Chiral Bifunctional Thioureas and Squaramides Grafted into Old Polymers of Intrinsic Microporosity for Novel Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Techniques
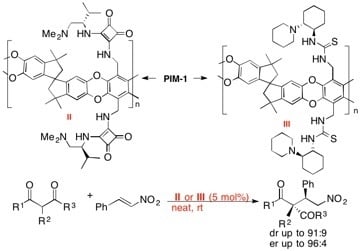
2.3. Synthesis of Grafted PIMs (I-VI)
2.3.1. Synthesis of Valine-Derived Thioureas (I) and (IV)
2.3.2. Synthesis of Valine-Derived Squaramides (II) and (V)
2.3.3. Synthesis of Cyclohexanediamine-Derived Thioureas (III) and (VI)
2.4. General Procedure for Asymmetric Reactions
2.4.1. General Procedure for Stereoselective Nitro-Michael Addition
2.4.2. General Procedure for Preparation of 2-Amino-4-(nitromethyl)-4H-chromene-3-carbonitrile from 2-(2-Nitrovinyl)phenol Derivative
2.5. Recyclability of the Grafted PIM’s Catalysts in the Asymmetric Reactions
3. Results and Discussion
3.1. Polymerization, Post-Modification, and Structural Characterization of PIMs
3.2. Evaluation of Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Budd, P.M.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E. Polymers of intrinsic porosity (PIMs): Robust, solution-processable, organic nanoporous materials. Chem. Commun. 2004, 2, 230–231. [Google Scholar] [CrossRef] [PubMed]
- McKeown, N.B.; Budd, P.M.; Msayib, K.J.; Ghanem, B.S.; Kingston, H.J.; Tattershall, C.E.; Makhseed, S.; Reynolds, K.J.; Friitsch, D. Polymers of Intrinsic Micropporosity (PIMs): Bridging the Void between Microporous and Polymeric Materials. Chem. Eur. J. 2005, 11, 2610–2620. [Google Scholar] [CrossRef] [PubMed]
- McKeown, N.B.; Budd, P.M. Exploitation of Intrinsic Microporosity in Polymer-Based Materials. Macromolecules 2010, 43, 5163–5176. [Google Scholar] [CrossRef]
- Dawson, R.; Cooper, A.I.; Adams, D.J. Nanoporous organic polymer networks. Progr. Polym. Sci. 2012, 37, 530–563. [Google Scholar] [CrossRef]
- Budd, P.M.; Msayib, K.J.; Tettershall, C.E.; Ghanem, B.S.; Reynolds, K.J.; McKeown, N.B.; Fritsch, D. Gas separation membranes from polymers of intrinsic microporosity. J. Membr. Sci. 2005, 251, 263–269. [Google Scholar] [CrossRef]
- Budd, P.M.; McKeown, N.B.; Ghanem, B.S.; Msayib, K.J.; Fritsch, D.; Starannikova, L.; Belov, N.; Sanfirova, O.; Yampolskii, Y.; Shantarovich, V. Gas permeation parameters and other physicochemical properties of a polymer of intrinsic microporosity: Polybenzodioxane PIM-1. J. Membr. Sci. 2008, 325, 851–860. [Google Scholar] [CrossRef]
- Ghanem, B.S.; McKeown, N.B.; Budd, P.M.; Selvie, J.D.; Fritsch, D. High-Performance Membranes from Polyimides with Intrinsic Microporosity. Adv. Mater. 2008, 20, 2766–2771. [Google Scholar] [CrossRef]
- Du, N.; Rpbertson, G.P.; Song, J.; Pinnau, I.; Thomas, S.; Guiver, M.D. Polymers of Intrinsic Microporosity Containig Trifluoromethyl and Phenylsulfone Groups as Materials for Membrane Gas Separation. Macromolecules 2008, 41, 9656–9662. [Google Scholar] [CrossRef]
- Bezzu, C.G.; Carta, M.; Tonkins, A.; Jansen, J.C.; Bernardo, P.; Bazzarelli, F.; McKeown, N.B. A Spirobifluorene-Based Polymer of Intrinsic Microporosity with Improved Performance for Gas Separation. Adv. Mater. 2012, 24, 5930–5933. [Google Scholar] [CrossRef]
