6896–6906. [Google Scholar] [CrossRef] [PubMed]
- Justynska, J.; Schlaad, H. Modular synthesis of functional block copolymers. Macromol. Rapid Commun. 2004, 25, 1478–1481. [Google Scholar] [CrossRef]
- Justynska, J.; Hordyjewicz, H.; Schlaad, H. Toward a toolbox of functional block copolymers via free-radical addition of mercaptans. Polymer 2005, 46, 12057–12064. [Google Scholar] [CrossRef]
- Justynska, J.; Hordyjewicz, H.; Schlaad, H. New functional diblock copolymers through radical addition of mercaptans. Macromol. Symp. 2006, 240, 41–46. [Google Scholar] [CrossRef]
- Li, C.Y.; Birnkrant, M.J.; Natarajan, L.V.; Tondiglia, V.P.; Lloyd, P.F.; Sutherland, R.L.; Bunning, T.J. Polymer crystallization/melting induced thermal switching in a series of holographically patterned bragg reflectors. Soft Matter 2005, 1, 238–242. [Google Scholar] [CrossRef]
- Birnkrant, M.J.; McWilliams, H.K.; Li, C.Y.; Natarajan, L.V.; Tondiglia, V.P.; Sutherland, R.L.; Lloyd, P.F.; Bunning, T.J. On the structure of holographic polymer-dispersed polyethylene glycol. Polymer 2006, 47, 8147–8154. [Google Scholar] [CrossRef]
- Charles, E.H.; Bowman, C.N. Thiol–ene click chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar]
- Andrew, L. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis. Polym. Chem. 2010, 1, 17–36. [Google Scholar]
- Natarajan, L.V.; Brown, D.P.; Wofford, J.M.; Tondiglia, V.P.; Sutherland, R.L.; Lloyd, P.F.; Bunning, T.J. Holographic polymer dispersed liquid crystal reflection gratings formed by visible light initiated thiol-ene photopolymerization. Polymer 2006, 47, 4411–4420. [Google Scholar] [CrossRef]
- Natarajan, L.V.; Shepherd, C.K.; Brandelik, D.M.; Sutherland, R.L.; Chandra, S.; Tondiglia, V.P.; Tomlin, D.; Bunning, T.J. Switchable holographic polymer-dispersed liquid crystal reflection gratings based on thiol-ene photopolymerization. Chem. Mater. 2003, 15, 2477–2484. [Google Scholar] [CrossRef]
- Senyurt, A.F.; Warren, G.; Whitehead, J.B., Jr.; Hoyle, C.E. Switchable holographic polymer-dispersed liquid crystal reflection gratings based on thiol-ene photopolymerization. Polymer 2006, 47, 2741–2749. [Google Scholar] [CrossRef]
- Yang, H.; Wang, L.X.; Shao, R.F.; Clark, N.A.; COrtega, J.; Etxebarria, J.; Albouy, P.A.; Walba, D.M.; Keller, P. Novel liquid-crystalline mesogens and main-chain chiral smectic thiol-ene polymers based on trifluoromethylphenyl moieties. J. Mater. Chem. 2009, 19, 7208–7215. [Google Scholar] [CrossRef]
- Yang, H.; Richardson, J.M.; Walba, D.M.; Zhu, C.H.; Shao, R.F.; Clark, N.A.; Ortegad, J.; Etxebarriae, J.; Keller, P. Synthesis and physical properties of a main-chain chiral smectic thiol-ene oligomer. Liq. Cryst. 2010, 37, 325–334. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Q.; Lin, B.P.; Fu, G.D.; Zhang, X.Q.; Guo, L.X. Thermo-sensitive electrospun fibers prepared by a sequential thiol-ene click chemistry approach. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 4182–4190. [Google Scholar] [CrossRef]
- Yang, H.; Liu, M.X.; Yao, Y.W.; Tao, P.Y.; Lin, B.P.; Keller, P.; Zhang, X.Q.; Sun, Y.; Guo, L.X. Polysiloxane-based liquid crystalline polymers and elastomers prepared by thiol−ene chemistry. Macromolecules 2013, 46, 3406–3416. [Google Scholar] [CrossRef]
- Yang, H.; Lv, Y.J.; Lin, B.P.; Zhang, X.Q.; Sun, Y.; Guo, L.X. Side-on main-chain liquid cystalline polymers prepared by acyclic diene metathesis polymerization and thiol-ene click step-growth polymerization. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1086–1098. [Google Scholar] [CrossRef]
- Yang, H.; Buguin, A.; Taulemesse, J.M.; Kaneko, K.; Méry, S.; Bergeret, A.; Keller, P. Micron-sized main-chain liquid crystalline elastomer actuators with ultralarge amplitude contractions. J. Am. Chem. Soc. 2009, 131, 15000–15004. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, J.J.; Wang, Z.F.; Guo, L.X.; Keller, P.; Lin, B.P.; Sun, Y.; Zhang, X.Q. Near-infrared-responsive gold nanorod/liquid crystalline elastomer composites prepared by sequential thiol-click chemistry. Chem. Commun. 2015, 51, 12126–12129. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Kamigaito, M.; Sawamoto, M.; Higashimura, T. Polymerization of methyl methacrylate with the carbon tetrachloride/dichlorotris- (triphenylphosphine)ruthenium(II)/methylaluminum bis(2,6-di-tert-butylphenoxide) initiating system: Possibility of living radical polymerization. Macromolecules 1995, 28, 1721–1723. [Google Scholar] [CrossRef]
- Wang, J.S.; Matyjaszewski, K. Controlled/”living” radical polymerization. atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- “The 2011 Wolf Prize in Chemistry”, Wolf Fund. Available online: https://en.wikipedia.org/wiki/Wolf_Prize_in_Chemistry (accessed on 21 February 2011).
- Cowie, J.M.G.; Arrighi, V. Polymers: Chemistry and Physics of Modern Materials, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 82–84. [Google Scholar]
- Matyjaszewski, K.; Tsarevsky, N.V. Nanostructured functional materials prepared by atom transfer radical polymerization. Nat. Chem. 2009, 1, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Beers, K.L.; Gaynor, S.G.; Matyjaszewski, K.; Sheiko, S.S.; Moller, M. The synthesis of densely grafted copolymers by atom transfer radical polymerization. Macromolecules 1998, 31, 9413–9415. [Google Scholar] [CrossRef]
- Hong, S.C.; Pakula, T.; Matyjaszewski, K. Preparation of polysubuten-graft-poly(methy methacrylate) and polyisobutene-graft-polystyrene with different compositions and side chain architectures through atom transfer redical polymerization (ATRP). Macromol. Chem. Phys. 2001, 202, 3392–3402. [Google Scholar] [CrossRef]
- Liu, S.S.; Sen, A. Synthesis of polyethylene-based graft copolymers by atom transfer radical polymerization. Polym. Prepr. Am. Chem. Soc. Div. Polym. Chem. 2000, 41, 1573–1577. [Google Scholar]
