Synthesis and Effect of Structure on Swelling Properties of Hydrogels Based on High Methylated Pectin and Acrylic Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
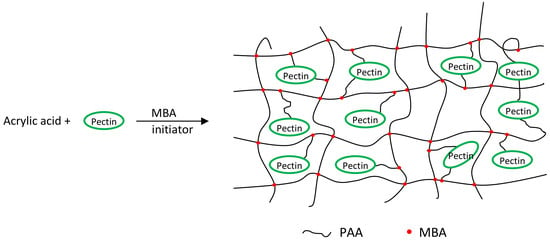
2.2. Pectin-Based Hydrogel Synthesis
2.3. Sol Content Determination
2.4. Molecular Weight and Molecular Mass Distribution of Water Soluble Fraction
2.5. FT-IR Spectroscopy
2.6. Swelling Properties
- Wt—swelling ratio at time t [g H2O/g dry gel]
- Mw—weight of the swollen hydrogel at time t [g]
- Mp—weight of the dried gel [g]
2.7. Thermal Characterization
2.8. Statistical Analyses
3. Results and Discussion
3.1. Swelling Characteristic of Poly(Acrylic Acid)/Pectin Hydrogels
3.2. Analysis of Sol Content in Pectin-Based Hydrogels
3.3. GPC Analysis of the Soluble Fraction
3.4. Thermal Analysis
3.5. FT–IR Spectra of Poly(Acrylic Acid)/Pectin Hydrogels
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Raafat, A.I.; Eid, M.; El-Arnaouty, M.B. Radiation synthesis of superabsorbent CMC based hydrogels for agriculture applications. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2012, 283, 71–76. [Google Scholar] [CrossRef]
- Bai, W.; Zhang, H.; Liu, B.; Wu, Y.; Song, J. Effects of super-absorbent polymers on the physical and chemical properties of soil following different wetting and drying cycles: Effects of SAPs on soil properties. Soil Use Manag. 2010, 26, 253–260. [Google Scholar] [CrossRef]
- Bajpai, A.; Giri, A. Water sorption behaviour of highly swelling (carboxy methylcellulose-g-polyacrylamide) hydrogels and release of potassium nitrate as agrochemical. Carbohydr. Polym. 2003, 53, 271–279. [Google Scholar] [CrossRef]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. A review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Peppas, N. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Tomić, S.L.; Mićić, M.M.; Dobić, S.N.; Filipović, J.M.; Suljovrujić, E.H. Smart poly(2-hydroxyethyl methacrylate/itaconic acid) hydrogels for biomedical application. Radiat. Phys. Chem. 2010, 79, 643–649. [Google Scholar] [CrossRef]
- Murphy, S.V.; Skardal, A.; Atala, A. Evaluation of hydrogels for bio-printing applications. J. Biomed. Mater. Res. Part A 2013, 101A, 272–284. [Google Scholar] [CrossRef]
- Li, Y.; Huang, G.; Zhang, X.; Li, B.; Chen, Y.; Lu, T.; Lu, T.J.; Xu, F. Magnetic Hydrogels and Their Potential Biomedical Applications. Adv. Funct. Mater. 2013, 23, 660–672. [Google Scholar] [CrossRef]
- Song, X.; Wang, K.; Tang, C.-Q.; Yang, W.-W.; Zhao, W.-F.; Zhao, C.-S. Design of Carrageenan-Based Heparin-Mimetic Gel Beads as Self-Anticoagulant Hemoperfusion Adsorbents. Biomacromolecules 2018, 19, 1966–1978. [Google Scholar] [CrossRef]