- Carta, M.; Malpass-Evans, R.; Croad, M.; Rogan, Y.; Jansen, J.C.; Bernatdo, P.; Bazzaralli, F.; McKeown, N.B. An Efficient Polymer Molecular Sieve for Membrane Gas Separations. Science 2013, 339, 303–307. [Google Scholar] [CrossRef]
- Carta, M.; Bernardo, P.; Clarizia, G.; Jansen, J.C.; McKeown, N.B. Gas Permeability of Hexaphenylbenzene Based Polymers of Intrinsic Microporosity. Macromolecules 2014, 47, 8320–8327. [Google Scholar] [CrossRef]
- Budd, P.M.; Elabas, E.S.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E.; Wang, D. Solution-Processed, Organophilic Membrane Derived from a Polymer of Intrinsic Microporosity. Adv. Mater. 2004, 16, 456–459. [Google Scholar] [CrossRef]
- McKeown, N.B.; Budd, P.M. Polymers of intrinsic microporosity (PIMs): Organoic materials for membrane separations, heterogeneous catalysis and hydrogen storage. Chem. Soc. Rev. 2006, 35, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Vandezande, P.; Gevers, L.E.M.; Vankelecom, I.F.J. Solvent resistant nanofiltration: Separating on a molecular level. Chem. Soc. Rev. 2008, 37, 365–405. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, D.; Meten, P.; Heinrich, K.; Lazar, M.; Priske, M. High performance organic solvent nanofiltration membranes: Development and thorough testing of thin film composite membranes made of polymers of intrinsic microporosity (PIMs). J. Membr. Sci. 2012, 401, 222–231. [Google Scholar] [CrossRef]
- McKeown, N.B.; Gahnem, B.; Msayib, K.J.; Budd, P.M.; Tattershall, C.E.; Mahmood, K.; Tan, S.; Book, D.; Langmi, H.W.; Walton, A. Towards Polymer-Based Hydrogen Storage Materials: Engineering Ultramicroporous Cavities within Polymers of Intrinsic Microporosity. Angew. Chem. Int. Ed. 2006, 45, 1804–1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budd, P.M.; Butler, A.; Selbie, J.; Mahmood, K.; McKeown, N.B.; Ghanem, B.; Msayid, K.; Book, D.; Walton, A. The potential of organic polymer-based hydrogen storage materials. Phys. Chem. Chem. Phys. 2007, 9, 1802–1808. [Google Scholar] [CrossRef]
- Ghanem, B.S.; Msayib, K.J.; McKeown, N.B.; Harris, K.D.M.; Pan, Z.; Budd, P.M.; Butler, A.; Selbie, J.; Book, D.; Walton, A. A triptycene-based polymer of intrinsic microposity that displays enhanced surface area and hydrogen adsorption. Chem. Commun. 2007, 1, 67–69. [Google Scholar] [CrossRef]
- McKeown, N.B.; Budd, P.M.; Book, D. Microporous Polymers as Potential Hydrogen Storage Materials. Macromol. Rapid Commun. 2007, 28, 995–1002. [Google Scholar] [CrossRef]
- Kaur, P.; Hupp, J.T.; Nguyen, S.T. Porous Organic Polymers in Catalysis: Opportunities and Challenges. ACS Catal. 2011, 1, 819–835. [Google Scholar] [CrossRef]
- McKeown, N.B. Polymers of Intrinsic Microporosity. ISRN Mater. Sci. 2012, 513986. [Google Scholar] [CrossRef]
- Du, N.; Robertson, G.P.; Song, J.; Pinnau, I.; Guiver, M.D. High-Performance Carboxylated Polymers of Intrinsic Microporosity (PIMs) with Tunable Gas Transport Properties. Macromolecules 2009, 42, 6038–6043. [Google Scholar] [CrossRef]
