PEG-assisted Sol-gel Synthesis of Compact Nickel Oxide Hole-Selective Layer with Modified Interfacial Properties for Organic Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Compact Nickel Oxide Layer
2.2. Fabrication of Photovoltaic Devices
2.3. Characterization
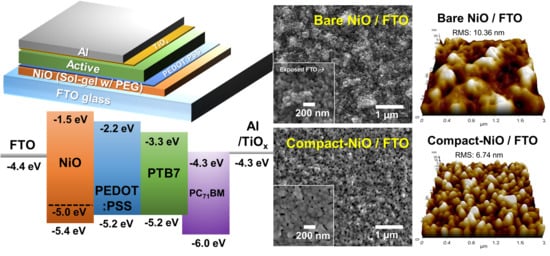
3. Results and Discussion
4. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Li, G.; Zhu, R.; Yang, Y. Polymer solar cells. Nat. Photonics 2012, 6, 153. [Google Scholar] [CrossRef]
- Wang, D.H.; Kim, J.K.; Seo, J.H.; Park, I.; Hong, B.H.; Park, J.H.; Heeger, A.J. Transferable graphene oxide by stamping nanotechnology: Electron-transport layer for efficient bulk-heterojunction solar cells. Angew. Chem. 2013, 125, 2946–2952. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, S.J.; Park, M.J.; Bae, S.; Cho, S.-P.; Du, Q.G.; Wang, D.H.; Park, J.H.; Hong, B.H. Surface-engineered graphene quantum dots incorporated into polymer layers for high performance organic photovoltaics. Sci. Rep. 2015, 5, 14276. [Google Scholar] [CrossRef] [PubMed]
- Sariciftci, N.S.; Smilowitz, L.; Heeger, A.J.; Wudl, F. Photoinduced electron transfer from a conducting polymer to buckminsterfullerene. Science 1992, 258, 1474–1476. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zeng, D.; Gao, X.; Li, P.; Zhang, Q.; Peng, X. Non-fullerene polymer acceptors based on perylene diimides in all-polymer solar cells. Sol. Energy Mater. Sol. Cells 2019, 189, 103–117. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, Y.; Zhang, Q.; Gao, X. Non-fullerene small molecule acceptors based on perylene diimides. J. Mater. Chem. A 2016, 4, 17604–17622. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; Shao, M.; Wu, Y.; Zeng, D.; Cai, X.; Duan, J.; Zhang, X.; Gao, X. Fine-tuning the quasi-3D geometry: Enabling efficient nonfullerene organic solar cells based on perylene diimides. ACS Appl. Mater. Interfaces 2018, 10, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kyu Kim, J.; Lim, Y.; Park, I.; Suk Choi, Y.; Hyeok Park, J. Tungsten oxide/pedot:Pss hybrid cascade hole extraction layer for polymer solar cells with enhanced long-term stability and power conversion efficiency. Sol. Energy Mater. Sol. Cells 2014, 122, 24–30. [Google Scholar] [CrossRef]
- Menke, S.M.; Ran, N.A.; Bazan, G.C.; Friend, R.H. Understanding energy loss in organic solar cells: Toward a new efficiency regime. Joule 2018, 2, 25–35. [Google Scholar] [CrossRef]
- Yin, C.; Pieper, B.; Stiller, B.; Kietzke, T.; Neher, D. Charge carrier generation and electron blocking at interlayers in polymer solar cells. Appl. Phys. Lett. 2007, 90, 133502. [Google Scholar] [CrossRef]
- Jin, F.; Su, Z.; Chu, B.; Cheng, P.; Wang, J.; Zhao, H.; Gao, Y.; Yan, X.; Li, W. Interface engineering of organic schottky barrier solar cells and its application in enhancing performances of planar heterojunction solar cells. Sci. Rep. 2016, 6, 26262. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gupta, S.K.; Garg, A. Inkjet printing of nio films and integration as hole transporting layers in polymer solar cells. Sci. Rep. 2017, 7, 1775. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, I.; Kim, W.; Wang, D.H.; Choi, D.-G.; Choi, Y.S.; Park, J.H. Enhanced performance and stability of polymer bhj photovoltaic devices from dry transfer of pedot:Pss. ChemSusChem 2014, 7, 1957–1963. [Google Scholar] [CrossRef]
- Lee, J.Y. Indium blocking in polymer light emitting diodes with a crosslinked poly(3,4-ethylenedioxythiophene)/silane hole transport layer. Synth. Met. 2006, 156, 537–540. [Google Scholar] [CrossRef]
- Carlé, J.E.; Helgesen, M.; Hagemann, O.; Hösel, M.; Heckler, I.M.; Bundgaard, E.; Gevorgyan, S.A.; Søndergaard, R.R.; Jørgensen, M.; García-Valverde, R.; et al. Overcoming the scaling lag for polymer solar cells. Joule 2017, 1, 274–289. [Google Scholar] [CrossRef]
