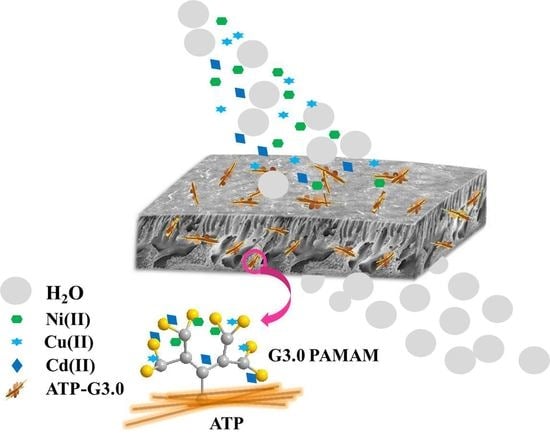
Preparation of PVDF/Hyperbranched-Nano-Palygorskite Composite Membrane for Efficient Removal of Heavy Metal Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Characterization
2.3.1. General Characterization
2.3.2. Heavy Metal Ion Adsorption
3. Results
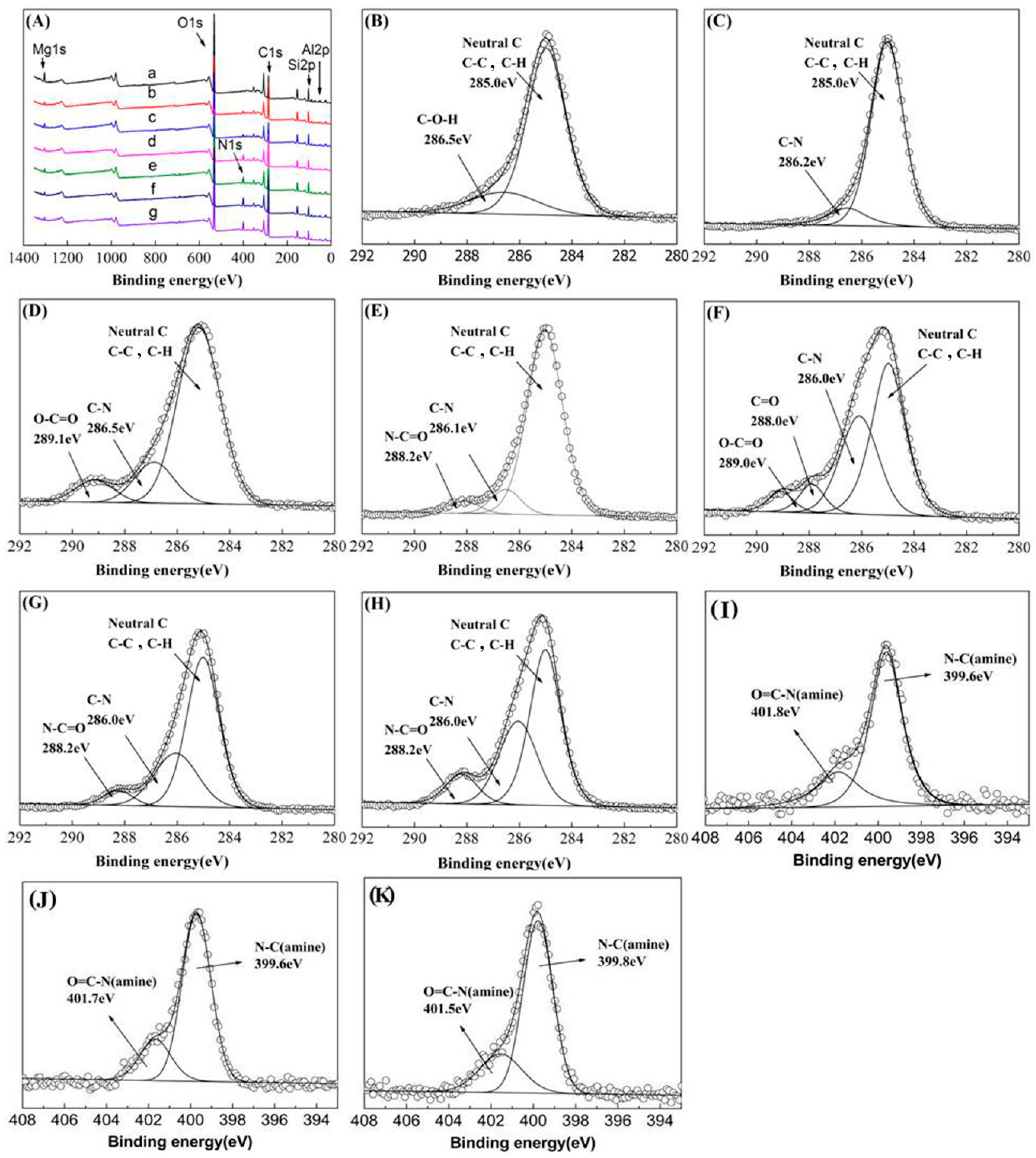
3.1. Characterization of the PAMAM-Pal
3.2. Membrane Structure and Performance
3.3. Heavy Metal Ions Adsorption and Adsorption Kinetic Model
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Al-Rashdi, B.; Somerfield, C.; Hilal, N. Heavy Metals Removal Using Adsorption and Nanofiltration Techniques. Sep. Purif. Rev. 2011, 40, 209–259. [Google Scholar] [CrossRef]
- Abdi, G.; Alizadeh, A.; Zinadini, S.; Moradi, G. Removal of dye and heavy metal ion using a novel synthetic polyethersulfone nanofiltration membrane modified by magnetic graphene oxide/metformin hybrid. J. Membr. Sci. 2018, 552, 326–335. [Google Scholar] [CrossRef]
- Katsou, E.; Malamis, S.; Haralambous, K.J.; Loizidou, M. Use of ultrafiltration membranes and aluminosilicate minerals for nickel removal from industrial wastewater. J. Membr. Sci. 2010, 360, 234–249. [Google Scholar] [CrossRef]
- He, Y.; Liu, J.; Han, G.; Chung, T.-S. Novel thin-film composite nanofiltration membranes consisting of a zwitterionic co-polymer for selenium and arsenic removal. J. Membr. Sci. 2018, 555, 299–306. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef]
- Camarillo, R.; Pérez, Á.; Cañizares, P.; de Lucas, A. Removal of heavy metal ions by polymer enhanced ultrafiltration. Desalination 2012, 286, 193–199. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Wan Ngah, W.S.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Chiang, Y.W.; Ghyselbrecht, K.; Santos, R.M.; Meesschaert, B.; Martens, J.A. Synthesis of zeolitic-type adsorbent material from municipal solid waste incinerator bottom ash and its application in heavy metal adsorption. Catal. Today 2012, 190, 23–30. [Google Scholar] [CrossRef]