- Weber, J.; Du, N.; Guiver, M.D. Influence of Intermolecular Interactions on the Observable Porosity in Intrinsically Microporous Polymers. Macromolecules 2011, 44, 1763–1767. [Google Scholar] [CrossRef] [Green Version]
- Mason, C.R.; Maynard-Atem, L.; Al Harbi, N.M.; Budd, P.M.; Bernardo, P.; Bazzarelli, F.; Clarizia, G.; Jansen, J.C. Polymer of Intrinsic Microporosity Incorporating Thioamide Functionality: Preparation and Gas Transport Properties. Macromolecules 2011, 44, 6471–6479. [Google Scholar] [CrossRef]
- Patel, H.A.; Yavuz, C.T. Noninvasive functionalization of polymers of intrinsic microporosity for enhanced CO2 capture. Chem. Commun. 2012, 48, 9989–9991. [Google Scholar] [CrossRef]
- Du, N.; Park, H.B.; Robertson, G.P.; Dal-Cin, M.M.; Visser, T.; Scoles, L.; Guiver, M.D. Polymer nanosieve membranes for CO2-capture applications. Nature Mater. 2011, 10, 372. [Google Scholar] [CrossRef]
- Du, N.; Robertson, G.P.; Dal-Cin, M.M.; Scoles, L.; Guiver, M.D. Polymers of intrinsic microporosity (PIMs) substituted with methyl tetrazole. Polymer 2012, 53, 4367–4372. [Google Scholar] [CrossRef] [Green Version]
- Yanaranop, P.; Santoso, B.; Etzion, R.; Jin, J. Facile conversion of nitrile to amide on polymers of intrinsic microporosity (PIM-1). Polymer 2016, 98, 244–251. [Google Scholar] [CrossRef]
- Mason, C.R.; Maynard-Atem, L.; Heard, K.W.J.; Satilmis, B.; Budd, P.; Friess, K.; Lanc, M.; Bernardo, P.; Clarizia, G.; Jansen, J.C. Enhancement of CO2 Affinity in a Polymer of Intrinsic Microporosity by Amine Modification. Macromolecules 2014, 47, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, J.; Pan, K. Chiral Helical Polymer Nanomaterials with Tunable Morphology: Prepared with Chiral Solvent to Induce Helix-Sense-Selective Precipitation Polymerization. Macromolecules 2018, 51, 8878–8886. [Google Scholar] [CrossRef]
- Xu, L.; Xu, X.-H.; Liu, N.; Zou, H.; Wu, Z.-Q. A Facile Synthetic Route to Multifunctional Poly(3- hexylthiophene)-b-poly(phenyl isocyanide) Copolymers: From Aggregation-Induced Emission to Controlled Helicity. Macromolecules 2018, 51, 7546–7555. [Google Scholar] [CrossRef]
- Yin, J.; Xu, L.; Han, X.; Zhou, L.; Li, C.; Wu, Z.-Q. A facile synthetic route to stereoregular helical poly(phenyl isocyanide)s with defined pendants and controlled helicity. Polym. Chem. 2017, 8, 545–556. [Google Scholar] [CrossRef]
- Zhou, L.; Shen, L.; Huang, J.; Liu, N.; Zhu, Y.-Y.; Wu, Z.-Q. Optically Active Helical Polyisocyanides Bearing Chiral Phosphine Pendants: Facile Synthesis and Application in Enantioselective Rauhut-Currier Reaction. Chin. J. Polym. Sci. 2018, 36, 163–170. [Google Scholar] [CrossRef]
- Zhou, L.; Chu, B.-F.; Xu, X.-Y.; Xu, L.; Liu, N.; Wu, Z.-Q. Significant Improvement on Enantioselectivity and Diastereoselectivity of Organocatalyzed Asymmetric Aldol Reaction Using Helical Polyisocyanides Bearing Proline Pendants. ACS Macro Lett. 2017, 6, 824–829. [Google Scholar] [CrossRef]