- Liu, S.; Sen, A. Syntheses of syndiotactic-polystyrene-graft-poly(methyl methacrylate), syndiotactic-polystyrene-graft-poly(methyl acrylate), and syndiotactic-polystyrene-graft-atactic-polystyrene with defined structures by atom transfer radical polymerization. Macromolecules 2000, 33, 5106–5110. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Miller, P.J.; Fossum, E.; Nakagawa, Y. Synthesis of block, graft and star polymers from inorganic macroinitiators. Appl. Organomet. Chem. 1998, 12, 667–673. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Miller, P.J.; Pyun, J.; Kickelbick, G.; Diamanti, S. Synthesis and characterization of star polymers with varying arm number, length, and composition from organic and hybrid inorganic/organic multifunctional initiators. Macromolecules 1999, 32, 6526–6535. [Google Scholar] [CrossRef]
- Hedrick, J.L.; Trollsås, M.; Hawker, C.J.; Atthuff, B.; Claesson, H.; Heise, A.; Miller, R.D.; Mecerreyes, D.; Jérôme, R.; Dubois, P. Dendrimer-like star block and amphiphilic copolymers by combination of ring opening and atom transfer radical polymerization. Macromolecules 1998, 31, 8691–8705. [Google Scholar] [CrossRef]
- Pan, Q.W.; Gao, L.C.; Chen, X.F.; Fan, X.H.; Zhou, Q.F. Star mesogen-jacketed liquid crystalline polymers with silsesquioxane core: synthesis and characterization. Macromolecules 2007, 40, 4887–4894. [Google Scholar] [CrossRef]
- Frechét, J.M.J.; Henmi, M.; Gitsov, I.; Aoshima, S.; Leduc, M.; Grubbs, R.B. Self-condensing vinyl polymerization: An approach to dendritic materials. Science 1995, 269, 1080–1083. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K.; Gaynor, S.G.; Mueller, A. Preparation of hyperbranched polyacrylates by atom transfer radical polymerization. 2. kinetics and mechanism of chain growth for the self-condensing vinyl polymerization of 2-((2-bromopropionyl)oxy)ethyl acrylate. Macromolecules 1997, 30, 7034–7041. [Google Scholar] [CrossRef]
- Cheng, G.; Simon, P.F.W.; Hartenstein, M.; Muller, A.H.E. Synthesis of hyperbranched poly(tert-butyl acrylate) by self-condensing atom transfer radical polymerization of a macroinimer. Macromol. Rapid Commun. 2000, 21, 846–852. [Google Scholar] [CrossRef]
- Cai, H.H.; Jiang, G.L.; Shen, Z.H.; Fan, X.H. Effects of dendron generation and salt concentration on phase structures of dendritic–linear block copolymers with a semirigid dendron containing PEG tails. Macromolecules 2012, 45, 6176–6184. [Google Scholar] [CrossRef]
- Wang, G.F.; Xiong, Y.; Tang, H.D. Synthesis and characterization of a graft side-chain liquid crystalline Polysiloxane. J. Organomet. Chem. 2015, 775, 50–54. [Google Scholar] [CrossRef]
- Trimmel, G.; Riegle, S.; Fuchs, G.; Slugovc, C. Liquid crystalline polymers by metathesis polymerization. Adv. Polym. Sci. 2005, 176, 43–87. [Google Scholar]
- Pugh, C.; Zhu, P.; Kim, G.; Zheng, J.X.; Rubal, M.J.; Cheng, S.Z.D. Synthesis of laterally attached side-chain liquid-crystalline polynorbornenes with high mesogen density by ring-opening metathesis polymerization. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 4076–4087. [Google Scholar] [CrossRef]
- Moller, M.; Tsukruk, V.I.; Wendling, J.; Wendorff, J.H.; Bengs, H.; Ringsdorf, H. Observation of a nematic phase displayed by a polysiloxane with trinitrofluorenones as side groups. Makrornol. Chem. 1992, 193, 2659–2668. [Google Scholar] [CrossRef]
- Hu, Y.J.; Tang, H.D.; Zhang, X.L. Studies on the syntheses and properties of side-chain liquid-crystallines with chromophore groups. J. Wuhan Univ. Nat. Sci. Ed. 2000, 46, 161–165. [Google Scholar]
- Zhuo, R.X.; Zhang, X.L.; Yuan, J.Y. Synthesis of cyclotetrasiloxane containing paired mesogenic side group. J. Wuhan Univ. Nat. Sci. Ed. 1989, 35, 125–128. [Google Scholar]
- Zhang, X.L.; Zhang, J.Q.; Wang, C.R. Synthesis and phase behaviors of 1,3-bis-(Mesogenes)-1,1,3,3-tetra methyldisiloxanes. Chem. J. Chin. Univ. 1995, 16, 471–476. [Google Scholar]
- Kato, T.; Kihara, H.; Uryu, T.; Fujishima, A.; FrØchet, J.M.J. Molecular self- assembly of liquid crystalline side-chain polymers through intermolecular hydrogen bonding. Polymeric complexes built from a polyacrylate and stilbazoles. Macromolecules 1992, 25, 6836–6841. [Google Scholar] [CrossRef]
- Kato, T.; Kihara, H.; Ujiie, S.; Uryu, T.; FrØchet, J.M.J. Structures and properties of supramolecular liquid-crystalline side-chain polymers built through intermolecular hydrogen bonds. Macromolecules 1996, 29, 8734–8739. [Google Scholar] [CrossRef]
- Kumar, U.; Kato, T.; FrØchet, J.M.J. Use of intermolecular hydrogen bonding for the induction of liquid crystallinity in the side chain of polysiloxanes. J. Am. Chem. Soc. 1992, 114, 6630–6639. [Google Scholar] [CrossRef]
- Kumar, U.; FrØchet, J.M.J.; Kato, T.; Ujiie, S.; Iimura, K. Induction of ferroelectricity in polymeric systems through hydrogen bonding. Angew. Chem. Int. Ed. Engl. 1992, 104, 1545–1547. [Google Scholar] [CrossRef]
- Kato, T.; Frechet, J.M.J. Hydrogen bonding and the self-assembly of supramolecular liquid-crystalline materials. Macromol. Syrnp. 1995, 98, 311–326. [Google Scholar] [CrossRef]
- Ito, T.; Otobe, S.; Oda, T.; Kojima, T.; Ono, S.; Watanabe, M.; Kiyota, Y.; Misawa, T.; Koguchi, S.; Higuchi, M.; et al. Polymerizable ionic liquid crystals comprising polyoxometalate clusters toward inorganic-organic hybrid solid electrolytes. Polymers 2017, 9, 290. [Google Scholar] [CrossRef]
- Lin, C.; Cheng, P.; Blumstein, A. Side chain liquid crystalline ionic polysiloxanes. Mol. Cryst. Liq. Cryst. 1995, 258, 173–183. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Guo, S.M.; Shao, B. Synthesis and characterization of liquid crystalline ionomers with polymethylhydrosiloxane main-chain- and side-chain-containing sulfonic acid groups. J. Appl. Polym. Sci. 1998, 68, 1555–1561. [Google Scholar] [CrossRef]
- Hu, J.S.; Zhang, B.Y.; Feng, Z.L.; Wang, H.G.; Zhou, A.J. Synthesis and characterization of chiral smectic side-chain liquid crystalline polysiloxanes and ionomers containing sulfonic acid groups. J. Appl. Polym. Sci. 2001, 80, 2335–2340. [Google Scholar] [CrossRef]