- Zhao, W.; Glavas, L.; Odelius, K.; Edlund, U.; Albertsson, A.-C. A robust pathway to electrically conductive hemicellulose hydrogels with high and controllable swelling behavior. Polymer 2014, 55, 2967–2976. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Song, X.; Shi, Z.; Tang, C.; Xiong, L.; Zhao, W.; Zhao, C. Reinforced-Concrete Structured Hydrogel Microspheres with Ultrahigh Mechanical Strength, Restricted Water Uptake, and Superior Adsorption Capacity. ACS Sustain. Chem. Eng. 2018, 6, 5950–5958. [Google Scholar] [CrossRef]
- Kulicke, W.-M.; Eidam, D.; Kath, F.; Kix, M.; Kull, A.H. Hydrocolloids and Rheology: Regulation of Visco-elastic Characteristics of Waxy Rice Starch in Mixtures with Galactomannans. Starch-Starke 1996, 48, 105–114. [Google Scholar] [CrossRef]
- Athawale, V.D.; Lele, V. Recent Trends in Hydrogels Based on Starchgraft-Acrylic Acid: A Review. Starch-Stärke 2001, 53, 7–13. [Google Scholar] [CrossRef]
- Kaur, I.; Sharma, M. Synthesis and characterization of graft copolymers of Sago starch and acrylic acid. Starch-Stärke 2012, 64, 441–451. [Google Scholar] [CrossRef]
- Ma, X.; Wei, R.; Cheng, J.; Cai, J.; Zhou, J. Synthesis and characterization of pectin/poly (sodium acrylate) hydrogels. Carbohydr. Polym. 2011, 86, 313–319. [Google Scholar] [CrossRef]
- Parvathy, P.C.; Jyothi, A.N. Synthesis, characterization and swelling behaviour of superabsorbent polymers from cassava starch-graft-poly(acrylamide). Starch-Stärke 2012, 64, 207–218. [Google Scholar] [CrossRef]
- Vityazev, F.V.; Fedyuneva, M.I.; Golovchenko, V.V.; Patova, O.A.; Ipatova, E.U.; Durnev, E.A.; Martinson, E.A.; Litvinets, S.G. Pectin-silica gels as matrices for controlled drug release in gastrointestinal tract. Carbohydr. Polym. 2017, 157, 9–20. [Google Scholar] [CrossRef]
- Kowalski, G.; Ptaszek, P. The effect of swelling time on rheological properties of hydrogels, consisting of high -amylose carboxymethyl corn starch and acrylic polymers: Starch acrylic hydrogels. Starch-Stärke 2016, 68, 381–388. [Google Scholar] [CrossRef]
- Buchholz, F.L.; Graham, A.T. Modern Superabsorbent Polymer Technology; Wiley-VCH: New York, NY, USA, 1998; ISBN 0-471-19411-5. [Google Scholar]
- Neufeld, L.; Bianco-Peled, H. Pectin—Chitosan physical hydrogels as potential drug delivery vehicles. Int. J. Biol. Macromol. 2017, 101, 852–861. [Google Scholar] [CrossRef]
- Almeida, E.A.M.S.; Bellettini, I.C.; Garcia, F.P.; Farinácio, M.T.; Nakamura, C.V.; Rubira, A.F.; Martins, A.F.; Muniz, E.C. Curcumin-loaded dual pH- and thermo-responsive magnetic microcarriers based on pectin maleate for drug delivery. Carbohydr. Polym. 2017, 171, 259–266. [Google Scholar] [CrossRef]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.N.; Ferreira, L.M.B.; Cardoso, V.M.O.; Boni, F.I.; Souza, A.L.R.; Gremião, M.P.D. Recent advances in smart hydrogels for biomedical applications: From self-assembly to functional approaches. Eur. Polym. J. 2018, 99, 117–133. [Google Scholar] [CrossRef]
- Jing, G.; Wang, L.; Yu, H.; Amer, W.A.; Zhang, L. Recent progress on study of hybrid hydrogels for water treatment. Colloids Surf. A Physicochem. Eng. Aspects 2013, 416, 86–94. [Google Scholar] [CrossRef]