- Emmott, C.J.M.; Röhr, J.A.; Campoy-Quiles, M.; Kirchartz, T.; Urbina, A.; Ekins-Daukes, N.J.; Nelson, J. Organic photovoltaic greenhouses: A unique application for semi-transparent PV? Energy Environ. Sci. 2015, 8, 1317–1328. [Google Scholar] [CrossRef]
- Andersson, A.; Johansson, N.; Bröms, P.; Yu, N.; Lupo, D.; Salaneck, W.R. Fluorine tin oxide as an alternative to indium tin oxide in polymer leds. Adv. Mater. 1998, 10, 859–863. [Google Scholar] [CrossRef]
- Docampo, P.; Ball, J.M.; Darwich, M.; Eperon, G.E.; Snaith, H.J. Efficient organometal trihalide perovskite planar-heterojunction solar cells on flexible polymer substrates. Nat. Commun. 2013, 4, 2761. [Google Scholar] [CrossRef] [Green Version]
- Van de Krol, R.; Grätzel, M. Photoelectrochemical Hydrogen Production; Springer: New York, NY, USA, 2011. [Google Scholar]
- Kim, W.; Kim, S.; Kang, I.; Jung, M.S.; Kim, S.J.; Kim, J.K.; Cho, S.M.; Kim, J.-H.; Park, J.H. Hybrid silver mesh electrode for ITO-free flexible polymer solar cells with good mechanical stability. ChemSusChem 2016, 9, 1042–1049. [Google Scholar] [CrossRef]
- Wang, D.H.; Kyaw, A.K.K.; Gupta, V.; Bazan, G.C.; Heeger, A.J. Enhanced efficiency parameters of solution-processable small-molecule solar cells depending on ito sheet resistance. Adv. Energy Mater. 2013, 3, 1161–1165. [Google Scholar] [CrossRef]
- Wang, K.-C.; Jeng, J.-Y.; Shen, P.-S.; Chang, Y.-C.; Diau, E.W.-G.; Tsai, C.-H.; Chao, T.-Y.; Hsu, H.-C.; Lin, P.-Y.; Chen, P.; et al. P-type mesoscopic nickel oxide/organometallic perovskite heterojunction solar cells. Sci. Rep. 2014, 4, 4756. [Google Scholar] [CrossRef] [PubMed]
- Ndione, P.F.; Garcia, A.; Widjonarko, N.E.; Sigdel, A.K.; Steirer, K.X.; Olson, D.C.; Parilla, P.A.; Ginley, D.S.; Armstong, N.R.; Richards, R.E.; et al. Highly-tunable nickel cobalt oxide as a low-temperature P-type contact in organic photovoltaic devices. Adv. Energy Mater. 2013, 3, 524–531. [Google Scholar] [CrossRef]
- Manders, J.R.; Tsang, S.-W.; Hartel, M.J.; Lai, T.-H.; Chen, S.; Amb, C.M.; Reynolds, J.R.; So, F. Solution-processed nickel oxide hole transport layers in high efficiency polymer photovoltaic cells. Adv. Funct. Mater. 2013, 23, 2993–3001. [Google Scholar] [CrossRef]
- Irwin, M.D.; Buchholz, D.B.; Hains, A.W.; Chang, R.P.H.; Marks, T.J. P-type semiconducting nickel oxide as an efficiency-enhancing anode interfacial layer in polymer bulk-heterojunction solar cells. Proc. Natl. Acad. Sci. USA 2008, 105, 2783–2787. [Google Scholar] [CrossRef]
- Zhai, Z.; Huang, X.; Xu, M.; Yuan, J.; Peng, J.; Ma, W. Greatly reduced processing temperature for a solution-processed niox buffer layer in polymer solar cells. Adv. Energy Mater. 2013, 3, 1614–1622. [Google Scholar] [CrossRef]
- Steirer, K.X.; Chesin, J.P.; Widjonarko, N.E.; Berry, J.J.; Miedaner, A.; Ginley, D.S.; Olson, D.C. Solution deposited nio thin-films as hole transport layers in organic photovoltaics. Org. Electron. 2010, 11, 1414–1418. [Google Scholar] [CrossRef]
- Pilban Jahromi, S.; Huang, N.M.; Kamalianfar, A.; Lim, H.N.; Muhamad, M.R.; Yousefi, R. Facile synthesis of porous-structured nickel oxide thin film by pulsed laser deposition. J. Nanomater. 2012, 2012, 97. [Google Scholar] [CrossRef]
- Kwon, U.; Kim, B.-G.; Nguyen, D.C.; Park, J.-H.; Ha, N.Y.; Kim, S.-J.; Ko, S.H.; Lee, S.; Lee, D.; Park, H.J. Solution-processible crystalline NiO nanoparticles for high-performance planar perovskite photovoltaic cells. Sci. Rep. 2016, 6, 30759. [Google Scholar] [CrossRef]
- Chen, Y.S.; Kang, J.F.; Chen, B.; Gao, B.; Liu, L.F.; Liu, X.Y.; Wang, Y.Y.; Wu, L.; Yu, H.Y.; Wang, J.Y.; et al. Microscopic mechanism for unipolar resistive switching behaviour of nickel oxides. J. Phys. D Appl. Phys. 2012, 45, 065303. [Google Scholar] [CrossRef]