- Chen, J.H.; Hsiue, G.H. Synthesis and thermal properties of ferroelectric side chain liquid crystalline polysiloxanes based on the phenyl ester mesogen and oligo(oxyethylene) spacers. 1. phenyl benzoate and Biphenyl Benzoate Mesogenic Groups. Macromolecules 1995, 28, 4366–4376. [Google Scholar] [CrossRef]
- Percec, V.; Rodenhouse, R. Liquid crystal polymers containing macroheterocyclic ligands. III. side chain liquid crystalline polymethacrylates containing mesogenic units based on diarylacetylenes and benzo-15-crown-5. J. Polym. Sci. Part A Polym. Chem. 1991, 29, 15–28. [Google Scholar] [CrossRef]
- Rodenhouse, R.; Percec, V. Liquid crystal polymers containing macroheterocyclic ligands, 7 Synthesis and characterization of 4-[(4-methacryloyloxy)-undecyloxy-2-methylphenylethynyl]phenyl (benzo-15-crown-5)-4′-carboxylate. Makromol. Chem. 1991, 192, 1873–1879. [Google Scholar] [CrossRef]
- Hsu, C.S.; Juang, T.M.; Lin, J.H. The influence of lateral substituents on the phase behavior of side-chain liquid crystalline polysiloxanes containing trans-2,5-disubstituted-1,3-dioxane based mesogenic side groups. J. Polym. Res. 1994, 1, 7–15. [Google Scholar] [CrossRef]
- Hsu, C.S.; Tsai, C.H. Effect of a lateral substituent on the mesomorphic properties of ferroelectric side chain liquid crystalline polysiloxanes. Liq. Cryst. 1997, 22, 669–677. [Google Scholar] [CrossRef]
- Nestor, G.; Gray, G.W.; Lacey, D.; Toyne, K.J. Liquid-crystalline polysiloxanes with fluorosubstituted side chains. Liq. Cryst. 1990, 7, 669–681. [Google Scholar] [CrossRef]
- Kossmehl, G.; Pithart, C. Synthesis and characterization of liquid crystalline polysiloxanes with stilbene units. Acta Polym. 1991, 42, 492–496. [Google Scholar] [CrossRef]
- Chen, J.H.; Hsiue, G.H.; Hwang, C.P. Synthesis and thermal properties of ferroelectric side-chain liquid-crystalline polysiloxanes based on naphthyl biphenylcarboxylate mesogenic groups and oligooxyethylene spacers. Chem. Mater. 1997, 9, 51–60. [Google Scholar] [CrossRef]
- Percec, V.; Heck, J. Liquid-crystalline polymers containing mesogenic units based on half-disk and rodlike moieties. 5. side-chain liquid-crystalline poly(methylsi1oxanes) containing hemiphasmidic mesogens based on 4-[[3,4,5-Tris(alkan-l-yloxby)enzoyl]oxy]-4′-[[p-(propan-l-y1oxy)-benzoyl] oxy] biphenyl groups. Macromolecules 1991, 24, 4957–4962. [Google Scholar]
- Lin, C.; Ringsdorf, H.; Ebert, M.; Kleppinger, R.; Wendorff, J.H. Structural variations of liquid crystalline polymers with phasmidic-type mesogens. Liq. Cryst. 1989, 5, 1841–1847. [Google Scholar] [CrossRef]
- Shibaev, V.P.; Bobrovsky, A.Y. Liquid crystalline polymers: Development trends and photocontrollable materials. Russ. Chem. Rev. 2017, 86, 1024–1072. [Google Scholar] [CrossRef]
- Shibaev, V.P.; Bobrovsky, A.Y.; Boiko, N.I. Photoactive liquid crystalline polymer systems with light-controllable structure and optical properties. Prog. Polym. Sci. 2003, 28, 729–836. [Google Scholar] [CrossRef]
- Hsiue, G.H.; Lee, R.H.; Jeng, R.J.; Chang, C.S. Dielectric study of ferroelectric side-chain liquid crystalline polysiloxanes with broad temperature ranges of the chiral smectic C phase 1. structure dependence of dielectric relaxation. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 555–563. [Google Scholar] [CrossRef]
- Hsu, C.S.; Leu, Y. Synthesis of liquid crystalline polysiloxanes containing naphthalene-based mesogens and chiral side chains. J. Mol. Cryst. Liy. Cryst. 1997, 300, 83–95. [Google Scholar] [CrossRef]
- Yao, W.H.; Gao, Y.Z.; Zhang, C.H.; Li, C.Y.; Li, F.S.; Yang, Z.; Zhang, L.Y. A series of novel side chain liquid crystalline polysiloxanes containing cyano- and cholesterol-terminated substituents: Where will the structure-dependence of terminal behavior of the side chain reappear? J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1765–1772. [Google Scholar] [CrossRef]
- Bubnov, A.; Kašpar, M.; Hamplová, V.; Glogarová, M.; Samaritani, S.; Galli, G.; Andersson, G.; Komitov, L. Polar liquid crystalline monomers with two or three lactate groups for the preparation of side chain polysiloxanes. Liq. Cryst. 2006, 33, 559–566. [Google Scholar] [CrossRef]
- Bubnov, A.; Novotná, V.; Pociecha, D.; Kašpar, M.; Hamplová, V.; Galli, G.; Glogarova, M. A liquid-crystalline co-polysiloxane with asymmetric bent side chains. Macrom. Chem. Phys. 2011, 212, 191–197. [Google Scholar] [CrossRef]
- Tóth-Katona, T.; Cigl, M.; Fodor-Csorba, K.; Hamplová, V.; Jánossy, I.; Kašpar, M.; Vojtylová, T.; Hampl, F.; Bubnov, A. Functional photochromic methylhydrosiloxane-based side-chain liquid-crystalline polymers. Macrom. Chem. Phys. 2014, 215, 742–752. [Google Scholar] [CrossRef]
- Petrova, I.; Gaj, A.; Pochiecha, D.; Shcherbina, M.; Makarova, N.N.; Bubnov, A. Design and self-assembling behaviour of comb-like stereoregular cyclolinear methylsiloxane copolymers with chiral lactate groups. Liq. Cryst. 2018. [Google Scholar] [CrossRef]
- Meng, F.B.; Sun, Y.H.; Gao, Y.M.; Song, X.G.; Zhang, B.Y. Synthesis and characterization of chiral liquid-crystalline polysiloxanes containing fluorinated units and sulfonic acid groups. Polym. Adv. Technol. 2008, 19, 1242–1249. [Google Scholar] [CrossRef]
- Tang, X.Q.; Du, X.Y.; Bai, L.; Zhang, L.; Meng, F.B. Liquid crystalline polyaniline and phthalocyanine-based polysiloxanes bearing lateral fluoro-substituted benzoic acid groups. Liq. Cryst. 2017, 44, 1259–1268. [Google Scholar] [CrossRef]
- Simon, R.; Coles, H.J. Investigations of comblike polysiloxanes. I. the influence of spacer length on dielectric relaxation studies of aligned samples. J. Polym. Sci. Part B Polym. Phys. 1989, 27, 1823–1836. [Google Scholar] [CrossRef]
- Parneix, J.P.; Njeumo, R.; Legrand, C.; Le Barny, P.; Dubois, J.C. Dielectric relaxation and molecular motion in comb-shaped liquid crystal polymers. Liq. Cryst. 1987, 2, 167–181. [Google Scholar] [CrossRef]
