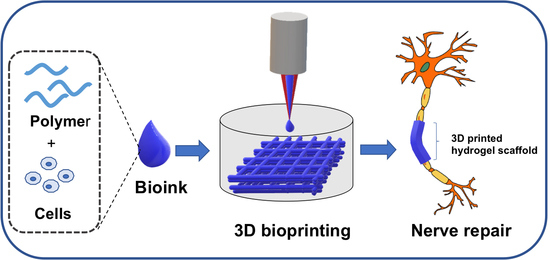
3D Printed Polymeric Hydrogels for Nerve Regeneration
Abstract
1. Introduction
2. 3D Printing Technology
3. Recent Reports on 3D Printing Technology for Nerve Regeneration
4. Basic Criteria for Hydrogel Selection
5. Conclusions and Future Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- National Institute of Neurological Disorders and Stroke. Peripheral Neuropathy Fact Sheet; National Institute of Neurological Disorders and Stroke: Bethesda, MA, USA, 2014.
- Haftek, J. Autogenous cable nerve grafting instead of end to end anastomosis in secondary nerve suture. Acta Neurochir. 1976, 34, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, G. A 25-year perspective of peripheral nerve surgery: Evolving neuroscientific concepts and clinical significance. J. Hand Surg. Am. 2000, 25, 391–414. [Google Scholar] [CrossRef] [PubMed]
- Milleisi, H. Techniques for nerve grafting. Hand Clin. 2000, 16, 73–91. [Google Scholar] [PubMed]
- Johnson, E.O.; Zoubos, A.B.; Soucacos, P.N. Regeneration and repair of peripheral nerves. Injury 2005, 36, S24–S49. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Han, K.; Tiel, R.L.; Murovic, J.A.; Kline, D.G. Surgical outcomes of 654 ulnar nerve lesions. J. Neurosurg. 2003, 98, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Li, M.G.; Guan, Y.J.; Schreyer, D.J.; Chen, X.B. Effects of laminin blended with chitosan on axon guidance on patterned substrates. Biofabrication 2010, 2, 045002. [Google Scholar] [CrossRef] [PubMed]
- Ciardelli, G.; Chiono, V. Materials for peripheral nerve regeneration. Macromol. Biosci. 2006, 6, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Caminero, R.A.; Herrera, L.P.; Martinez, C.A.R.; Almodovar, J. Polymeric scaffolds for three-dimensional culture of nerve cells: A model of peripheral nerve regeneration. MRS Commun. 2017, 7, 391–415. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Stenberg, L.; Gonzalez-Perez, F.; Wrobel, S.; Ronchi, G.; Udina, E.; Suganuma, S.; Geuna, S.; Navarro, X.; Dahlin, L.B.; et al. Chitosan-film enhanced chitosan nerve guides for long-distance regeneration of peripheral nerves. Biomaterials 2016, 76, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sandner, B.; Nicholson, L.S.; Schackel, T.; Tenenbaum, L.; Puttagunta, R.; Müller, R.; Weidner, N.; Blesch, A. Regulated viral brain-derived neurotrophic factor delivery in combination with Schwann cells promotes axonal regeneration through alginate capillary hydrogels after spinal cord injury. Acta Biomater. 2017, 60, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Günther, M.I.; Weidner, N.; Müller, R.; Blesch, A. Cell-seeded alginate hydrogel scaffolds promote directed linear axonal regeneration in the injured rat spinal cord. Acta Biomater. 2015, 27, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Prang, P.; Müller, R.; Eljaouhari, A.A.; Heckmann, K.; Kunz, W.; Weber, T.; Faber, C.; Vroemen, M.; Bogdahn, U.; Weidner, N. