Star Shaped Long Chain Branched Poly (lactic acid) Prepared by Melt Transesterification with Trimethylolpropane Triacrylate and Nano-ZnO
Abstract
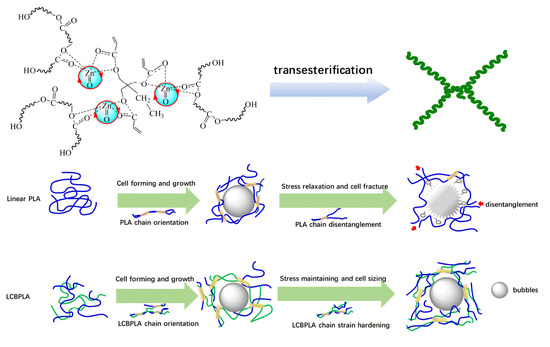
1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation and Reactive Processing
2.3. Purification of Sample
2.4. Gel Determination
2.5. 1H Nuclear Magnetic Resonance Spectroscopy
2.6. X-ray Photoelectron Spectroscopy
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.8. Size Exclusion Chromatography (SEC)
2.9. Rheological Measurement
2.9.1. Oscillatory Shear Rheology
2.9.2. Melt Strength Measurement
2.10. Differential Scanning Calorimetry (DSC)
2.11. Extrusion Foaming Process and Scanning Electron Microscope (SEM)
3. Results and Discussions
3.1. Torque Curves and Reaction Evolution
3.2. 1H-NMR Spectrum
3.3. X-ray Photoelectron Spectroscopy (XPS)
3.4. FTIR Spectroscopy
3.5. Proposed Mechanism
3.6. Size Exclusion Chromatography (SEC)
3.7. Rheological Measurements
3.8. SEM Images of Foaming PLA
3.9. Crystallization Behavior
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mi, H.Y.; Salick, M.R.; Jing, X.; Jacques, B.R.; Crone, W.C.; Peng, X.F.; Turng, L.S. Characterization of thermoplastic polyurethane/polylactic acid (TPU/PLA) tissue engineering scaffolds fabricated by microcellular injection molding. Mater. Sci. Eng. C 2013, 33, 4767–4776. [Google Scholar] [CrossRef] [PubMed]
- Bordes, P.; Pollet, E.; Averous, L. Nano-biocomposites: Biodegradable polyester/nanoclay systems. Prog. Polym. Sci. 2009, 34, 125–155. [Google Scholar] [CrossRef]
- Numata, K.; Srivastava, R.K.; Finne-Wistrand, A.; Albertsson, A.C.; Doi, Y.; Abe, H. Branched Poly(lactide) Synthesized by Enzymatic Polymerization: Effects of Molecular Branches and Stereochemistry on Enzymatic Degradation and Alkaline Hydrolysis. Biomacromolecules 2007, 8, 3115–3125. [Google Scholar] [CrossRef] [PubMed]
- Pilla, S.; Kramschuster, A.; Lee, J.; Clemons, C.; Gong, S.; Turng, L.S. Microcellular processing of polylactide–hyperbranched polyester–nanoclay composites. J. Mater. Sci. 2010, 45, 2732–2746. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; He, Y.; Fan, Z.; Li, S. Non-isothermal crystallization kinetics of poly(L-lactide). Polym. Int. 2010, 59, 1616–1621. [Google Scholar] [CrossRef]
- Auras, R.; Lim, L.T.; Selke, S.E.M.; Tsuji, H. Poly (Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; ISBN 978-0-470-29366-9. [Google Scholar]
- Li, Z.Q.; Ye, L.; Zhao, X.W.; Coates, P.; Caton-Rose, F.; Martyn, M. High Orientation of Long chain branched Poly (lactic acid) with Enhanced Blood Compatibility and Bionic Structure. J. Biomed. Mater. Res. A 2016, 104, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Cai, X.; Zhang, Y.; Wang, S.; Dong, W.; Chen, M.; Lemstra, P.J. In-situ compatibilization of poly (lactic acid) and poly(butylene adipate-co-terephthalate) blends by using dicumyl peroxide as a free-radical initiator. Polym. Degrad. Stab. 2014, 102, 145–151. [Google Scholar] [CrossRef]
- Li, J.F.; Li, Z.Q.; Ye, L.; Zhao, X.W.; Coates, P.; Caton-Rose, F.; Martyn, M. Structure evolution and orientation mechanism of long-chain-branched poly (lactic acid) in the process of solid die drawing. Eur. Polym. J. 2017, 90, 54–65. [Google Scholar] [CrossRef]