- Pedrosa, R.; Andrés, J.M.; Gamarra, A.; Manzano, R.; Pérez-López, C. Novel sulfonylpolystyrene-supported prolinamides as catalysts for enantioselective aldol reaction in water. Tetrahedron 2013, 69, 10811–10819. [Google Scholar] [CrossRef]
- Pedrosa, R.; Andrés, J.M.; Ávila, D.P.; Ceballos, M.; Pindado, R. Chiral ureas and thioureas supported on polystyrene for enantioselective aza-Henry reactions under solvent-free conditions. Green Chem. 2015, 17, 2217–2225. [Google Scholar] [CrossRef]
- Andrés, J.M.; de la Cruz, N.; Valle, M.; Pedrosa, R. Bottom-Up Synthesis of Supported Thioureas and Their Use in Enantioselective Solvent-Free Aza-Henry and Michael Additions. ChemPlusChem 2016, 81, 86–92. [Google Scholar] [CrossRef]
- Andrés, J.M.; Ceballos, M.; Maestro, A.; Sanz, I.; Pedrosa, R. Supported bifunctional thioureas as recoverable and reusable catalysts for enantioselective nitro-Michael reactions. Beilstein J. Org. Chem. 2016, 12, 628. [Google Scholar] [CrossRef]
- Emmler, T.; Heinrich, K.; Fritsch, D.; Budd, P.M.; Chaukura, N.; Ehlers, D.; Rätzke, K.; Faupel, F. Free Volume Investigation of Polymers of Intrinsic Microporosity (PIMs): PIM-1 and PIM1 Copolymers Incorporating Ethanoanthracene Units. Macromolecules 2010, 43, 6075–6084. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Malerich, J.P.; Rawal, V.M. Squaramide-Catalyzed Enantioselective Michael Addition of Diphenyl Phosphite to Nitroalkenes. Angew. Chem. Int. Ed. 2010, 122, 157–160. [Google Scholar] [CrossRef]
- Niederl, J.B.; Nagel, R.H. Indano-indanes. J. Am. Chem. Soc. 1940, 43, 3070–3072. [Google Scholar] [CrossRef]
- Andrés, J.M.; Maestro, A.; Rodríguez-Ferrer, P.; Simón, I.; Pedrosa, R. Short Synthesis of Novel Recyclable Chiral Bifunctional Thioureas from Aminoalkyl Polystyrene and their use as Organocatalysts in Stereoselective aza-Henry Reaction. ChemistrySelect 2016, 1, 5057–5061. [Google Scholar] [CrossRef]
- Andrés, J.M.; Losada, J.; Maestri, A.; Rodríguez-Ferrer, P.; Pedrosa, R. Supported and Unsupported Chiral Squaramides as Organocatalysts for Stereoselective Michael Additions: Synthesis of Enantiopure Chromenes and Spirochromanes. J. Org. Chem. 2017, 82, 8444–8454. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, D.; Bengtson, G.; Carta, M.; McKeown, N.B. Synthesis and Gas Permeation Properties of Spirobischromane-Based Polymers of Intrinsic Microporosity. Macromol. Chem. Phys. 2011, 212, 1137–1146. [Google Scholar] [CrossRef]
- Kasaplar, P.; Ozkal, E.; Rodríguez-Escrich, C.; Pericàs, M.A. Enantioselective α-amination of 1,3-dicarbonyl compounds in batch and flow with immobilized thiourea organocatalysts. Green Chem. 2015, 17, 3122–3129. [Google Scholar] [CrossRef] [Green Version]
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Gómez, C.; Guillena, G. Recent Advances in Asymmetric Organocatalyzed Conjugate Additions to Nitroalkenes. Molecules 2017, 22, 895. [Google Scholar] [CrossRef] [PubMed]
- Kemnitzer, W.; Kasibhatla, S.; Jiang, S.; Zhang, H.; Zhao, J.; Jia, S.; Xu, L.; Grongan-Grundy, C.; Denis, R.; Barriault, N.; et al. Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. 2. Structure-activity relationship for the 7- and 5-, 6-, 8 positions. Bioorg. Med. Chem. Lett. 2005, 15, 4745–4751. [Google Scholar] [CrossRef]