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials 2006, 27, 3560–3569. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.C.; Vu, P.; Modi, P.; Chung, P.E.; Landis, R.C.; Khaing, Z.Z.; Hardy, J.G.; Schmidt, C.E. Sacrificial crystal templated hyaluronic acid hydrogels as biomimetic 3D tissue scaffolds for nerve tissue regeneration. ACS Biomater. Sci. Eng. 2017, 3, 1451–1459. [Google Scholar] [CrossRef]
- Ozgenel, G.Y. Effects of hyaluronic acid on peripheral nerve scarring and regeneration in rats. Microsurgery 2003, 23, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wan, A.C.; Xu, X.; Gao, S.; Mao, H.Q.; Leong, K.W.; Yu, H. A new nerve guide conduit material composed of a biodegradable poly(phosphoester). Biomaterials 2001, 22, 1157–1169. [Google Scholar] [CrossRef]
- Wan, A.C.; Mao, H.Q.; Wang, S.; Leong, K.W.; Ong, L.K.; Yu, H. Fabrication of poly(phosphoester) nerve guides by immersion precipitation and the control. Biomaterials 2001, 22, 1147–1156. [Google Scholar] [CrossRef]
- Chamberlain, L.J.; Yannas, I.V.; Arrizabalaga, A.; Hsu, H.P.; Norregaard, T.V.; Spector, M. Early peripheral nerve healing in collagen and silicone tube implants: Myofibroblasts and the cellular response. Biomaterials 1998, 19, 1393–1403. [Google Scholar] [CrossRef]
- Yoshii, S.; Oka, M. Peripheral nerve regeneration along collagen filaments. Brain Res. 2001, 888, 158–162. [Google Scholar] [CrossRef]
- Keeley, R.D.; Nguyen, K.D.; Stephanides, M.J.; Padilla, J.; Rosen, J.M. The artificial nerve graft: A comparison of blended elastomer hydrogel with polyglycolic acid conduits. J. Reconstr. Microsurg. 1991, 7, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kiyotani, T.; Nakamura, T.; Shimizu, Y.; Endo, K. Experimental study of nerve regeneration in a biodegradable tube made from collagen and polyglycolic acid. ASAIO J. 1995, 41, M657–M661. [Google Scholar] [CrossRef] [PubMed]
- Hadlock, T.; Elisseeff, J.; Langer, R.; Vacanti, J.; Cheney, M. A tissue-engineered conduit for peripheral nerve repair. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Hadloc, K.T.; Sundback, C.; Hunter, D.; Cheney, M.; Vacanti, J.P. A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng. 2000, 6, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Mligiliche, N.L.; Tabata, Y.; Kitada, M.; Endoh, K.; Okamato, K.; Fujimoto, E.; Ide, C. Poly lactic acid–caprolactone copolymer tube with a denatured skeletal muscle segment inside as a guide for peripheral nerve regeneration: A morphological and electrophysiological evaluation of the regenerated nerves. Anat. Sci. Int. 2003, 78, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.J.; Hollister, S.J.; Niedner, M.F.; Mahani, M.G.; Park, A.H.; Mehta, D.K.; Ohye, R.G.; Green, G.E. Mitigation of tracheobronchomalacia with 3D-printed personalized medical devices in pediatric patients. Sci. Transl. Med. 2015, 7, 285ra264. [Google Scholar] [CrossRef] [PubMed]