- Khamsarn, T.; Supthanyakul, R.; Matsumoto, M.; Chirachanchai, S. PLA with high elongation induced by multi-branched poly(ethylene imine) (mPEI) containing poly(l-lactic acid) (PLLA) terminals. Polymer 2017, 112, 87–91. [Google Scholar] [CrossRef]
- Tian, J.H.; Yu, W.; Zhou, C.X. The preparation and rheology characterization of long chain branching polypropylene. Polymer 2006, 47, 7962–7969. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, L.; He, L.; Gong, W.; Luo, Z.; Liu, W. Influence of different functionality degree grafting monomers on polypropylene melt radical branching reaction and their structure. Polym. Adv. Technol. 2018, 29, 551–559. [Google Scholar] [CrossRef]
- Fang, H.G.; Zhang, Y.Q.; Bai, J.; Wang, Z.K.; Wang, Z.G. Bimodal architecture and rheological and foaming properties for gamma-irradiated long-chain branched polylactides. RSC Adv. 2013, 3, 8783–8795. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Tsuji, H. Crystallization behavior and physical properties of linear 2-arm and branched 4-arm poly(l-lactide)s: Effects of branching. Polymer 2013, 54, 2422–2434. [Google Scholar] [CrossRef]
- Liu, J.Y.; Lou, L.J.; Yu, W.; Liao, R.G.; Li, R.M.; Zhou, C.X. Long chain branching polylactide: Structures and properties. Polymer 2010, 51, 5186–5197. [Google Scholar] [CrossRef]
- Liu, J.Y.; Zhang, S.J.; Zhang, L.Y.; Bai, Y.Q. Preparation and rheological characterization of long chain branching polylactide. Polymer 2014, 55, 2472–2480. [Google Scholar] [CrossRef]
- Cailloux, J.; Santana, O.O.; Franco-Urquiza, E.; Bou, J.J.; Gámez-Pérez, J.; Maspoch, M.L. Sheets of branched poly (lactic acid) obtained by one step reactive extrusion calendering process: Melt rheology analysis. Express Polym. Lett. 2013, 7, 304–318. [Google Scholar] [CrossRef]
- Cailloux, J.; Santana, O.O.; Maspoch, M.L.; Bou, J.J.; Carrasco, F. Using viscoelastic properties to quantitatively estimate the amount of modified poly (lactic acid) chains through reactive extrusion. J. Rheol. 2015, 59, 1191–1227. [Google Scholar] [CrossRef]
- You, J.X.; Lou, L.J.; Yu, W.; Zhou, C.X. The preparation and crystallization of long chain branching polylactide made by melt Radicals reaction. J. Appl. Polym. Sci. 2013, 129, 1959–1970. [Google Scholar] [CrossRef]
- Li, S.J.; He, G.J.; Liao, X.; Park, C.B.; Yang, Q.; Li, G.X. Introduction of a long-chain branching structure by ultraviolet-induced reactive extrusion to improve cell morphology and processing properties of polylactide foam. RSC Adv. 2017, 11, 6266–6277. [Google Scholar] [CrossRef]
- Gu, L.L.; Xu, Y.W.; Fahnhorst, G.W.; Macosko, C.W. Star vs long chain branching of poly (lactic acid) with multifunctional aziridine. J. Rheol. 2017, 61, 785–796. [Google Scholar] [CrossRef]
- Harada, M.; Iida, K.; Okamoto, K.; Hayashi, H.; Hirano, K. Reactive compatibilization of biodegradable poly (lactic acid)/poly(ε-caprolactone) blends with reactive processing agents. Polym. Eng. Sci. 2008, 48, 1359–1368. [Google Scholar] [CrossRef]
- Shieh, Y.T.; Lin, Y.T. Transesterification and polymerization reactions of aliphatic polyesters in supercritical CO2 fluids without the presence of a catalyst. Eur. Polym. J. 2007, 43, 1847–1856. [Google Scholar] [CrossRef]