- Percec, V.; Tomazos, D. Synthesis and characterization of liquid crystalline polymethacrylates, polyacrylates, and polysiloxanes containing 4-hydroxy-4′-methoxy-cu-methylstilben based mesogenic groups. Macromolecules 1989, 22, 2062–2069. [Google Scholar] [CrossRef]
- Percec, V.; Rodenhouse, R. Liquid crystal polymers containing macroheterocyclic ligands. 2. side chain liquid crystal polysiloxanes and polymethacrylates containing 4-(w-Alkan-l-yloxy)-4′-(4′-carboxybenzo-15-crown-5) biphenyl side groups. Macromolecules 1989, 22, 4408–4412. [Google Scholar] [CrossRef]
- Percec, V.; Pugh, C. Side Chain Liquid Crystal Polymers; McArdle, C.B., Ed.; Blackie and Son: Glasgow, UK, 1989; p. 30. [Google Scholar]
- Percec, V.; Hahn, B. Liquid crystalline polymers containing heterocycloalkanediyl groups as mesogens. 7. molecular weight and composition effects on the phase transitions of poly(methylsi1oxane)s and poly(methylsi1oxane-co-dimethylsi1oxane)s containing 2-[4-(2(S)-Methyl-l-butoxy)phenyl]-5-(11-undecanyl)-1,3,2-dioxaborinane side groups. Macromolecules 1989, 22, 1588–1599. [Google Scholar]
- Percec, V.; Tomazos, D.; Pugh, C. Influence of molecular weight on the thermotropic mesophases of poly[6-[4-(4-methoxy-beta-methylstyryl)phenoxy]hexyl methacrylate]. Macromolecules 1989, 22, 3259–3267. [Google Scholar] [CrossRef]
- Hsu, C.S.; Lin, J.H.; Chou, L.R. Synthesis and characterization of ferroelectric liquid crystalline polysiloxanes and polymethacrylates containing [(S)-2-methyl-1-butoxylphenyl 4-(alkyloxy) biphenyL4′-carboxylate side groups. Macromolecules 1992, 25, 7126–7134. [Google Scholar] [CrossRef]
- Lewthwaite, R.A.; Goodby, J.W.; Toyne, K.J. Effect of backbone flexibility and the position of the lateral group on the mesophase stability of laterally attached, side-chain polymers. J. Mater. Chem. 1993, 3, 241–245. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Chen, S.; Zhao, H.; Shen, Z.H.; Chen, X.F.; Fan, X.H.; Zhou, Q.F. Synthesis and properties of a series of mesogen-jacketed liquid crystalline polymers with polysiloxane backbones. Macromolecules 2010, 43, 6024–6032. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, H.L.; Huang, Y.; Chen, E.Q.; Lu, Y.F.; Shen, D.Y.; Wan, X.H.; Shen, Z.H.; Cheng, S.Z.D.; Zhou, Q.F. Molecular weight dependence of phase structures and transitions of mesogen-jacketed liquid crystalline polymers based on 2-vinylterephthalic acids. Macromolecules 2004, 37, 7188–7196. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Fan, X.H.; Wan, X.H.; Chen, X.F.; Y, Y.; Wang, L.S.; Xia, D.; Zhou, Q.F. Unusual phase behavior of a mesogen-jacketed liquid crystalline polymer synthesized by atom transfer radical polymerization. Macromolecules 2006, 39, 948–956. [Google Scholar] [CrossRef]
- Chai, C.P.; Zhu, X.Q.; Wang, P.; Ren, M.Q.; Chen, X.F.; Xu, Y.D.; Fan, X.H.; Ye, C.; Chen, E.Q.; Zhou, Q.F. Synthesis and phase structures of mesogen-jacketed liquid crystalline polymers containing 1,3,4-oxadiazole based side chains. Macromolecules 2007, 40, 9361–9370. [Google Scholar] [CrossRef]
- Chen, S.; Gao, L.C.; Zhao, X.D.; Chen, X.F.; Fan, X.H.; Xie, P.Y.; Zhou, Q.F. Synthesis and properties of mesogen-jacketed liquid crystalline polymers with asymmetry mesogenic core. Macromolecules 2007, 40, 5718–5725. [Google Scholar] [CrossRef]
- Zhang, Q.K.; Tian, H.J.; Li, C.F.; Zhu, Y.F.; Liang, Y.R.; Shen, Z.H.; Fan, X.H. Synthesis and phase behavior of a new 2-vinylbiphenyl-based mesogen-jacketed liquid crystalline polymer with a high glass transition temperature and low threshold molecular weight. Polym. Chem. 2014, 5, 4526–4533. [Google Scholar] [CrossRef]
- Kossmehl, G.; Schulz, M.; Vieth, H.M.; VanDerEst, A. Liquid crystalline side chain polymers with fluorene as mesogenic group. Mol. Cryst. Liq. Cryst. 1990, 193, 171–175. [Google Scholar] [CrossRef]
- KoJmehl, G.; Schulz, M. Liquid-crystalline polysiloxanes with fluorene units, and related monomeric compounds. Makmrnol. Chem. 1990, 191, 3107–3113. [Google Scholar]
- Hahn, B.; Percec, V. Liquid-crystalline polymers containing heterocycloalkane mesogenic groups. 5. synthesis of biphasic chiral smectic polysiloxanes containing 2,5-disubstituted-l,3-dioxane and 2,5-disubstituted-l,3,2-dioxaborinane-Based mesogenic groups. Macromolecules 1987, 20, 2961–2968. [Google Scholar] [CrossRef]
- Percec, V.; Hahn, B.; Ebert, M.; Wendorff, J.H. Liquid-crystalline polymers containing heterocycloalkanediyl groups as mesogens. 8. morphological evidence for microphase separation in poly(methylsi1oxane-cedimethylsi1oxane)s containing 2-[4-(2(S)-methyl-1-butoxy)-phenyl]-5-(11-undecany1)-1,3,2-dioxaborinane side groups. Macromolecules 1990, 23, 2092–2095. [Google Scholar]
- Percec, V.; Tomazos, D. Can the rigidity of a side-chain liquid-crystalline polymer backbone influence the mechanism of distortion of its random-coil conformation? Polymer 1990, 31, 1658–1662. [Google Scholar] [CrossRef]
- Engel, M.; Hisgen, B.; Keller, R.; Kreuder, W.; Reck, B.; Ringsdorf, H.; Schmidt, H.W.; Tschirner, P. Synthesis, structure and properties of liquid crystalline polymers. Pure Appl. Chem. 1985, 57, 1009–1014. [Google Scholar] [CrossRef]
- Diele, S.; Oekiner, S.; Kuschel, F. X-ray investigations of liquid crystalline homo- and copolysiloxanes with paired mesogens. Makromol. Chem. 1987, 188, 993–2000. [Google Scholar] [CrossRef]
- Yonetake, K.; Nakagomi, M.; Masuko, T. Growth of liquid crystal in poly[[6-(4′-cyanobiphenyl-4-oxy)hexyl]-methylsiloxane]. Polym. J. 1995, 27, 1157–1159. [Google Scholar] [CrossRef]
- Yonetake, K.; Nakagomi, M.; Ueda, M.; Masuko, T. Effects of the degree of substitution on the properties and structures of side chain liquid crystalline polysiloxanes. Polym. J. 1997, 29, 240–244. [Google Scholar] [CrossRef]
- Yao, W.H.; Gao, Y.Z.; Yuan, X.; He, B.F.; Yu, H.F.; Zhang, L.Y.; Shen, Z.H.; He, W.L.; Yang, Z.; Yang, H.; et al. Synthesis and self-assembly behaviours of side-chain smectic thiol–ene polymers based on the polysiloxane backbone. J. Mater. Chem. C 2016, 4, 1425–1440. [Google Scholar] [CrossRef]