- Siemionow, M.; Bozkurt, M.; Zor, F. Regeneration and repair of peripheral nerves with different biomaterials: Review. Microsurgery 2010, 30, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Thomas, D. Advances in medical polymer technology towards the panacea of complex 3D tissue and organ manufacture. Am. J. Surg. 2018. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Atala, A. Printing technologies for medical applications. Trends Mol. Med. 2016, 22, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I.T. Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol. 2015, 33, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.N.; Lancaster, K.Z.; Zhen, G.; He, J.; Gupta, M.K.; Kong, Y.L.; Engel, E.A.; Krick, K.D.; Ju, A.; Meng, F.; et al. A 3D printed anatomical nerve regeneration pathways. Adv. Funct. Mater. 2015, 25, 6205–6217. [Google Scholar] [CrossRef] [PubMed]
- Yurie, H.; Ikeguchi, R.; Aoyama, T.; Kaizawa, Y.; Tajino, J.; Ito, A.; Ohta, S.; Oda, H.; Takeuchi, H.; Akieda, S.; et al. The efficacy of a scaffold-free Bio 3D conduit developed from human fibroblasts on peripheral nerve regeneration in a rat sciatic nerve model. PLoS ONE 2017, 12, e0171448. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wu, Y.; Gou, Z.; Tao, J.; Zhang, J.; Liu, Q.; Kang, T.; Jiang, S.; Huang, S.; He, J.; et al. 3D-engineering of cellularized conduits for peripheral nerve regeneration. Sci. Rep. 2016, 6, 32184. [Google Scholar] [CrossRef] [PubMed]
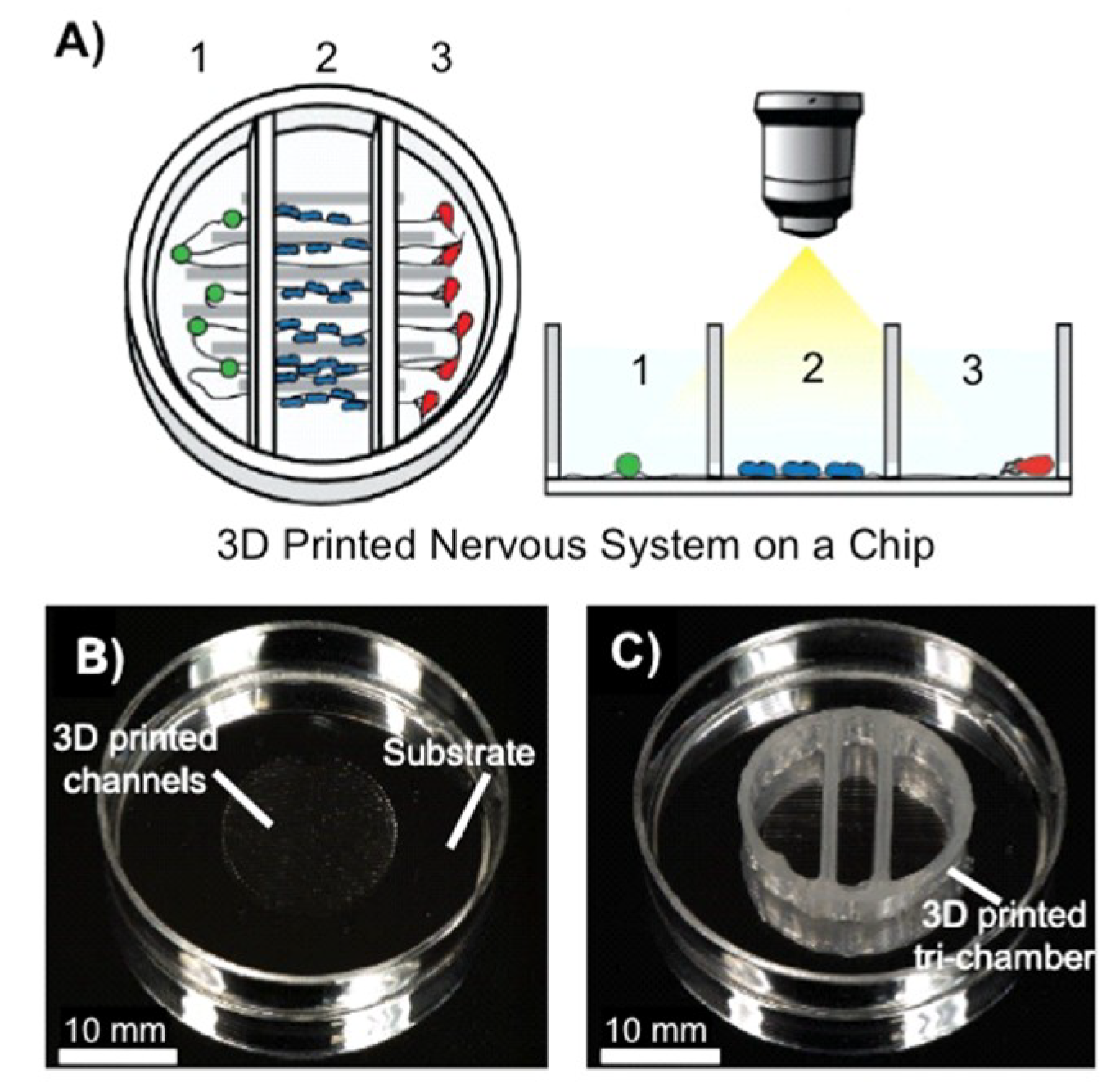
- Johnson, B.N.; Lancaster, K.Z.; Hogue, I.B.; Meng, F.; Kong, Y.L.; Enquist, L.W.; McAlpine, M.C. 3D printed nervous system on a chip. Lab Chip 2016, 16, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.C.; Minner, E.J.; Wiseman, S.L.; Klank, R.L.; Gilbert, R.J. Agarose and methylcellulose hydrogel blends for nerve regeneration applications. J. Neural Eng. 2008, 5, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Soucy, J.R.; Sani, E.S.; Lara, R.P.; Diaz, D.; Dias, F.; Weiss, A.S.; Koppes, A.N.; Koppes, R.A.; Annabi, N. Photocrosslinkable Gelatin/Tropoelastin Hydrogel Adhesives for Peripheral Nerve Repair. Tissue Eng. Part A 2018, 24. [Google Scholar] [CrossRef] [PubMed]
- Belkasa, J.S.; Munroa, C.A.; Shoichetb, M.S.; Midha, R. Peripheral nerve regeneration through a synthetic hydrogel nerve tube. Restor. Neurol. Neurosci. 2005, 23, 19–29. [Google Scholar]
- Klein, S.; Vykoukal, J.; Felthaus, O.; Dienstknecht, T.; Prantl, L. Collagen type I conduits for the regeneration of nerve defects. Materials 2016, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Madduri, S.; Feldman, K.; Tervoort, T. Collagen nerve conduits releasing the neurotrophic factors GDNF and NGF. J. Control. Release 2010, 143, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Matsiko, A.; Haugh, M.G.; Gleeson, J.P.; O’Brien, F.J. Mesenchymal stem cell fate is regulated by the composition and mechanical properties of collagen-glycosaminoglycan scaffolds. J. Mech. Behav. Biomed. Mater. 2012, 11, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.W.; Kim, G.H. New strategy for enhancing in situ cell viability of cell-printing process via piezoelectric transducer-assisted three-dimensional printing. Biofabrication 2016, 8, 025010. [Google Scholar] [CrossRef] [PubMed]