- Chauhan, G.S.; Kumari, A.; Sharma, R. Pectin and acrylamide based hydrogels for environment management technologies: Synthesis, characterization, and metal ions sorption. J. Appl. Polym. Sci. 2007, 106, 2158–2168. [Google Scholar] [CrossRef]
- Liu, L.; Cooke, P.H.; Coffin, D.R.; Fishman, M.L.; Hicks, K.B. Pectin and polyacrylamide composite hydrogels: Effect of pectin on structural and dynamic mechanical properties. J. Appl. Polym. Sci. 2004, 92, 1893–1901. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Barzegar, S. Smart Pectin-based Superabsorbent Hydrogel as a Matrix for Ibuprofen as an Oral Non-steroidal Anti-inflammatory Drug Delivery. Starch-Stärke 2009, 61, 173–187. [Google Scholar] [CrossRef]
- Fares, M.M.; Assaf, S.M.; Abul-Haija, Y.M. Pectin grafted poly(N-vinylpyrrolidone): Optimization and in vitro controllable theophylline drug release. J. Appl. Polym. Sci. 2010, 117, 1945–1954. [Google Scholar] [CrossRef]
- Peppas, N.A. (Ed.) Hydrogels in Medicine and Pharmacy; CRC Press: Boca Raton, FL, USA, 1986; ISBN 978-0-8493-5546-2. [Google Scholar]
- Pourjavadi, A.; Barzegar, S.; Zeidabadi, F. Synthesis and properties of biodegradable hydrogels of κ-carrageenan grafted acrylic acid-co-2-acrylamido-2-methylpropanesulfonic acid as candidates for drug delivery systems. React. Funct. Polym. 2007, 67, 644–654. [Google Scholar] [CrossRef]
- Wu, J.; Lin, J.; Li, G.; Wei, C. Influence of the COOH and COONa groups and crosslink density of poly(acrylic acid)/montmorillonite superabsorbent composite on water absorbency. Polym. Int. 2001, 50, 1050–1053. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Brzeźnicki, S.; Bonczarowska, M.; Gromiec, J. Oznaczanie kwasu akrylowego w powietrzu środowiska pracy metodą wysokosprawnej chromatografii cieczowej. Podstawy i Metody Oceny Środowiska Pracy 2013, 75, 139–151. [Google Scholar] [CrossRef]
- Lawal, O.S.; Lechner, M.D.; Hartmann, B.; Kulicke, W.-M. Carboxymethyl Cocoyam Starch: Synthesis, Characterisation and Influence of Reaction Parameters. Starch-Stärke 2007, 59, 224–233. [Google Scholar] [CrossRef]
- Passauer, L.; Liebner, F.; Fischer, K. Starch Phosphate Hydrogels. Part I: Synthesis by Mono-phosphorylation and Cross-linking of Starch. Starch-Stärke 2009, 61, 621–627. [Google Scholar] [CrossRef]
- Scranton, A.B.; Klier, J.; Peppas, N.A. Soluble chain fractions in hydrophilic polymer networks: Origin and effect on dynamic uptake overshoots. Polymer 1990, 31, 1288–1293. [Google Scholar] [CrossRef]
- Chauhan, K.; Kumar, R.; Kumar, M.; Sharma, P.; Chauhan, G.S. Modified pectin-based polymers as green antiscalants for calcium sulfate scale inhibition. Desalination 2012, 305, 31–37. [Google Scholar] [CrossRef]