- Lipik, V.T.; Widjaja, L.K.; Liow, S.S.; Abadie, M.J.M.; Venkatraman, S.S. Effects of transesterification and degradation on properties and structure of polycaprolactone-polylactide copolymers. Polym. Degrad. Stab. 2010, 95, 2596–2602. [Google Scholar] [CrossRef]
- Schreck, K.M.; Hillmyer, M.A. Block copolymers and melt blends of polylactide with Nodax™ microbial polyesters: Preparation and mechanical properties. J. Biotechnol. 2007, 132, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Coltelli, M.B.; Toncelli, C.; Ciardelli, F.; Bronco, S. Compatible blends of biorelated polyesters through catalytic transesterification in the melt. Polym. Degrad. Stab. 2011, 96, 982–990. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Rheological, morphological, and interfacial properties of compatibilized PLA/PBAT blends. Rheol. Acta 2014, 53, 501–517. [Google Scholar] [CrossRef]
- Kim, T.J.; Kim, T.H.; Sang, G.K.; Seo, K.H. Structural, Thermal, and Mechanical Properties of PLA/PBAT/MEA Blend. Polym. Korea 2016, 40, 371–379. [Google Scholar] [CrossRef]
- Lin, S.; Guo, W.N.; Chen, C.Y.; Ma, J.L.; Wang, B.B. Mechanical properties and morphology of biodegradable poly (lactic acid)/poly(butylene adipate-co-terephthalate) blends compatibilized by transesterification. Mater. Des. 2012, 36, 604–608. [Google Scholar] [CrossRef]
- Signori, F.; Coltelli, M.B.; Bronco, S. Thermal degradation of poly (lactic acid) (PLA) and poly(butylene adipate-co-terephthalate) (PBAT) and their blends upon melt processing. Polym. Degrad. Stab. 2009, 94, 74–82. [Google Scholar] [CrossRef]
- Slivniak, R.; Domb, A.J. Lactic Acid and Ricinoleic Acid Based Copolyesters. Macromolecules 2005, 38, 5545–5553. [Google Scholar] [CrossRef]
- Slivniak, R.; Langer, R.; Domb, A.J. Lactic and Ricinoleic Acid Based Copolyesters Stereocomplexation. Macromolecules 2005, 38, 5634–5639. [Google Scholar] [CrossRef]
- Slivniak, R.; Ezra, A.; Domb, A.J. Hydrolytic Degradation and Drug Release of Ricinoleic Acid-Lactic Acid Copolyesters. Pharm. Res. 2006, 23, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Coullerez, G.; Lowe, C.; Pechy, P.; Kausch, H.H.; Hilborn, J. Synthesis of acrylate functional telechelic poly (lactic acid) oligomer by transesterification. J. Mater. Sci.-Mater. Med. 2000, 11, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Elkouly, S.M.; Mohamed, G.M.; Fathy, N.A.; Fagal, G.A. Effect of nanosized CeO2 or ZnO loading on adsorption and catalytic properties of activated carbon. Adsorpt. Sci. Technol. 2017, 35. [Google Scholar] [CrossRef]
- Qu, M.; Tu, H.L.; Amarante, M.; Song, Y.Q.; Zhu, S.S. Zinc oxide nanoparticles catalyze rapid hydrolysis of poly (lactic acid) at low temperatures. J. Appl. Polym. Sci. 2014, 131, 2928–2935. [Google Scholar] [CrossRef]
- And, S.S.; Ishida, M.; Anderson, M.A. Visible Luminescence and Surface Properties of Nanosized ZnO Colloids Prepared by Hydrolyzing Zinc Acetate. J. Phys. Chem. B 1998, 102, 10169–10175. [Google Scholar]
- Li, Y.; Yao, Z.; Chen, Z.H.; Qiu, S.L.; Zeng, C.C.; Cao, K. High melt strength polypropylene by ionic modification: Preparation, rheological properties and foaming behaviors. Polymer 2015, 70, 207–214. [Google Scholar] [CrossRef]
- Montaudo, G.; Puglisi, C.; Samperi, F. Mechanism of Exchange in PBT/PC and PET/PC Blends. Composition of the Copolymer Formed in the Melt Mixing Process. Macromolecules 1998, 31, 650–661. [Google Scholar] [CrossRef]