- Patil, R.A.; Chang, C.-P.; Devan, R.S.; Liou, Y.; Ma, Y.-R. Impact of nanosize on supercapacitance: Study of 1D nanorods and 2D thin-films of nickel oxide. ACS Appl. Mater. Interfaces 2016, 8, 9872–9880. [Google Scholar] [CrossRef]
- Kim, J.K.; Shin, K.; Cho, S.M.; Lee, T.-W.; Park, J.H. Synthesis of transparent mesoporous tungsten trioxide films with enhanced photoelectrochemical response: Application to unassisted solar water splitting. Energy Environ. Sci. 2011, 4, 1465–1470. [Google Scholar] [CrossRef]
- Kim, B.; Jang, W.; Wang, D. Facile niox sol-gel synthesis depending on chain length of various solvents without catalyst for efficient hole charge transfer in perovskite solar cells. Polymers 2018, 10, 1227. [Google Scholar] [CrossRef]
- Kim, J.K.; Chai, S.U.; Ji, Y.; Levy-Wendt, B.; Kim, S.H.; Yi, Y.; Heinz, T.F.; Nørskov, J.K.; Park, J.H.; Zheng, X. Resolving hysteresis in perovskite solar cells with rapid flame-processed cobalt-doped TiO2. Adv. Energy Mater. 2018, 8, 1801717. [Google Scholar] [CrossRef]
- Wang, D.H.; Choi, D.G.; Lee, K.-J.; Park, O.O.; Park, J.H. Active layer transfer by stamping technique for polymer solar cells: Synergistic effect of tiox interlayer. Org. Electron. 2010, 11, 599–603. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, K.M.; Jang, W.; Ahn, S.; Kim, Y.Y.; Park, O.O.; Wang, D.H. Vacuum-process-based dry transfer of active layer with solvent additive for efficient organic photovoltaic devices. J. Mater. Chem. C 2017, 5, 1106–1112. [Google Scholar] [CrossRef]
- Kim, J.K.; Cho, Y.; Jeong, M.J.; Levy-Wendt, B.; Shin, D.; Yi, Y.; Wang, D.H.; Zheng, X.; Park, J.H. Rapid formation of a disordered layer on monoclinic BiVO4: Co-catalyst-free photoelectrochemical solar water splitting. ChemSusChem 2018, 11, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Hu, L.; Yun, J.S.; Zhang, M.; Lau, C.F.J.; Bing, J.; Deng, X.; Ma, Q.; Cho, Y.; Fu, W.; et al. Solution-processed, silver-doped niox as hole transporting layer for high-efficiency inverted perovskite solar cells. ACS Appl. Energy Mater. 2018, 1, 561–570. [Google Scholar] [CrossRef]
- Karthikeyan, B.; Pandiyarajan, T.; Hariharan, S.; Ollakkan, M.S. Wet chemical synthesis of diameter tuned nio microrods: Microstructural, optical and optical power limiting applications. CrystEngComm 2016, 18, 601–607. [Google Scholar] [CrossRef]
- Rawool, S.A.; Pai, M.R.; Banerjee, A.M.; Arya, A.; Ningthoujam, R.S.; Tewari, R.; Rao, R.; Chalke, B.; Ayyub, P.; Tripathi, A.K.; et al. Pn heterojunctions in NiO:TiO2 composites with type-II band alignment assisting sunlight driven photocatalytic H2 generation. Appl. Catal. B Environ. 2018, 221, 443–458. [Google Scholar] [CrossRef]





© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.K. PEG-assisted Sol-gel Synthesis of Compact Nickel Oxide Hole-Selective Layer with Modified Interfacial Properties for Organic Solar Cells. Polymers 2019, 11, 120. https://doi.org/10.3390/polym11010120
Kim JK. PEG-assisted Sol-gel Synthesis of Compact Nickel Oxide Hole-Selective Layer with Modified Interfacial Properties for Organic Solar Cells. Polymers. 2019; 11(1):120. https://doi.org/10.3390/polym11010120
Chicago/Turabian StyleKim, Jung Kyu. 2019. "PEG-assisted Sol-gel Synthesis of Compact Nickel Oxide Hole-Selective Layer with Modified Interfacial Properties for Organic Solar Cells" Polymers 11, no. 1: 120. https://doi.org/10.3390/polym11010120
APA StyleKim, J. K. (2019). PEG-assisted Sol-gel Synthesis of Compact Nickel Oxide Hole-Selective Layer with Modified Interfacial Properties for Organic Solar Cells. Polymers, 11(1), 120. https://doi.org/10.3390/polym11010120