Improve the Performance of Soy Protein-Based Adhesives by a Polyurethane Elastomer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
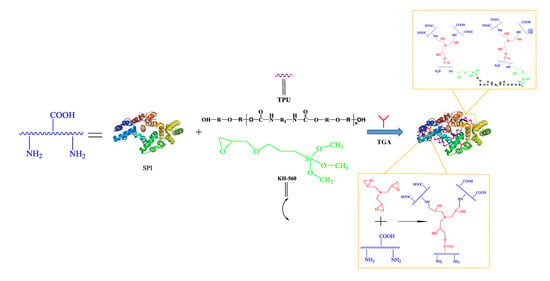
2.2. Preparation of the TGA
2.3. Preparation of the Different Adhesives
2.4. Preparation of Three-Ply Plywood Samples
2.5. Adhesive Strength Measurement
2.6. Determination of the Non Hydrolysable Residue
2.7. Fourier Transform Infrared (FTIR) Spectroscopy
2.8. Scanning Electron Microscopy (SEM)
2.9. Thermal Stability Measurement
2.10. Cracks Observation
3. Results
3.1. FTIR Analysis
3.2. Non Hydrolysable Residue of Different Cured Adhesives
3.3. Thermal Stability Analysis
3.4. SEM Analysis
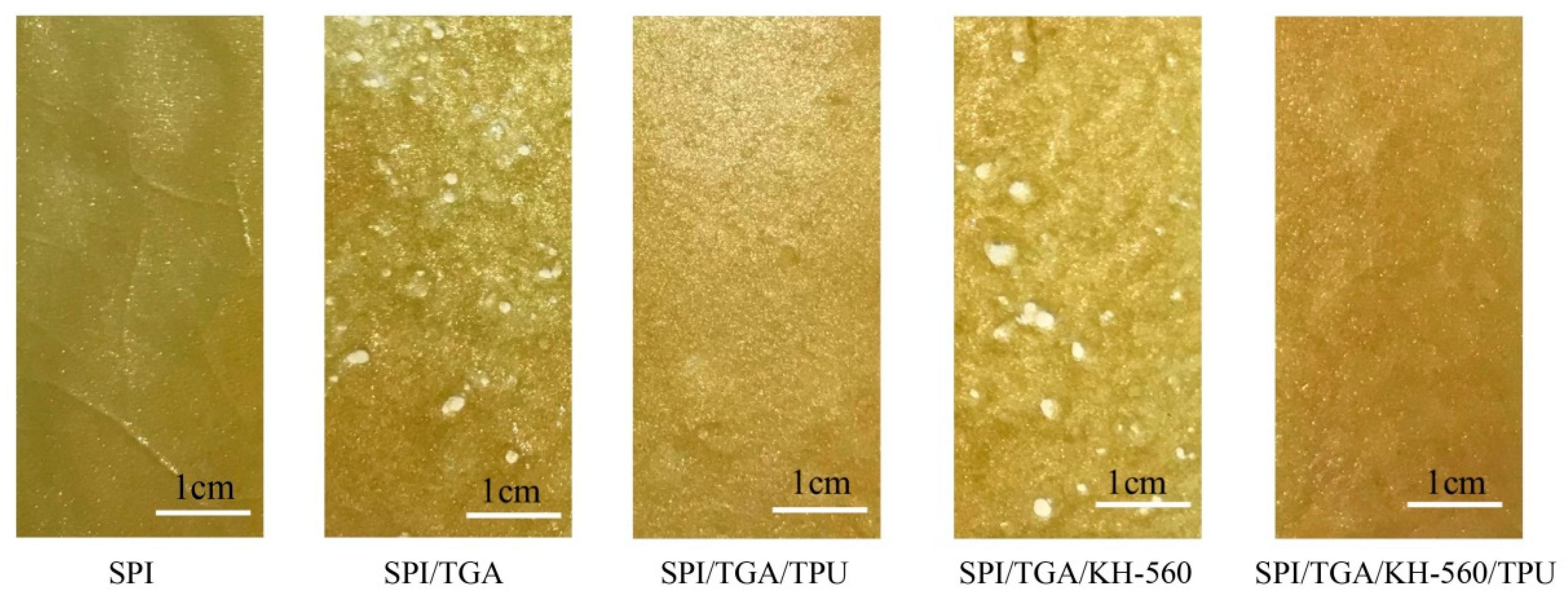
3.5. Cracks Observation of Cured Adhesive
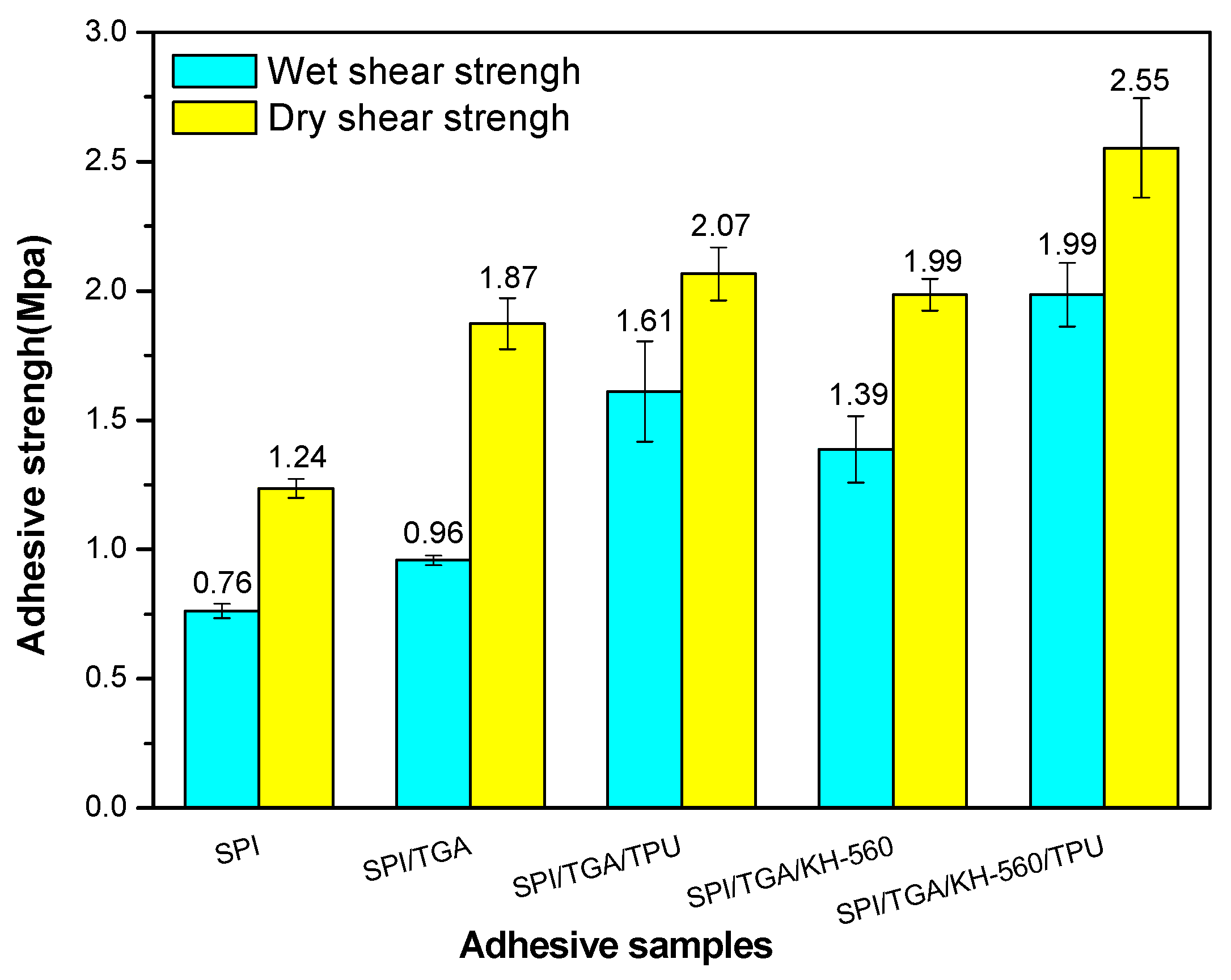
3.6. Shear Strength Measurement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, M.; Luo, J.; Shi, R.; Zhang, J.; Gao, Q.; Li, J. Improved adhesion performance of soy protein-based adhesives with a larch tannin-based resin. Polymers 2017, 9, 408. [Google Scholar] [CrossRef]
- Li, Y.; Chen, F.; Zhang, L.; Yao, Y. Effect of surface changes of soy protein materials on water resistance. Mater. Lett. 2015, 149, 120–122. [Google Scholar] [CrossRef]
- Bacigalupe, A.; Poliszuk, A.K.; Eisenberg, P.; Escobar, M.M. Rheological behavior and bonding performance of an alkaline soy protein suspension. Int. J. Adhes. Adhes. 2015, 62, 1–6. [Google Scholar] [CrossRef]