- Guvendiren, M.; Lu, H.D.; Burdick, J.A. Shear-thinning hydrogels for biomedical applications. Soft Matter 2012, 8, 260–272. [Google Scholar] [CrossRef]
- Rezende, R.A.; Bartolo, P.J.; Mendes, A.; Maciel, R. Rheological behavior of alginate solutions for biomanufacturing. J. Appl. Polym. Sci. 2009, 113, 3866–3871. [Google Scholar] [CrossRef]
- Khalil, S.; Sun, W. Bioprinting endothelial cells with alginate for 3D tissue constructs. J. Biomech. Eng. 2009, 131, 111002. [Google Scholar] [CrossRef] [PubMed]
- Boland, T.; Xu, T.; Damon, B.; Cui, X. Application of inkjet printing to tissue engineering. Biomater. Biotechnol. J. 2006, 1, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Remy, M.; Bordenave, L.; Amedee, J.; et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yan, M.; Wang, Y.; Fu, J.; Suo, H. 3D Bioprinting of low-concentration cell-laden gelatin methacrylate (GelMA) bioinks with a two-step cross-linking strategy. ACS Appl. Mater. Interfaces 2018, 10, 6849–6857. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Colosi, C.; Shin, S.R.; Manoharan, V.; Massa, S.; Costantini, M.; Barbetta, A.; Dokmeci, M.R.; Dentini, M.; Khademhosseini, A. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv. Mater. 2016, 28, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Lim, K.S.; Farrugia, B.L.; Hooper, G.J.; Woodfield, T.B. Covalent incorporation of heparin improves chondrogenesis in photocurable gelatinmethacryloyl hydrogels. Macromol. Biosci. 2017, 17, 1700158. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Breitenkamp, K.; Finn, M.G.; Lotz, M.; D’Lima, D.D. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng. Part A 2012, 18, 1304. [Google Scholar] [CrossRef] [PubMed]
- Hockaday, L.A.; Kang, K.H.; Colangelo, N.W.; Cheung, P.Y.; Duan, B.; Malone, E.; Wu, J.L.; Girardi, N.; Bonassar, L.J.; Lipson, H.; et al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication 2012, 4, 035005. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Zhang, J.; McCoard, L.; Xu, X.; Oottamasathien, S.; Prestwich, G.D. Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng. Part A 2010, 16, 2675–2685. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Hutmacher, D.W.; Melchels, F.P.W.; Klein, T.J.; Malda, J. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Pescosolido, L.; Schuurman, W.; Malda, J.; Matricardi, P.; Alhaique, F.; Coviello, T.; van Weeren, P.R.; Dhert, W.J.; Hennink, W.E.; Vermonden, T. Hyaluronic acid and dextran-based semi-IPN hydrogels as biomaterials for bioprinting. Biomacromolecules 2011, 12, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Zhang, J.; Prestwich, G.D. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials 2010, 31, 6173–6181. [Google Scholar] [CrossRef] [PubMed]