- Cui, S.; Yao, B.; Gao, M.; Sun, X.; Gou, D.; Hu, J.; Zhou, Y.; Liu, Y. Effects of pectin structure and crosslinking method on the properties of crosslinked pectin nanofibers. Carbohydr. Polym. 2017, 157, 766–774. [Google Scholar] [CrossRef]
- Mishra, R.K.; Datt, M.; Pal, K.; Banthia, A.K. Preparation and characterization of amidated pectin based hydrogels for drug delivery system. J. Mater. Sci. Mater. Med. 2008, 19, 2275–2280. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S.; Al-Deyab, S.S. Preparation of poly(acrylic acid)/starch hydrogel and its application for cadmium ion removal from aqueous solutions. React. Funct. Polym. 2014, 75, 1–8. [Google Scholar] [CrossRef]
- Sinitsya, A.; Čopíková, J.; Prutyanov, V.; Skoblya, S.; Machovič, V. Amidation of highly methoxylated citrus pectin with primary amines. Carbohydr. Polym. 2000, 42, 359–368. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds: Tables of Spectral Data, 4th ed.; Springer: Berlin, Germany, 2009; ISBN 978-3-540-93809-5. [Google Scholar]







| Sample | Pectin, g | MBA, g | AA, g |
|---|---|---|---|
| P1 | 5 | 0.0048 | 30 |
| P2 | 0.0098 | ||
| P3 | 0.0188 | ||
| P4 | 0.0293 | ||
| P5 | 0.0504 | ||
| P6 | 0.0804 | ||
| P3A | 0 | 0.0188 | |
| P3B | 1 | ||
| P3C | 2 | ||
| P3D | 3 | ||
| P3E | 5 | ||
| P3F | 7 | ||
| P3G | 10 |
| Sample | Mn × 10−5, g/mol | Mw × 10−5, g/mol | Ɖ |
|---|---|---|---|
| Pectin | 0.88 | 7.60 | 8.6 |
| P1 | 0.55 | 1.39 | 2.5 |
| P2 | 0.40 | 0.92 | 2.3 |
| P3 | 0.54 | 1.55 | 2.8 |
| P4 | 0.48 | 1.60 | 3.3 |
| P5 | 0.85 | 2.92 | 3.4 |
| P6 | 0.73 | 2.97 | 4.1 |
| P3A | 0.53 | 1.66 | 3.1 |
| P3B | 0.58 | 1.94 | 3.4 |
| P3C | 0.61 | 2.05 | 3.4 |
| P3D | 0.84 | 3.45 | 4.1 |
| P3E | 0.54 | 1.63 | 3.0 |
| P3F | 0.73 | 3.33 | 4.6 |
| P3G | 0.71 | 2.63 | 3.7 |
| Sample | Pectin, g/100 g | MBA, g/100 g | TON, °C | TMID, °C | TINF, °C | TEND, °C | TEND—TON, °C | ΔCP, J·g−1·K−1 |
|---|---|---|---|---|---|---|---|---|
| P1 | 5 | 0.0048 | 66.7 ± 0.2 c | 74.9 ± 0.1 d | 75.6 ± 0.1 c | 81.5 ± 0.2 c | 14.8 ± 0.3 bc | 0.613 ± 0.009 c |
| P2 | 5 | 0.0098 | 69.9 ± 0.2 e | 79.0 ± 0.1 j | 80.0 ± 0.3 g | 86.5 ± 0.2 g | 16.6 ± 0.3 e | 0.660 ± 0.001 d |
| P3 | 5 | 0.0188 | 67.0 ± 0.1 c | 75.4 ± 0.0 e | 76.3 ± 0.1 d | 82.5 ± 0.1 d | 15.5 ± 0.2 cd | 0.615 ± 0.000 c |
| P4 | 5 | 0.0293 | 64.8 ± 0.0 b | 73.0 ± 0.0 b | 73.6 ± 0.2 a | 79.7 ± 0.1 b | 14.9 ± 0.1 bc | 0.621 ± 0.011 c |
| P5 | 5 | 0.0504 | 73.7 ± 0.7 f | 82.9 ± 0.1 k | 84.1 ± 0.1 h | 90.4 ± 0.2 h | 16.7 ± 0.7 e | 0.672 ± 0.004 d |
| P6 | 5 | 0.0804 | 63.4 ± 0.3 a | 71.9 ± 0.1 a | 73.1 ± 0.3 a | 78.9 ± 0.0 a | 15.5 ± 0.3 cd | 0.621 ± 0.001 c |
| P3A | 0 | 0.0183 | 78.1 ± 0.1 g | 85.0 ± 0.0 l | 86.7 ± 0.1 i | 91.1 ± 0.1 i | 13.1 ± 0.2 a | 0.742 ± 0.009 g |
| P3B | 1 | 0.0181 | 69.3 ± 0.1 e | 77.1 ± 0.1 h | 78.4 ± 0.1 f | 83.4 ± 0.1 ef | 14.1 ± 0.2 b | 0.715 ± 0.010 f |
| P3C | 2 | 0.0185 | 69.7 ± 0.2 e | 77.4 ± 0.0 i | 78.0 ± 0.2 f | 83.8 ± 0.1 f | 14.1 ± 0.2 b | 0.690 ± 0.004 e |