- Costa, N.A.; Pereira, J.; Ferra, J.; Cruz, P.; Martins, J.; Magalhães, F.D.; Mendes, A.; Carvalho, L.H. Scavengers for achieving zero formaldehyde emission of wood-based panels. Wood Sci. Technol. 2013, 47, 1261–1272. [Google Scholar] [CrossRef]
- Ghahri, S.; Pizzi, A. Improving soy-based adhesives for wood particleboard by tannins addition. Wood Sci. Technol. 2018, 5, 261–279. [Google Scholar] [CrossRef]
- Adhikari, B.; Appadu, P.; Kislitsin, V.; Chae, M.; Choi, P.; Bressler, D. Enhancing the adhesive strength of a plywood adhesive developed from hydrolyzed specified risk materials. Polymers 2016, 8, 285. [Google Scholar] [CrossRef]
- Li, F.; Li, X.P.; Wang, W.H. Soy flour adhesive modified with urea, citric acid and boric acid. Pigm. Resin Technol. 2010, 39, 223–227. [Google Scholar] [CrossRef]
- Verker, R.; Rivkin, A.; Zilberman, G.; Shoseyov, O. Insertion of nano-crystalline cellulose into epoxy resin via resilin to construct a novel elastic adhesive. Cellulose 2014, 21, 4369–4379. [Google Scholar] [CrossRef]
- Barthes, J.; Mutschler, A.; Dollinger, C.; Gaudinat, G.; Lavalle, P.; Le, H.V.; Brian McGuinness, G.; Engin Vrana, N. Establishing contact between cell-laden hydrogels and metallic implants with a biomimetic adhesive for cell therapy supported implants. Biomed. Mater. 2017, 13, 015015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Xi, X.; Lei, H.; Du, G. Soy-based adhesive cross-linked by phenol-formaldehyde-glutaraldehyde. Polymers 2017, 9, 169. [Google Scholar] [CrossRef]