- Law, N.; Doney, B.; Glover, H.; Qin, Y.; Aman, Z.M.; Sercombe, T.B.; Liew, L.J.; Dilley, R.J.; Doyle, B.J. Characterisation of hyaluronic acid methylcellulose hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 77, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Suri, S.; Han, L.-H.; Zhang, W.; Singh, A.; Chen, S.; Schmidt, C.E. Solid freeform fabrication of designer scaffold of hyaluronic acid for nerve tissue engineering. Biomed. Microdevices 2011, 13, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Censi, R.; Schuurman, W.; Malda, J.; di Dato, G.; Burgisser, P.E.; Dhert, W.J.A.; van Nostrum, C.F.; di Martino, P.; Vermonden, T.; Hennink, W.E. A printable photopolymerizable thermosensitive p(HPMAm-lactate)-PEG hydrogel for tissue engineering. Adv. Funct. Mater. 2011, 21, 1833–1842. [Google Scholar] [CrossRef]
- Lee, S.-J.; Nowicki, M.; Harris, B.; Zhang, L.G. Fabrication of a highly aligned neural scaffold via a table top stereolithography 3D printing and electrospinning. Tissue Eng. Part A 2017, 23, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, F.-Y.; Lin, H.-H.; Hsu, S.-H. 3D bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials 2015, 71, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Eisenberg, H.M.; Jia, X. Advances and future applications of augmented peripheral nerve Regeneration. Int. J. Mol. Sci. 2016, 17, 1494. [Google Scholar] [CrossRef] [PubMed]
- Mobini, S.; Spearman, B.S.; Lacko, C.S.; Schmidt, C.E. Recent advances in strategies for peripheral nerve tissue engineering. Curr. Opin. Biomed. Eng. 2017, 4, 134–142. [Google Scholar] [CrossRef]
- Johnson, B.N.; McAlpine, M.C. From print to patient: 3D-printed personalized nerve regeneration. Biochemist 2016, 38, 28–31. [Google Scholar]




| Hydrogel | Applied Material Oncentration (w/v) | Mechanical Roperties a | Viscosity (Pa/s) | Gelation Method b | Bioprinting Technique c | Cell type Density Cells/mL d | Cytocompatibility /Biodegradability | Refs. |
|---|---|---|---|---|---|---|---|---|
| Alginate | Alginate/Ca2+ 1%–3%/0.5% | Elastic modulus λ = 21.35 kPa | 2.9 at a shear rate of 91 s−1 | IC | Inject | RHECs 500,000 | 83%/yes | [44,45] |
| Sodium alginate 1% | - | 0.12 shear rate not reported | IC | Laser-assisted | HUEVCs (Eahy926), 6 × 107 and Rabbit carcinoma cells (B16) 4 × 107 | High, day 1/- | [46] | |
| Gelatin | Methacrylate GelMA/gelatin 5%/8% | Young’s modulus Y = 4.85 ± 0.41 kPa | 10−100 at a shear rate of 1–500 s−1 | PC | EB | BMSCs 5.0 × 106 | <90%/yes | [47] |
| GelMA/alginate/4-arm PEGTA 5%–7%/1%–3%/1%–3% | Compressive moduli = 24.2–50.7 kPa | 28−54 at a shear rate of 7.74 s−1 0.08 Pa s−1 | PC | Inject | HUVECs MSCs 3 × 106 | 80%–90%, day 7/yes | [48] | |