- Yao, W.H.; Gao, Y.Z.; Li, F.S.; Zhang, L.Y.; Yang, Z.; Yang, H. Influence of shorter backbone and cholesteric monomer percentage on the phase structures and thermal-optical properties of linear siloxane tetramers containing cholesterol and benzene methyl ether groups. RSC Adv. 2016, 6, 87502–87512. [Google Scholar] [CrossRef]
- Sledzifiska, I.; Soltysiak, J.; Staficzyk, W. Liquid crystal oligo(cyclosiloxanes). J. Inorg. Organomet. Polym. 1994, 4, 199–204. [Google Scholar] [CrossRef]
- Richards, D.C.; Hawthorne, W.D.; Hill, J.D.; White, M.S.; Lacey, D.; Semlyen, J.A.; Gray, G.W.; Kendrick, T.C. Liquid crystalline cyclic polymers. J. Chem. Soc. Chem. Commun. 1990, 2, 95–97. [Google Scholar] [CrossRef]
- Gresham, K.D.; Mchugh, C.M.; Bunning, T.J.; Crane, R.L.; Klei, H.E.; Samulskl, E.T. Phase behavior of cyclic siloxane-based liquid crystalline compounds. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 2039–2047. [Google Scholar] [CrossRef]
- He, X.Z.; Zhang, B.Y.; Hu, J.S.; Yao, D.S.; Hu, Z.F. Side-chain copolymers containing smectic monomer and chiral reagent—Synthesis and characterization. J. Appl. Polym. Sci. 2008, 108, 1265–1272. [Google Scholar] [CrossRef]
- Medeiros, D.R.; Hale, M.A.; Hung, R.J.P.; Leitko, J.K.; Willson, C.G. Ferroelectric cyclic oligosiloxane liquid crystals. J. Mater. Chem. 1999, 9, 1453–1460. [Google Scholar] [CrossRef]
- Pan, Q.W.; Chen, X.F.; Fan, X.H.; Shen, Z.H.; Zhou, Q.F. Organic–inorganic hybrid bent-core liquid crystals with cubic silsesquioxane cores. J. Mater. Chem. 2008, 18, 3481–3488. [Google Scholar] [CrossRef]
- Chen, S.; Tong, X.Q.; He, H.W.; Ma, M.; Shi, Y.Q.; Wang, X. Body temperature controlled optical and thermal information storage light scattering display with fluorescence effect and high mechanical strength. ACS Appl. Mater. Interfaces 2017, 9, 11924–11932. [Google Scholar] [CrossRef] [PubMed]
- Chojnowski, J.; Cypryk, M.; Fortuniak, W.; Ścibiorek, M.; Rózga-Wijas, K. Synthesis of branched polysiloxanes with controlled branching and functionalization by anionic ring-opening polymerization. Macromolecules 2003, 36, 3890–3897. [Google Scholar] [CrossRef]
- Stochmal, E.; Strzezikb, J.; Krowiak, A. Physicochemical and catalytic properties of polysiloxane network—Pt systems. RSC Adv. 2017, 7, 26342–26360. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Cao, H.; Zhao, D.Y.; Hu, W.; He, W.L.; Yuan, X.T.; Xiao, J.M.; Zhang, H.Q.; Yang, H. Liquid crystalline and thermo-optical properties of cyclic siloxane tetramers containing cholestryl-4-allyloxy-benzoate and biphenyl-4-yl 4-allyloxybenzoate. Liq. Cryst. 2011, 38, 9–15. [Google Scholar] [CrossRef]
- Finkelmann, H.; Ringsdorf, H.; Siol, W.; Wendorff, J.H. Enantiotropic (liquid crystalline) polymers: Synthesis and models. In Mesomorphic Order in Polymers and Polymerization in Liquid Crystalline Media; Blumstein, A., Ed.; American Chemical Society: Washington, DC, USA, 1978; Volume 74, pp. 22–32. [Google Scholar]
- Shibaev, V.P.; Plate, N.A.; Friedson, Y.S. Thermotropic liquid crystalline polymers. I. Cholesterol-containing polymers and copolymers. J. Polym. Sci. Part A Polym. Chem. 1979, 17, 1655–1670. [Google Scholar] [CrossRef]
- Shibaev, V.P.; Moiseenko, V.M.; Freidzon, Y.S.; Platé, N.A. Specific features of liquid-crystalline comb-like polymers with mesogenic groups. Eur. Polym. J. 1980, 16, 277–281. [Google Scholar] [CrossRef]
- Hsu, C.S.; Chu, P.H.; Chang, H.L.; Hsieh, T.H. Effect of lateral substituents on the mesomorphic properties of side-chain liquid crystalline polysiloxanes containing 4-[(S)-2-methyl-1-butoxy]phenyl 4-(Alkenyloxy)benzoate side groups. J. Polym. Sci. Part A Polym. Chem. 1997, 35, 2793–2800. [Google Scholar] [CrossRef]
- Lecommandoux, S.; Achard, M.F.; Hardouin, F. Side-on fixed polysiloxanes and ‘diluted’ copolysiloxanes with nematic and smectic C phases. Liq. Cryst. 1998, 25, 85–94. [Google Scholar] [CrossRef]
- Hsiue, G.H.; Hsieh, P.J.; Wu, S.L.; Hsu, C.S. Synthesis and X-ray diffraction of ferroelectric liquid crystalline polysiloxanes containing 4′-(2-chloro-3-methylpentanoyloxy)-4-alkanyloxybiphenyl side groups. Polym. Bull. 1994, 33, 159–166. [Google Scholar] [CrossRef]
- Sutherland, H.H.; Adib, Z.A.; Gray, G.W.; Lacey, D.; Nestor, G.; Toyne, K.J. A structural investigation of some terminally cyano-substituted side chain liquid-crystalline polysiloxanes. Liq. Cryst. 1988, 3, 1293–1300. [Google Scholar] [CrossRef]
- Percec, V.; Tomazos, D. Transformation of a monotropic mesophase into an enantiotropic mesophase by copolymerization of the parent polymers’ monomer pair containing constitutional isomeric mesogenic side groups. Macromolecules 1989, 22, 1512–1514. [Google Scholar] [CrossRef]
- Hsu, C.S.; Rodriguez-Parada, J.M.; Percec, V. Liquid crystalline polymers containing heterocycloalkanediyl groups as mesogens, 1 Liquid crystalline polymethacrylates and polyacrylates containing 1,3-dioxane-2,5-diyl groups as mesogens in the side chain. Makromol. Chem. 1987, 188, 1017–1031. [Google Scholar] [CrossRef]
- Hsu, C.S.; Rodriguez-Parada, J.M.; Percec, V. Liquid crystalline polymers containing heterocycloalkane mesogens. 2. side-chain liquid crystalline polysiloxanes containing 2,5-disubstituted-l,3-dioxanMe mesogen. J. Polym. Sci. Part A Polym. Chem. 1987, 25, 2425–2445. [Google Scholar] [CrossRef]
- HSU, C.S.; Lu, Y.H. Synthesis and characterization of side-chain liquid crystalline polysiloxanes containing 4-alkanyloxyphenyl trans-4-alkylcyclohexanoate side groups. J. Polym. Sci. Part A Polym. Chem. 1991, 29, 977–986. [Google Scholar] [CrossRef]
- White, M.S.; Clarson, S.J.; Semlyen, J.A.; Horwood, E. Siloxane Polymers; PTR Prentice Hall: Englewoods Cliffs, NJ, USA, 1993. [Google Scholar]
- Day, G.M.; Jackson, W.R.; Jacobs, H.A.; Kim, J.H.; Simon, G.P.; Sarna, R.; Watson, K.G. Preparation of thermotrophic liquid crystalline polymers involving a novel spacer unit based on vinylacetic acid. Polym. Bull. 1992, 29, 21–25. [Google Scholar] [CrossRef]