- Czub, P.; Kasza, P. Study on mechanical properties of the crosslinked with isocyanates product of the reaction of modified soybean oil with epoxy resin. Polimery 2014, 59, 466–476. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, Y.; Deng, L.; Li, Z.; Chen, Z. Properties of soy-based wood adhesives enhanced by waterborne polyurethane modification. J. Biobased Mater. Bioenergy 2017, 11, 330–335. [Google Scholar] [CrossRef]
- Sienkiewicz, A.; Czub, P. Novel bio-based epoxy-polyurethane materials from modified vegetable oils—Synthesis and characterization. Express Polym. Lett. 2017, 11, 308–319. [Google Scholar] [CrossRef]
- Gao, Q.; Qin, Z.; Li, C.; Zhang, S.; Li, J. Preparation of wood adhesives based on soybean meal modified with pegda as a crosslinker and viscosity reducer. Bioresources 2013, 8, 5380–5391. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Li, X.; Li, K.; Gao, Q.; Li, J. Toughening improvement to a soybean meal-based bioadhesive using an interpenetrating acrylic emulsion network. J. Mater. Sci. 2016, 51, 9330–9341. [Google Scholar] [CrossRef]
- Test Methods of Evaluating the Poperties of Wood-Based Panels and Surface Decorated Wood-Based Panels; GB/T 17657; Standardization Administration of the People’s Republic of China: Beijing, China, 2013.