| GelMA/alginate/Ca2+ 4.5%/1%–4%/0.3–0.6 M | λ = 15 – 55 kPa | 0.08 shear rate not reported | PC/IC | EB | HUVECs- | 75%, day 5/- | [49] | |
| GelMA/GelSH & heparin 10%/1% | Compressive moduli = 1 ± 2 kPa | - | Thiol-ene | - | Human articular chondrocytes 15 × 106 | 74%–86%, week 5/- | [50] | |
| Poly (ethylene glycol) | Dimethacrylate 10%; 20% | Compressive moduli = 395.73 ± 80.40 kPa | - | PC | Inject | Human articular chondrocytes 5 × 106 | 89%, day 1/- | [51] |
| Diacrylate/alginate 20%/12.5% | λ = 5.3 ± 0.9 to 74.6 ± 1.5 kPa | - | PC | EB | PAVIC 20 × 106 | ca. 100%, day 21/- | [52] | |
| Hyaluron-ic acid (HA) | Methacrylate (HA-MA)/GelMA 1.5%/- | Storage modulus G′ = 80–90 Pa Loss modulus G″ = 40 Pa | - | PC | Inject | HepG2 C3A Int-407 NIH 3T3 2.5 × 105 | Cell proliferation p < 0.05/yes | [53] |
| Gelatin-methacrylamide/HA 20%/2.4% | Compressive modulus = 7995 kPa | - | PC | Inject | Chondrocytes 5 × 106 | 82% ± 8%, day 3/yes | [54] | |
| HA/hydroxyethyl-methacrylate derivatized-dextran (dex-HEMA) 2%–6%/10% | G′ = 10 kPa | 70 at a shear rate of 0.1 s−1 and >10 at a shear rate <10 s−1 for 2% HA and 10% DexHEMA | PC | EB | Chondrocytes- | 75%±19%, day 3/yes | [55] | |
| PEG-tetraacrylate/yaluronic acid 3%–5%/1.5%–2.5% | G′ = 100–800 Pa | - | Michael addition | Microcapillary tube-style printing | NIH 3T3; HepG2 C3A; Int 407 25 × 106 | ca. 100%, week 4/yes | [56] | |
| HA/methyl cellulose 0.25%–2.0%/0.5%–9% | G′ = 10–1000 Pa | - | Thermal | EB | MSCs- | 75%, day 15/- | [57] | |
| Hyaluronic acid hydrogels grafted with laminin- | - | - | PC | Photopatterned layer-by-layer | Schwann cells- | Cells retained at 36 h/yes (enzymatically) | [58] | |
| p(HPMAm-lac)-PEG-p(HPMAm-lac) | 25%–35% | λ = 119 kPa | - | Thermal/PC | EB | Chondrocytes 5.0 × 106 | 94%, day 1/yes | [59] |
| Polycaprolactone (PCL) | PCL with gelatin/PEGDA- | Y = 1.43 ± 0.33 mPa | - | PC | Stereolithography and electrospinning | NE-4C NSCs - | Enhancement in cell proliferation, day 5/- | [60] |
| Polyurethane | Polyurethane with PCL 25%–30% | G′ = 680–4000 Pa | - | Supramolecular (hydrogen bonding) | Fused-deposition manufacturing | NSCs 4 × 106 | ca. 100%, day 3/yes | [61] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiti, B.; Díaz Díaz, D. 3D Printed Polymeric Hydrogels for Nerve Regeneration. Polymers 2018, 10, 1041. https://doi.org/10.3390/polym10091041
Maiti B, Díaz Díaz D. 3D Printed Polymeric Hydrogels for Nerve Regeneration. Polymers. 2018; 10(9):1041. https://doi.org/10.3390/polym10091041
Chicago/Turabian StyleMaiti, Binoy, and David Díaz Díaz. 2018. "3D Printed Polymeric Hydrogels for Nerve Regeneration" Polymers 10, no. 9: 1041. https://doi.org/10.3390/polym10091041
APA StyleMaiti, B., & Díaz Díaz, D. (2018). 3D Printed Polymeric Hydrogels for Nerve Regeneration. Polymers, 10(9), 1041. https://doi.org/10.3390/polym10091041