- Fabre, B. Simonet, Electroactive polymers containing crown ether or polyether ligands as cation-responsive materials. J. Coord. Chem. Rev. 1998, 178, 1211–1250. [Google Scholar] [CrossRef]
- Funahashi, M.; Shimura, H.; Yoshio, M.; Kato, T. Functional liquid-crystalline polymers for ionic and electronic conduction. In Liquid Crystalline Functional Assemblies and Their Supramolecular Structures; Springer: Berlin/Heidelberg, Germany, 2008; Volume 128, pp. 151–179. [Google Scholar]
- Liang, X.C.; Chen, X.F.; Li, C.Y.; Shen, Z.H.; Fan, X.H.; Zhou, Q.F. Mesogen-jacketed liquid crystalline polymers substituted with oligo(oxyethylene) as peripheral chain. Polymer 2010, 51, 3693–3705. [Google Scholar] [CrossRef]
- Ping, J.; Pan, H.B.; Hou, P.P.; Zhang, M.Y.; Wang, X.; Wang, C.; Chen, J.T.; Wu, D.C.; Shen, Z.H.; Fan, X.H. Solid polymer electrolytes with excellent high-temperature properties based on brush block copolymers having rigid side chains. ACS Appl. Mater. Interfaces 2017, 9, 6130–6137. [Google Scholar] [CrossRef] [PubMed]
- Percec, V.; Tomazos, D. Mesomorphic polyelectrolytes based on side-chain liquid-crystalline polymers containing end-on fixed mesogens and oligooxyethylenic spacers. J. Mater. Chem. 1993, 3, 633–642. [Google Scholar] [CrossRef]
- Percec, V.; Tomazos, D. Mesomorphic Polyelectrolytes based on side-chain liquid-crystalline polymers containing side-on fixed mesogens and oligooxyethylenic spacers. J. Mater. Chem. 1993, 3, 643–650. [Google Scholar] [CrossRef]
- Hardouin, F.; Mery, S.; Achard, M.F.; Noirez, L.; Keller, P. Evidence for a jacketed nematic polymer. J. Phys. II 1991, 1, 511–520. [Google Scholar] [CrossRef]
- Milano, J.C.; Robert, J.M.; Vernet, J.L.; Gallot, B. The thioether spacer in liquid crystalline polysiloxanes with cyano- and nitrobiphenyl mesogens. Macromol. Chem. Phys. 1999, 200, 1580–1586. [Google Scholar] [CrossRef]
- Gilles, P.P.; Milano, J.C.; Vernet, J.L. Synthesis and dielectric study of new liquid-crystalline polysiloxanes presenting a thioether spacer. Macromol. Chem. Phys. 2003, 204, 2222–2232. [Google Scholar] [CrossRef]
- Fischer, H.; Poser, S.; Arnold, M.; Frank, W. On the influence of the morphological structure on the liquid crystalline behavior of liquid crystalline side chain block copolymers. Macromolecules 1994, 27, 7133–7138. [Google Scholar] [CrossRef]
- Wong, G.C.L.; Commandeur, J.; Fischer, H.; de Jeu, W.H. Orientational wetting in hybrid liquid crystalline block copolymers. Phys. Rev. Lett. 1996, 77, 5221–5224. [Google Scholar] [CrossRef] [PubMed]





























| Polymer | Spacer Length | Phase Transitions (°C) | Activation Energies (kJ mol−1) | |||
| At Low Temperatures in LC Phase | At 0.98Tcl | Isotropic Phase | ||||
| polysiloxane | 5 | G 19 N 65 I | 202 | 172 | 142 | |
| polysiloxane | 6 | G 7 N 48 I | 238 | 190 | 144 | |
| polysiloxane | 8 | G 9 S 102 I | 146 | 95 | –– | |
| Polymer | Spacer Length | Phase Transitions (°C) | Activation Energies (kJ mol−1) | |||
| NRe | SAd | N | I | |||
| polyacrylate | 6 | G 32 NRe 80 SAd 124.5 N 132 I | 241 | 126 | 193 | 83 |
| Polymethacrylates (4′-n-PMA) | GPC | Phase Transitions(°C) and Corresponding Enthalpy Changes (kcal/mru−1) | ||
|---|---|---|---|---|
| 10−3n | w/n | Heating | Cooling | |
| 4′-11-PMA | 23.4 | 2.0 | G 12 S 116 (1.25) I | I 105 (1.13) S 4 G |
| 4′-11-PMA a | 23.4 | 2.0 | G 15 K 55 (0.55) K 83 (0.39) S 119 (0.99) I | I 105 (1.13) S 4 G |
| 4′-8-PMA | 24.1 | 2.0 | G 18 S 55 (0.29) N 112 (0.43) I | I 108 (0.43) N 53 (0.21) S 15 G |
| 4′-6-PMA | 24.7 | 2.5 | G 32 N 101 (0.26) I | I 97 (0.21) N 24 G |
| 4′-3-PMA | 43.6 | 2.5 | G 55 S 85 (0.05) N 115 (0.17) I | I 110 (0.16) N 75 (0.03) S 42 G |
| Polyacrylates (4′-n-PAC) | 10−3n | w/n | Heating | Cooling |
| 4′-11-PAC | 15.9 | 2.0 | G 12 K 107 (2.86) S 115 c (0.80) I | I 107 (0.72) S 37 (1.08) K 3 G |
| 4′-8-PAC | 12.2 | 1.8 | G 11 S 66 (0.03) N 83 (0.39) K 99 (0.55) N 104 (0.07) c | I 97 (0.23) N 63 (0.03) S 6 G |
| 4′-6-PAC | 14.0 | 2.0 | G 18 N 101 (0.11) I | I 97 (0.10) N 14 G |
| 4′-6-PAC b | 14.0 | 2.0 | K 55 (1.09) N 103 (0.06) I | I 97 (0.10) N 14 G |
| 4′-3-PAC | 16.3 | 1.9 | G 38 K 59 (0.02) N 81 (0.03) I | I 76 (0.10) N 14 G |
| Polysiloxanes (4′-n-PS) | 10−3n | w/n | Heating | Cooling |
| 4′-11-PS | 13.4 | 1.8 | K 127 (3.81) S 138 (1.20) d I | I 131 (0.76) S 90 (3.75) K |
| 4′-8-PS | 16.4 | 2.0 | K 128 (4.46) I | I 117 (0.22) N 89 (3. 54) K |
| 4′-6-PS | 19.6 | 1.9 | G 10 K 124 (3.95) I | I 103 (0.09) N 81 (3.09) K |
| 4′-3-PS | 14.3 | 2.0 | G 22 K 84 (1.43) N 110 (0.12) I | I 107 (0.10) N 65 (1.33) K |
| Compounds | GPC | Phase Transitions(°C) and Corresponding Enthalpy Changes (kcal/mru−1) | ||
|---|---|---|---|---|
| n | w/n | Heating | Cooling | |
| PSC-11 | 5700 | 1.3 | G 50 S1 106 (0.60) S2 168 (0.80) N 216 (0.18) I | I 208 (0.18) N 159 (0.86) S2 88 (0.64) S1 |
| PMC-11 | 14,600 | 2.8 | G 50 S 129 (0.54) N 166 (0.37) I | I 160 (0.39) N 123 (0.57) S 49 G |
| Spacer Length (n) | Polysiloxanes | Polymethacrylates |
|---|---|---|
| Phase Transitions/°C (Enthalpies/kcal mru−1) Heating/Cooling | Phase Transitions/°C (Enthalpies/kcal mru−1) Heating/Cooling | |
| 3 | G 25 SA 117 (1.82) I | K 81 (6.65) SB 86 (0.58) SA 147 (1.07) I |
| I 99 (1.80) SA | I 145 (1.01) SA 85 (0.55) SB 34 (4.08) K | |
| 4 | G 18 SA 84 (0.78) I | |
| I 80 (0.42) SA | ||
| 5 | G 22 SB 99 (0.89) SA 212 (0.86) I | |
| I 204 (0.68) SA 89 (0.82) SB | ||
| 6 | G 16 SB120 (1.73) SC* 166 (–) a SA 244 (1.24) I | K 101 (1.97) SB 123 (0.58) SA 149 (0.82) I |
| I 236 (0.98) SA 164 (–) a SC* 109 (1.47) SB | I 142 (0.97) SA 119 (0.53) SB 96 (2.00) K | |
| 11 | G 20 SB109 (0.78) SC* 143 (–) a SA 218 (0.86) I | K 88 (1.45) SC* 125 (–) a SA 169 (0.66) I |
| I 210 (0. 89) SA 142 (–) a SC* 103 (0. 74) SB | I 165 (0.61) SA 120 (–) a SC* 81 (0.63) SB 65 (0.07) K |
| Polymers | Transition Temperatures/°C (Enthalpies/J g−1) | Molecular Weight (n) | Polydispersity (γ) |
|---|---|---|---|
| PS | G 22.6 Ch 123.8 (0.27) I | 16,300 | 1.30 |