- Kryvoruchko, A.P.; Atamanenko, I.D.; Yurlova, L.Y. Concentration/purification of Co(II) ions by reverse osmosis and ultrafiltration combined with sorption on clay mineral montmorillonite and cation-exchange resin KU-2-8n. J. Membr. Sci. 2004, 228, 77–81. [Google Scholar] [CrossRef]
- Xiang, L.; Pan, Y.; Zeng, G.; Jiang, J.; Chen, J.; Wang, C. Preparation of poly(ether-block-amide)/attapulgite mixed matrix membranes for CO2/N2 separation. J. Membr. Sci. 2016, 500, 66–75. [Google Scholar] [CrossRef]
- Sen Gupta, S.; Bhattacharyya, K.G. Adsorption of metal ions by clays and inorganic solids. RSC Adv. 2014, 4, 28537–28586. [Google Scholar] [CrossRef]
- Wu, M.; Ma, T.; Su, Y.; Wu, H.; You, X.; Jiang, Z.; Kasher, R. Fabrication of composite nanofiltration membrane by incorporating attapulgite nanorods during interfacial polymerization for high water flux and antifouling property. J. Membr. Sci. 2017, 544, 79–87. [Google Scholar] [CrossRef]
- Pan, D.; Fan, Q.; Fan, F.; Tang, Y.; Zhang, Y.; Wu, W. Removal of uranium contaminant from aqueous solution by chitosan@attapulgite composite. Sep. Purif. Technol. 2017, 177, 86–93. [Google Scholar] [CrossRef]
- Ali, I. New generation adsorbents for water treatment. Chem. Rev. 2012, 112, 5073–5091. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Liu, P.; Wang, A. Facile approach to magnetic attapulgite-Fe3O4/polystyrene tri-component nanocomposite. Mater. Lett. 2012, 85, 11–13. [Google Scholar] [CrossRef]
- Deng, Y.; Gao, Z.; Liu, B.; Hu, X.; Wei, Z.; Sun, C. Selective removal of lead from aqueous solutions by ethylenediamine-modified attapulgite. Chem. Eng. J. 2013, 223, 91–98. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, B.; Li, H.; Zhang, W.; Lv, L.; Wu, J. Selective removal of Cu (II) ions by using cation-exchange resin-supported polyethyleneimine (PEI) nanoclusters. Environ. Sci. Technol. 2010, 44, 3508–3513. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Zhang, D.-Y.; Liu, X.-Q.; Shi, L.-Y.; Sun, L.-B. A tandem demetalization–desilication strategy to enhance the porosity of attapulgite for adsorption and catalysis. Chem. Eng. Sci. 2016, 141, 184–194. [Google Scholar] [CrossRef]