- Lagendijk, R.P.; Hogt, A.H.; Buijtenhuijs, A.; Gotsis, A.D. Peroxydicarbonate modification of polypropylene and extensional flow properties. Polymer 2001, 42, 10035–10043. [Google Scholar] [CrossRef]
- Liang, X.K.; Luo, Z.; Yang, L.; Wei, J.T.; Yuan, X.; Zheng, Q. Rheological properties and crystallization behaviors of long chain branched polyethylene prepared by melt branching reaction. J. Polym. Eng. 2017, 38, 7–17. [Google Scholar] [CrossRef]
- Su, F.H.; Huang, H.X. Rheology and melt strength of long chain branching polypropylene prepared by reactive extrusion with various peroxides. Polym. Eng. Sci. 2010, 50, 342–351. [Google Scholar] [CrossRef]
- Wang, L.Y.; Jing, X.B.; Cheng, H.B.; Hu, X.L.; Yang, L.X.; Huang, Y.B. Blends of Linear and Long-Chain Branched Poly(l-lactide)s with High Melt Strength and Fast Crystallization Rate. Ind. Eng. Chem. Res. 2012, 51, 10088–10099. [Google Scholar] [CrossRef]
- Van Gurp, M.; Palmen, J. Time-temperature superposition for polymeric blends. Rheol. Bull. 1998, 67, 5–8. [Google Scholar]
- Liu, J.Y.; Yu, W.; Zhou, C.X. Polymer chain topological map as determined by linear viscoelasticity. J. Rheol. 2011, 55, 545–570. [Google Scholar] [CrossRef]
- Kempf, M.; Ahirwal, D.; Cziep, M.; Wilhelm, M. Synthesis and Linear and Nonlinear Melt Rheology of Well-Defined Comb Architectures of PS and PpMS with a Low and Controlled Degree of Long-Chain Branching. Macromolecules 2013, 46, 4978–4994. [Google Scholar] [CrossRef]
- Kempf, M.; Barroso, V.C.; Wilhelm, M. Anionic Synthesis and Rheological Characterization of Poly(p-methylstyrene) Model Comb Architectures with a Defined and Very Low Degree of Long Chain Branching. Macromol. Rapid Commun. 2010, 31, 2140–2145. [Google Scholar] [CrossRef] [PubMed]
- Christos, J.T.; Alexandros, D.G. Rheological Characterization of Long Chain Branching in a Melt of Evolving Molecular Architecture. Macromolecules 2001, 34, 4685–4687. [Google Scholar]
- Pearson, D.S.; Helfand, E. Viscoelastic properties of star-shaped polymers. Macromolecules 1984, 17, 888–895. [Google Scholar] [CrossRef]
- Ball, R.C.; Mcleish, T.C.B. Dynamic dilution and the viscosity of star-polymer melts. Macromolecules 1989, 5, 1911–1913. [Google Scholar] [CrossRef]
- Mcleish, T.C.B. Molecular rheology of H-polymers. Macromolecules 1988, 21, 1062–1070. [Google Scholar] [CrossRef]
- Mcleish, T.C.B. Hierarchical relaxation in tube models of branched polymers. Europhys. Lett. 1988, 6, 511–516. [Google Scholar] [CrossRef]
- Heshmati, V.; Zolali, A.M.; Favis, B.D. Morphology development in poly (lactic acid)/polyamide11 biobased blends: Chain mobility and interfacial interactions. Polymer 2017, 170, 197–208. [Google Scholar] [CrossRef]
- Hepperle, J.; Münstedt, H. Rheological properties of branched polystyrenes: Nonlinear shear and extensional behavior. Rheol. Acta 2006, 45, 717–727. [Google Scholar] [CrossRef]
- And, W.A.; Dealy, J.M. Using Rheological Data to Determine the Branching Level in Metallocene Polyethylenes. Macromolecules 2000, 33, 7481–7488. [Google Scholar]
- Taki, K.; Tabata, K.; Kihara, S.; Ohshima, M. Bubble coalescence in foaming process of polymers. Polym. Eng. Sci. 2006, 46, 680–690. [Google Scholar] [CrossRef]