| PA | G 30.1 Ch 110.8 (0.36) I | 9250 | 1.35 |
| PMA | G 37.8 Ch 101.5 (0.18) I | 18,900 | 1.66 |
| Conventional MJLCPs. | Chemical Structures | Mesogen-Jacketed Polyailoxanes | Chemical Structures |
|---|---|---|---|
| PMPCS |  | Si-MPCS |  |
| PBPCS |  | Si-BPCS |  |
| OXD-C12p |  | Si-OXD-C12p |  |
| 4C2Vp |  | Si-4C2Vp |  |
| 12C2Vp |  | Si-12C2Vp |  |
| Polymers | Molecular Weight (n) | Tg (°C) | LC Phase | d-Spacing |
|---|---|---|---|---|
| PMPCS | 15,900 | 118 | ΦN | 1.59 |
| PBPCS | 12,700 | 98 | ΦHN | 1.87 |
| OXD-C12p | 142,000 | 167 | SA | 3.44 |
| 4C2Vp | 83,000 | 105 | SA | 3.00 |
| 12C2Vp | 80,000 | 86 | SC | 4.20 |
| Si-MPCS | 16,600 | 111 | ΦN | 1.58 |
| Si-BPCS | 19,500 | 92 | ΦHN | 1.86 |
| Si-OXD-C12p | 29,200 | 156 | SC | 3.53 |
| Si-4C2Vp | 22,200 | 96 | No | No |
| Si-12C2Vp | 30,000 | 76 | SA | 4.24 |
| Polymers | Transition Temperatures/°C Heating/Cooling |
|---|---|
| 1a | G 130–140 S 170–191 I |
| I 180–157 SA 142–138 SE 135–116 G | |
| 1b | G 137–143 S 174–194 I |
| I 192–166 SA 139–130 G | |
| 2a | G 141–153 SE 153–158 SA 168–186 I |
| I 177–155 SA 154–147 SE 143–133 G | |
| 2b | G 97–133 SE 152–163 SA 168–194 I |
| I 182–163 SA 153–141 SE 121–80 G |
| DP (NMR) | w/ | Phase Transitions (°C) and Corresponding Enthalpy Changes (kcal/mru−1) | |
|---|---|---|---|
| Heating | Cooling | ||
| 7 | 1.17 | G -5 S 10 (0.12) S 72 (1.90) I | I 64 (1.90) S 6 (0.10) S |
| 15 | 1.14 | G 9 S 25 (0.16) S 93 (2.10) I | I 85 (2.00) S 20 (0.15) S |
| 22 | 1.17 | G 10 S 24 (0.15) S 98 (2.00) I | I 87 (2.00) S 16 (0.13) S |
| 27 | 1.45 | G 10 S 21 (0.11) S 95 (1.80) I | I 85 (1.80) S 13 (0.10) S |
| 43 | 1.60 | G 10 S 21 (0.10) S 99 (1.90) I | I 90 (1.80) S 15 (0.09) S |
| 54 | 1.63 | G 10 S 21 (0.08) S 98 (1.60) I | I 87 (1.50) S 16 (0.09) S |
| 56 | 1.81 | G 10 S 21 (0.13) S 98 (1.80) I | I 90 (1.70) S 14 (0.10) S |
| 92 | 1.72 | G 10 S 24 (0.11) S 104 (1.80) I | I 94 (1.70) S 17 (0.12) S |
| Structural Units Containing Side Groups | Phase Transitions (°C) and Corresponding Enthalpy Changes (kcal/mru−1) | ||||
|---|---|---|---|---|---|
| mol % a | wt % a | w | w/n | Heating | Cooling |
| 23.3 | 65 | 26,800 | 2.95 | G1 −98 G2 −8 S −7 (0.16) S 37 (1.30) I | I 31 (1.30) S −14 (0.12) S |
| 38.0 | 79 | 21,400 | 2.02 | G1 −81 G2 −8 S −6 (0.22) S 47 (1.30) I | I 41 (1.20) S −14 (0.21) S |
| 56.0 | 89 | 25,900 | 2.17 | G1 −66 G2 5 S 9 (0.23) S 75 (1.80) I | I 67 (1.80) S 2 (0.19) S |
| 82.0 | 97 | 22,800 | 1.49 | G 8 S 18 (0.24) S 92 (2.00) I | I 83 (2.00) S 13 (0.26) S |
| 100.0 | 100 | 25,500 | 2.10 | G 8 S 20 (0.13) S 90 (1.80) I | I 81 (1.70) S 13 (0.12) S |
| Linear Polysiloxane Tetramers | Phase Transitions a (°C) | PMMS-Based Polysiloxane Copolymers | Phase Transitions a (°C) |
|---|---|---|---|
| Heating | Heating | ||
| Cooling | Cooling | ||
| PS4-Xchol-1.00 | G55.63SmA234.94I | PMMS-Xchol-1.00 | G35.7SmAd139.9SmAs204.8I |
| I234.13SmA56.74G | I197.9SmAs136.65SmAd34.6G | ||
| PS4-Xchol-0.90 | G49.94SmA231.35I | PMMS-Xchol-0.90 | G35.6SmAd’59.6SmAd152.2SmAs189.9I |
| I230.32SmA56.51G | I185.5SmAs154.9SmAd59.0SmAd’34.1G | ||
| PS4-Xchol-0.80 | G45.10SmA206.39I | PMMS-Xchol-0.80 | G31.9SmAd145.1SmAs174.4I |
| I206.98SmA43.35G | I171.8SmAs144.6SmAd27.8G | ||
| PS4-Xchol-0.70 | G50.75SmA187.32Ch213.48I | PMMS-Xchol-0.70 | G30.3SmAd164.4I |
| I211.63Ch187.25SmA45.37G | I161.5SmAd28.9G | ||
| PS4-Xchol-0.60 | G42.62SmA141.65Ch185.21I | PMMS-Xchol-0.60 | G22.8SmAd132.4I |
| I183.69Ch141.39SmA39.57G | I129.8SmAd22.6G | ||
| PS4-Xchol-0.50 | G40.51Ch178.89I | PMMS-Xchol-0.50 | G21.9SmAd114.7I |
| I177.38Ch39.01G | I117.7SmAd20.1G | ||
| PS4-Xchol-0.40 | G34.48Ch156.72I | PMMS-Xchol-0.40 | G20.2SmAs110.7I |
| I155.20Ch32.59G | I108.3SmAs18.6G | ||
| PS4-Xchol-0.30 | G24.00Ch106.50I | PMMS-Xchol-0.30 | G16.0SmE55.9SmAs102.5I |
| I105.40Ch23.00G | I99.83SmAs5I.3SmE11.8G | ||
| PS4-Xchol-0.20 | G18.39Ch82.50I | PMMS-Xchol-0.20 | G7.9SmE70.6SmAs80.0I |
| I81.42Ch15.99G | I72.5SmAs63.4SmE9.6G | ||
| PS4-Xchol-0.10 | G11.41Ch64.06I | PMMS-Xchol-0.10 | G11.4SmE’46.8SmE71.9SmAs90.0I |
| I62.53Ch8.98G | I76.5SmAs71.9SmE57.4SmE’8.91G | ||
| PS4-Xchol-0.00 | G6.97N32.09I | PMMS-Xchol-0.00 | G17.1SmE83.4SmAs95.5I |
| I30.04N4.80G | I80.5SmAs68.6SmE15.2G |
| x | d (nm) | Phase Transitions (°C) |
|---|---|---|
| 4 | 3.13 | K 50 S 118 I |
| 5 | 3.16 | K 44 S 116 I |
| 6 | 3.15 | K 43 S 124 I |
| 7 | 3.26 | K 37 S 119 I |
| 8 | 3.27 | K 38 S 118 I |
| 5R | 4R | ||
|---|---|---|---|
| Compound a | Thermal Transitions (°C) | Compound a | Thermal Transitions (°C) |
| 5R1 | G 66 N 135 I | 4R1 | G 110 Viscous melt |
| 5R2 | K1 115 K2 130 N 175 I | 4R2 | K 180 N 200 I |
| 5R3 | K 148 N 175 I I 172 N 140 SC 120 K | 4R3 | K 188 I I 178 N 148 K |
| 5R4 | K1 80 K2 103 SA 172 I | 4R3 | K 116 SA 169 I |
| 5R5 | G 76 SA 247 I | 4R5 | G 100 SA 232 I |
| 5R6 | G 61 SA 228 N* 246 I | 4R6 | G 76 SA 270 N* 277 I |
| 5R7 | G 50 SA 255 I | 4R7 | G 58 SA 263 I |
| 5R8 | G 49 S*C 100 SA 252 I | 4R8 | G 55 S*C 100 SA 246 I |
| 5R1/5 | G 80 N* 204 I | 4R1/5 | G 75 N* 204 I |
| 5R2/6 | G 50 N* 220 I | 4R2/6 | G 57 N* 237 I |
| 5R3/7 | G 41 S*C 130 SA 198 N* 228 I | 4R3/7 | G 35 N* 220 I |
| 5R4/8 | G 26 SA 199 N* 209 I | 4R4/8 | G 30 SA 204 N* 210 I |
| Linear Polysiloxane Tetramers | Phase Transitions a (°C) | Cyclic Polysiloxane Tetramers | Phase Transitions a (°C) |
|---|---|---|---|
| Heating | Heating | ||
| Cooling | |||
| PS4-Xchol-1.00 | G55.63SmA234.94I | PCS4-Xchol-1.00 | G65.5SmA233.1Ch248.0BPs266.5I |
| I234.13SmA56.74G | |||
| PS4-Xchol-0.90 | G49.94SmA231.35I | PCS4-Xchol-0.875 | G56.4SmA220.2Ch245.4BPs251.0I |
| I230.32SmA56.51G | |||
| PS4-Xchol-0.80 | G45.10SmA206.39I | PCS4-Xchol-0.75 | G657.3SmA197.7Ch237.5BPs242.5I |
| I206.98SmA43.35G | |||
| PS4-Xchol-0.70 | G50.75SmA187.32Ch213.48I | PCS4-Xchol-0.625 | G55.2Ch230.5BPs234.5I |
| I211.63Ch187.25SmA45.37G | |||
| PS4-Xchol-0.60 | G42.62SmA141.65Ch185.21I | PCS4-Xchol-0.50 | G43.4Ch205.2BPs208.0I |
| I183.69Ch141.39SmA39.57G | |||