- Sonsona, I.G.; Marqués-López, E.; Herrera, R.P. Enantioselective Organocatalyzed Synthesis of 2-Amino-3-cyano-4H-chromene Derivatives. Symmetry 2015, 7, 1519–1535. [Google Scholar] [CrossRef] [Green Version]






| Entry | 5a–c | Catalyst | t (H) | Product (Yield) b | Drc | Er d (Conf) |
|---|---|---|---|---|---|---|
| 1 | 5a | I | 12 | 7a (71) | - | 79:21 (S) |
| 2 | 5a | II | 12 | 7a (69) | - | 81:19 (S) |
| 3 | 5a | III | 12 | 7a (79) | - | 93:7 (R) |
| 4 | 5a | IV | 6 | 7a (69) | - | 68:32 (S) |
| 5 | 5a | V | 12 | 7a (75) | 80:20 (S) | |
| 6 | 5a | VI | 12 | 7a (86) | - | 89:11 (R) |
| 7 | 5b | I | 1 | 7b (68) | 74:26 (S) | |
| 8 | 5b | II | 1 | 7b (72) | - | 96:4 (S) |
| 9 | 5b | V | 1 | 7b (75) | - | 92:8 (S) |
| 10 | 5b | VI | 1 | 7b (72) | 87:13 | 71:29 (R) |
| 11 | 5c | I | 1 | 7c (93) | 89:11 | 79:21 (S,R) e |
| 12 | 5c | II | 1 | 7c (89) | 91:9 | 81:19 (S,R) e |
| 13 | 5c | III | 1 | 7c (94) | 92:8 | 65:35 (R,S) e |
| 14 | 5c | VI | 1 | 7c (87) | 88:12 | 68:32 (R,S) e |
| 15 f | 5a | III | 12 | 7a (79) | - | 89:11 (R) |
| 16 f | 5a | III | 12 | 7a (82) | - | 86:14 (R) |
| 17 f | 5a | III | 12 | 7a (71) | - | 89:11 (R) |
| 18 f | 5a | III | 12 | 7a (68) | - | 90:10 (R) |
| 19 f | 5a | III | 12 | 7a (65) | - | 89:11 (R) |
| 20 g | 5b | II | 1 | 7b (81) | - | 95:5 (S) |
| 21 g | 5b | II | 1 | 7b (83) | - | 96:4 (S) |
| 22 g | 5b | II | 1 | 7b (79) | - | 94:6 (S) |
| 23 g | 5b | II | 1 | 7b (86) | - | 96:4 (S) |
| 24g | 5b | II | 1 | 7b (86) | - | 96:4 (S) |
| Entry | Solvent | Catalyst | t (H) | Yield a | Erb (Conf) |
|---|---|---|---|---|---|
| 1 | CH2Cl2 | I | 2 | 77 | 80:20 (S) |
| 2 | CH2Cl2 | II | 2 | 75 | 67:33 (S) |
| 3 | CH2Cl2 | III | 2 | 83 | 75:25 (R) |
| 4 | CH2Cl2 | IV | 2 | 70 | 68:32 (S) |
| 5 | CH2Cl2 | V | 2 | 68 | 65:35 (S) |
| 6 | CH2Cl2 | VI | 2 | 76 | 71:29 (R) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valle, M.; Martín, L.; Maestro, A.; Andrés, J.M.; Pedrosa, R. Chiral Bifunctional Thioureas and Squaramides Grafted into Old Polymers of Intrinsic Microporosity for Novel Applications. Polymers 2019, 11, 13. https://doi.org/10.3390/polym11010013
Valle M, Martín L, Maestro A, Andrés JM, Pedrosa R. Chiral Bifunctional Thioureas and Squaramides Grafted into Old Polymers of Intrinsic Microporosity for Novel Applications. Polymers. 2019; 11(1):13. https://doi.org/10.3390/polym11010013
Chicago/Turabian StyleValle, María, Laura Martín, Alicia Maestro, José M. Andrés, and Rafael Pedrosa. 2019. "Chiral Bifunctional Thioureas and Squaramides Grafted into Old Polymers of Intrinsic Microporosity for Novel Applications" Polymers 11, no. 1: 13. https://doi.org/10.3390/polym11010013
APA StyleValle, M., Martín, L., Maestro, A., Andrés, J. M., & Pedrosa, R. (2019). Chiral Bifunctional Thioureas and Squaramides Grafted into Old Polymers of Intrinsic Microporosity for Novel Applications. Polymers, 11(1), 13. https://doi.org/10.3390/polym11010013