- Nofar, M.; Zhu, W.L.; Park, C.B.; Randall, J. Crystallization Kinetics of Linear and Long-Chain-Branched Polylactide. Ind. Eng. Chem. Res. 2011, 50, 13789–13798. [Google Scholar] [CrossRef]
- Fang, H.G.; Zhang, Y.Q.; Bai, J.; Wang, Z.G. Shear-Induced Nucleation and Morphological Evolution for Bimodal Long Chain Branched Polylactide. Macromolecules 2013, 46, 6555–6565. [Google Scholar] [CrossRef]
- Bai, J.; Fang, H.G.; Zhang, Y.Q.; Wang, Z.G. Studies on crystallization kinetics of bimodal long chain branched polylactides. CrystEngComm 2014, 16, 2452–2461. [Google Scholar] [CrossRef]














| Sample | PLA Pellet (g) | Nano-ZnO (g) | TMPTA (g) | Sampling Time (s) a | GP (%) | MFR b (g/10 min) | η0 (Pa·s) c |
|---|---|---|---|---|---|---|---|
| PLA | 100 | 0 | 0 | 400 | 0 | 4.10 | 4571.41 |
| Z1 | 100 | 0.40 | 0 | 400 | 0 | 4.70 | 4387.42 |
| T1 | 100 | 0 | 2.00 | 400 | 0 | 4.52 | 4408.62 |
| B1 | 100 | 0.20 | 2.00 | 161 * | 0.29 | 1.08 | 11,206.2 |
| B2 | 100 | 0.40 | 2.00 | 145 * | 0.38 | 0.98 | 11,963.8 |
| B3 | 100 | 0.60 | 2.00 | 150 * | 0.33 | 1.54 | 8179.90 |
| B4 | 100 | 0.80 | 2.00 | 145 * | 0.35 | 2.44 | 5955.33 |
| B5 | 100 | 1.00 | 2.00 | 155 * | 0.26 | 2.82 | 6672.38 |
| B6 | 100 | 0.40 | 2.00 | 400 | 0.36 | 5.92 | 2176.05 |
| Sample | Mn (g/mol) | Mw (g/mol) | Mw/Mn |
|---|---|---|---|
| PLA | 108,994 | 185,293 | 1.70 |
| B2 | 97,055 | 178,689 | 1.84 |
| B6 | 74,277 | 126,084 | 1.82 |
| Sample | Tg (°C) | Tonset (°C) | Tcp (°C) | Tm (°C) | ∆Hm (J/g) | ∆Hm − ∆Hc (J/g) | v × 10−3 (s−1) |
|---|---|---|---|---|---|---|---|
| PLA | 62.60 | 106.99 | 124.10 | 151.54 | 15.520 | 1.260 | 10.30 |
| Z1 | 62.61 | 105.60 | 120.84 | 152.32 | 18.731 | 2.424 | 10.60 |
| B1 | 62.79 | 108.17 | 128.49 | 152.79 | 11.600 | 1.899 | 9.96 |
| B2 | 62.63 | 109.58 | 129.67 | 152.47 | 8.710 | 0.544 | 9.57 |
| B3 | 62.68 | 110.98 | 131.94 | 152.90 | 4.394 | 0.499 | 8.17 |
| B4 | 62.67 | 112.83 | 131.01 | 153.45 | 6.803 | 0.903 | 9.16 |
| B5 | 62.63 | 111.00 | 131.21 | 152.68 | 5.053 | 0.421 | 8.05 |
| B6 | 62.64 | 109.76 | 126.51 | 153.04 | 14.31 | 0.640 | 10.20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Yang, Z.; Zhang, F.; Xie, L.; Luo, Z.; Zheng, Q. Star Shaped Long Chain Branched Poly (lactic acid) Prepared by Melt Transesterification with Trimethylolpropane Triacrylate and Nano-ZnO. Polymers 2018, 10, 796. https://doi.org/10.3390/polym10070796
Yang L, Yang Z, Zhang F, Xie L, Luo Z, Zheng Q. Star Shaped Long Chain Branched Poly (lactic acid) Prepared by Melt Transesterification with Trimethylolpropane Triacrylate and Nano-ZnO. Polymers. 2018; 10(7):796. https://doi.org/10.3390/polym10070796
Chicago/Turabian StyleYang, Le, Zaijun Yang, Feng Zhang, Lijin Xie, Zhu Luo, and Qiang Zheng. 2018. "Star Shaped Long Chain Branched Poly (lactic acid) Prepared by Melt Transesterification with Trimethylolpropane Triacrylate and Nano-ZnO" Polymers 10, no. 7: 796. https://doi.org/10.3390/polym10070796
APA StyleYang, L., Yang, Z., Zhang, F., Xie, L., Luo, Z., & Zheng, Q. (2018). Star Shaped Long Chain Branched Poly (lactic acid) Prepared by Melt Transesterification with Trimethylolpropane Triacrylate and Nano-ZnO. Polymers, 10(7), 796. https://doi.org/10.3390/polym10070796