| PS4-Xchol-0.50 | G40.51Ch178.89I | PCS4-Xchol-0.375 | G53.7Ch208.3I |
| I177.38Ch39.01G | |||
| PS4-Xchol-0.40 | G34.48Ch156.72I | PCS4-Xchol-0.25 | G51.5Ch192.7I |
| I155.20Ch32.59G | |||
| PS4-Xchol-0.30 | G24.00Ch106.50I | PCS4-Xchol-0.125 | G55.5Ch189.2I |
| I105.40Ch23.00G | |||
| PS4-Xchol-0.20 | G18.39Ch82.50I | PCS4-Xchol-0 | Cr145.6Cr2156.9N194.4I |
| I81.42Ch15.99G | |||
| PS4-Xchol-0.10 | G11.41Ch64.06I | ||
| I62.53Ch8.98G | |||
| PS4-Xchol-0.00 | G6.97N32.09I | ||
| I30.04N4.80G |
| Polymers | n × 103 (GPC) | w/n (GPC) | Thermal Transitions/°C and Corresponding Enthalpy Changes (kcal mru−1) in Parentheses a | |
|---|---|---|---|---|
| Heating | Cooling | |||
| P-11a-2 | 2.7 | 1.51 | G18K52(0.98)SA298(0.72) b I | I268(0.95)SA16G |
| P-11a-2/3-2(8/2) | 4.9 | 1.51 | K51(1.56)SA235(0.41)I | I224(0.49)SA10G |
| g 7 SA 231 (0.45) I | ||||
| P-11a-2/3-2(6/4) | 6.1 | 1.84 | G-2K41(0.64)SA158(0.05) I | I156(0.33)SA-10G |
| G-6SA159(0.20)I | ||||
| P-11a-2/3-2(4/6) | 9.5 | 1.86 | K40(0.82)SA92(0.02) c I | I92(0.13) c SA-21G |
| G-19K31(0.02)SA95(0.01) c I | ||||
| P-11a-3 | 3.0 | 1.12 | K51(2.20)SA265(0.94)I | I257(0.95)SA5G |
| G10SA264(0.86)1 | ||||
| P-11a-3/3-3(8/2) | 3.3 | 1.07 | K47(1.58)SA204(0.37)I | I190(0.26)SA-11G |
| G-5K41(0.03)SA195(0.45)I | ||||
| P-11a-3/3-3(6/4) | 3.5 | 1.10 | K36(0.91)SA105(0.05) c I | I107(0.14) c SA-20G |
| G-16K26(0.08) d K34(0.10)SA108(0.05) c I | ||||
| P-11b-2 | 14.4 | 1.97 | K57(0.46)K121(1.98)SA164(0.18)N212(0.35)I | I209(0.20)N157(0.15)SA92(2.03)K68(0.34)K |
| K76(0.39)K123(2.32)SA163(0.15)N213(0.22)I | ||||
| P-11b-3 | 10.4 | 2.00 | K64(0.40)K107(2.00)N160(0.18)I | I155(0.16)N83(1.79)K52 (0.40)K |
| K63(0.38)K107(1.98)N159(0.16)I | ||||
| Mole of Li+CF3SO3− Per Mole Repeat of P-11a-3 | Thermal Transitions/°C and Corresponding Enthalpy Changes (kcal mru−1) in Parentheses a | |
|---|---|---|
| Heating | Cooling | |
| 0 | K51(2.20)SA287 b I | SA5I |
| G10SA287 b I | ||
| 0.3 | G15SA270 b I | SA18I |
| G23SA270 b I | ||
| 0.7 | K48K55(1.13) c SA237 b I | SA30I |
| G36SA237 b I | ||
| 1.1 | K53(0.65)SA234 b I | SA29I |
| G38SA234 b I | ||
| 1.7 | K47K58(0.44) c SA231 b I | SA39I |
| G45SA231 b I | ||
| 2.0 | K48K57K65(0.83) c SA228 b I | SA40I |
| G45SA228 b I | ||
| 2.5 | K58(0.16)K66(0.43)SA222 b I | SA41I |
| G44SA222 b 1 | ||
| 2.7 | K56(0.31)K64(0.34)SA 220 b I | SA38I |
| G44SA220 b I | ||
| 3.0 | K56(0.31)K64(0.21)SA221 b I | SA45I |
| G46SA221bI | ||
| 3.3 | K55(0.35)K64(0.21)SA215 b I | SA44I |
| G47SA215 b I | ||
| 4.5 | K55K64(0.59) c SA 14 b I | SA41I |
| G43SA214 b I | ||
| Polymers | n × 103 (GPC) | w/n (GPC) | Thermal Transitions/°C and Corresponding Enthalpy Changes (kcal mru−1) in Parentheses a | |
|---|---|---|---|---|
| Heating | Cooling | |||
| P-12-1 | 7.8 | 1.97 | G41K88(0.29)N122(0.13)I | I125(0.19)N47G |
| G50N129(0.18)I | ||||
| P-12-2 | 9.7 | 1.27 | K54(0.45)K90(2.33)N109(0.31)I | I107(0.20)N35G |
| G39N111(0.19)I | ||||
| P-12-3 | 12.0 | 1.84 | K53(0.21)K83(2.99)N104(0.17)I | I98(0.19)N26G |
| G31N103(0.23)I | ||||
| Mole of Li+CF3SO3− Per Mole Repeat of P-12-3 | Thermal Transitions/°C and Corresponding Enthalpy Changes (kcal mru−1) in Parentheses a | |
|---|---|---|
| Heating | Cooling | |
| 0 | K53(0.21)K83(2.99)N104(0.17)I | I98(0.19)N26G |
| G31N103(0.23)I | ||
| 0.1 | K70(3.68)I | I67(0.06)N16G |
| G19N71(0.07)I | ||
| 0.2 | K66(3.16)I | I60(0.07)N20G |
| G22N 65(0.05) | ||
| 0.3 | K60(2.67)I | I55(0.06)N20G |
| G26N61(0.05) | ||
| 0.4 | K60(1.87)I | I48(0.05)N24G |
| G28N54(0.06)I | ||
| 0.5 | K45(0.87)I | I26G |
| G28I | ||
| 0.6 | K48(0.35)I | I31G |
| G31I | ||
| 0.7 | - b | I33G |
| G33I | ||
| 0.8 | - b | I43G |
| G48I | ||
| Polymers (Pn,m) | Tg/°C | Tk/°C | Ti/°C | ΔT/°C | Mesophase | |
|---|---|---|---|---|---|---|
| (1) | (2) | |||||
| P6,2 | −15 | - | 101 | 116 | - | SmA |
| P8,2 | −20 | - | 118 | 138 | - | SmA |
| P10,2 | −21 | - | 125 | 146 | - | SmA |
| P10,3 | −10 | - | 135 | 145 | - | SmA |
| P11,2 | −22 | 45 | 121 | - | 76 | SmA |
| P11,3 | −15 | 68 | 140 | - | 72 | SmA |
| P11,- | - | 52 | 181 | - | 129 | SmA |
| Polymers | Phase Transitions (°C) a | |
|---|---|---|
| Heating | Cooling | |
| PMMS-g-LC1 | SmX 62 I | I 59 SmX |
| PMMS-g-LC2 | SmX 82 I | I 80 SmX |
| PMMS-g-LC3 | N 70 I | I 68 N |
| PMMS-g-LC4 | N73 I | I 71 N |
| PMMS-g-LC5 | N 56 I | I 54 N |
| Polymers | Xchola | Mn (g/mol) | Phase Transitions (°C) d | |||
|---|---|---|---|---|---|---|
| In Feed | CALC b | GPC c | CALC b | Heating | Cooling | |
| P1 | 0.00 | 0.000 | 2200 | 18,747 | G2.4SmAs92.1I | I81.3SmAs-1.4G |
| P2 | 0.20 | 0.221 | 3300 | 21,412 | G10.5SmAs96.3 I | I98.5SmAs8.8G |
| P3 | 0.40 | 0.425 | 3700 | 23,872 | G18.7SmAs111.7I | I113.4SmAs16.4G |
| P4 | 0.60 | 0.604 | 4000 | 26,031 | G23.6SmAs176.8I | I175.8SmAs19.5G |
| P5 | 0.80 | 0.819 | 4400 | 28,624 | G33.1SmAs189.4I | I186.7SmAs29.9G |
| P6 | 1.00 | 1.000 | 4800 | 30,852 | G35.7SmAd139.9SmAs204.8I | I197.9SmAs136.6SmAd34.6G |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Yao, W.; Gao, Y.; Zhang, C.; Yang, H. Polysiloxane-Based Side Chain Liquid Crystal Polymers: From Synthesis to Structure–Phase Transition Behavior Relationships. Polymers 2018, 10, 794. https://doi.org/10.3390/polym10070794
Zhang L, Yao W, Gao Y, Zhang C, Yang H. Polysiloxane-Based Side Chain Liquid Crystal Polymers: From Synthesis to Structure–Phase Transition Behavior Relationships. Polymers. 2018; 10(7):794. https://doi.org/10.3390/polym10070794
Chicago/Turabian StyleZhang, Lanying, Wenhuan Yao, Yanzi Gao, Cuihong Zhang, and Huai Yang. 2018. "Polysiloxane-Based Side Chain Liquid Crystal Polymers: From Synthesis to Structure–Phase Transition Behavior Relationships" Polymers 10, no. 7: 794. https://doi.org/10.3390/polym10070794
APA StyleZhang, L., Yao, W., Gao, Y., Zhang, C., & Yang, H. (2018). Polysiloxane-Based Side Chain Liquid Crystal Polymers: From Synthesis to Structure–Phase Transition Behavior Relationships. Polymers, 10(7), 794. https://doi.org/10.3390/polym10070794