| P3D | 3 | 0.0185 | 68.0 ± 0.2 d | 75.7 ± 0.1 f | 76.9 ± 0.4 e | 82.2 ± 0.4 d | 14.2 ± 0.4 b | 0.665 ± 0.002 d |
| P3E | 5 | 0.0188 | 67.0 ± 0.1 c | 75.4 ± 0.0 e | 76.3 ± 0.1 d | 82.5 ± 0.01 d | 15.5 ± 0.2 cd | 0.615 ± 0.0000 c |
| P3F | 7 | 0.0181 | 67.1 ± 0.1 c | 76.1 ± 0.1 g | 76.9 ± 0.0 e | 83.4 ± 0.1 e | 16.3 ± 0.2 de | 0.577 ± 0.010 b |
| P3G | 10 | 0.0178 | 65.0 ± 0.2 b | 74.4 ± 0.2 c | 75.1 ± 0.2 b | 81.7 ± 0.1 c | 16.8 ± 0.3 e | 0.504 ± 0.010 a |
| One-way ANOVA—p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Sample | Pectin [g/100 g] | MBA [g/100 g] | TON, °C | TP, °C | TEND, °C | TEND—TON, °C | −ΔH, J·g−1 |
|---|---|---|---|---|---|---|---|
| P1 | 5 | 0.0048 | 189.5 ± 1.0 abcd | 192.2 ± 1.1 bc | 200.0 ± 0.6 b | 10.5 ± 0.5 | 185.6 ± 10.0 cd |
| P2 | 5 | 0.0098 | 191.1 ± 1.3 bcde | 193.7 ± 1.1 bcde | 200.8 ± 1.7 b | 9.7 ± 0.6 | 173.2 ± 1.3 e |
| P3 | 5 | 0.0188 | 188.4 ± 0.6 ab | 191.8 ± 1.0 ab | 200.9 ± 0.1 bc | 12.5 ± 0.9 | 183.9 ± 1.6 d |
| P4 | 5 | 0.0293 | 188.1 ± 1.0 ab | 191.3 ± 1.1 ab | 200.9 ± 0.2 b | 12.8 ± 1.3 | 194.1 ± 2.3 b |
| P5 | 5 | 0.0504 | 192.6 ± 0.9 de | 195.1 ± 1.1 de | 202.2 ± 0.5 bc | 9.6 ± 1.6 | 163.8 ± 2.7 f |
| P6 | 5 | 0.0804 | 189.3 ± 1.8 abc | 192.3 ± 2.1 bcd | 200.5 ± 1.7 b | 11.3 ± 0.4 | 193.9 ± 2.3 bc |
| P3A | 0 | 0.0183 | 197.8 ± 0.5 f | 200.4 ± 0.6 f | 207.9 ± 0.3 d | 10.1 ± 0.3 | 120.2 ± 0.1 h |
| P3B | 1 | 0.0181 | 193.8 ± 1.6 e | 196.2 ± 1.2 e | 203.7 ± 1.6 c | 10.0 ± 0.4 | 143.9 ± 6.0 g |
| P3C | 2 | 0.0185 | 192.3 ± 0.1 cde | 194.8 ± 0.3 cde | 201.9 ± 1.7 bc | 9.6 ± 2.0 | 159.0 ± 3.5 f |
| P3D | 3 | 0.0185 | 189.1 ± 2.6 ab | 191.9 ± 3.3 b | 199.8 ± 0.9 b | 10.8 ± 2.5 | 165.7 ± 1.8 ef |
| P3E | 5 | 0.0188 | 188.4 ± 0.6 ab | 191.8 ± 1.0 ab | 200.9 ± 0.1 b | 12.5 ± 0.9 | 183.9 ± 1.6 d |
| P3F | 7 | 0.0181 | 189.8 ± 0.6 bcd | 192.4 ± 0.1 bcd | 200.2 ± 1.9 b | 10.4 ± 2.0 | 184.0 ± 0.3 d |
| P3G | 10 | 0.0178 | 186.4 ± 1.7 a | 188.9 ± 1.8 a | 196.55 ± 1.9 a | 10.15 ± 0.0 | 203.8 ± 2.2 a |
| One-way ANOVA—p | <0.001 | <0.001 | <0.001 | 0.307 | <0.001 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalski, G.; Kijowska, K.; Witczak, M.; Kuterasiński, Ł.; Łukasiewicz, M. Synthesis and Effect of Structure on Swelling Properties of Hydrogels Based on High Methylated Pectin and Acrylic Polymers. Polymers 2019, 11, 114. https://doi.org/10.3390/polym11010114
Kowalski G, Kijowska K, Witczak M, Kuterasiński Ł, Łukasiewicz M. Synthesis and Effect of Structure on Swelling Properties of Hydrogels Based on High Methylated Pectin and Acrylic Polymers. Polymers. 2019; 11(1):114. https://doi.org/10.3390/polym11010114
Chicago/Turabian StyleKowalski, Grzegorz, Karolina Kijowska, Mariusz Witczak, Łukasz Kuterasiński, and Marcin Łukasiewicz. 2019. "Synthesis and Effect of Structure on Swelling Properties of Hydrogels Based on High Methylated Pectin and Acrylic Polymers" Polymers 11, no. 1: 114. https://doi.org/10.3390/polym11010114