- Chen, N.; Lin, Q.; Rao, J.; Zeng, Q. Water resistances and bonding strengths of soy-based adhesives containing different carbohydrates. Ind. Crops Prod. 2013, 50, 44–49. [Google Scholar] [CrossRef]
- Li, J.; Luo, J.; Li, X.; Yi, Z.; Gao, Q.; Li, J. Soybean meal-based wood adhesive enhanced by ethylene glycol diglycidyl ether and diethylenetriamine. Ind. Crops Prod. 2015, 74, 613–618. [Google Scholar] [CrossRef]
- Luo, J.; Li, C.; Li, X.; Luo, J.; Gao, Q.; Li, J. A new soybean meal-based bioadhesive enhanced with 5,5-dimethyl hydantoin polyepoxide for the improved water resistance of plywood. RSC Adv. 2015, 5, 62957–62965. [Google Scholar] [CrossRef]
- Su, J.F.; Zhen, H.; Yuan, X.Y.; Wang, X.Y.; Min, L. Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by maillard reactions. Carbohydr. Polym. 2010, 79, 145–153. [Google Scholar] [CrossRef]
- Qi, G.; Sun, X.S. Soy protein adhesive blends with synthetic latex on wood veneer. J. Am. Oil Chem. Soc. 2011, 88, 271–281. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, W.; Lu, Y.; Gao, Z.; Gu, J. Nano-scale blocking mechanism of mmt and its effects on the properties of polyisocyanate-modified soybean protein adhesive. Ind. Crops Prod. 2014, 57, 35–42. [Google Scholar] [CrossRef]
- Gao, Z.H.; Zhang, Y.H.; Fang, B.; Zhang, L.P.; Shi, J. The effects of thermal-acid treatment and crosslinking on the water resistance of soybean protein. Ind. Crops Prod. 2015, 74, 122–131. [Google Scholar] [CrossRef]
- Gao, Q.; Shi, S.; Zhang, S.; Li, J.; Liang, K. Improved plywood strength and lowered emissions from soybean meal/melamine urea-formaldehyde adhesives. For. Prod. J. 2011, 61, 688–693. [Google Scholar] [CrossRef]







| Process | Time/s | Pressure/MPa |
|---|---|---|
| Booster | 5 | 0.8 |
| Packing | 485 | 0.8 |
| Decompression | 490 | 0.4 |
| Packing | 720 | 0.4 |
| Decompression | 725 | 0 |
| Insulate | 735 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Xu, Y.; Zhu, W.; Zhang, W.; Gao, Q.; Li, J. Improve the Performance of Soy Protein-Based Adhesives by a Polyurethane Elastomer. Polymers 2018, 10, 1016. https://doi.org/10.3390/polym10091016
Xu Y, Xu Y, Zhu W, Zhang W, Gao Q, Li J. Improve the Performance of Soy Protein-Based Adhesives by a Polyurethane Elastomer. Polymers. 2018; 10(9):1016. https://doi.org/10.3390/polym10091016
Chicago/Turabian StyleXu, Yecheng, Yantao Xu, Wenjie Zhu, Wei Zhang, Qiang Gao, and Jianzhang Li. 2018. "Improve the Performance of Soy Protein-Based Adhesives by a Polyurethane Elastomer" Polymers 10, no. 9: 1016. https://doi.org/10.3390/polym10091016
APA StyleXu, Y., Xu, Y., Zhu, W., Zhang, W., Gao, Q., & Li, J. (2018). Improve the Performance of Soy Protein-Based Adhesives by a Polyurethane Elastomer. Polymers, 10(9), 1016. https://doi.org/10.3390/polym10091016