- Bu, J.; Li, R.; Quah, C.W.; Carpenter, K.J. Propagation of PAMAM Dendrons on Silica Gel: A Study on the Reaction Kinetics. Macromolecules 2004, 37, 6687–6694. [Google Scholar] [CrossRef]
- Kaneko, Y.; Imai, Y.; Shirai, K.; Yamauchi, T.; Tsubokawa, N. Preparation and properties of hyperbranched poly(amidoamine) grafted onto a colloidal silica surface. Colloids Surf. A Physicochem. Eng. Asp. 2006, 289, 212–218. [Google Scholar] [CrossRef]
- Yoo, H.; Kwak, S.-Y. Surface functionalization of PTFE membranes with hyperbranched poly(amidoamine) for the removal of Cu2+ ions from aqueous solution. J. Membr. Sci. 2013, 448, 125–134. [Google Scholar] [CrossRef]
- Yantasee, W.; Lin, Y.; Fryxell, G.E.; Alford, K.L.; Busche, B.J.; Johnson, C.D. Selective Removal of Copper(II) from Aqueous Solutions Using Fine-Grained Activated Carbon Functionalized with Amine. Ind. Eng. Chem. Res. 2004, 43, 2759–2764. [Google Scholar] [CrossRef]
- Cao, J.S.; Wang, C.; Fang, F.; Lin, J.X. Removal of heavy metal Cu(II) in simulated aquaculture wastewater by modified palygorskite. Environ. Pollut. 2016, 219, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Qian, Y.; Li, Q.; Zhang, Q.; Zhai, J. Adsorption of aqueous Hg(II) by a polyaniline/attapulgite composite. Chem. Eng. J. 2012, 211–212, 216–223. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, J.; Deng, B. Enhanced mercury ion adsorption by amine-modified activated carbon. J. Hazard. Mater. 2009, 166, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.-L.; Ang, M.B.M.Y.; Hung, H.-C.; Huang, S.-H.; An, Q.-F.; Lee, K.-R.; Lai, J.-Y. Bio-inspired deposition of polydopamine on PVDF followed by interfacial cross-linking with trimesoyl chloride as means of preparing composite membranes for isopropanol dehydration. J. Membr. Sci. 2018, 557, 58–66. [Google Scholar] [CrossRef]
- Wu, H.; Mansouri, J.; Chen, V. Silica nanoparticles as carriers of antifouling ligands for PVDF ultrafiltration membranes. J. Membr. Sci. 2013, 433, 135–151. [Google Scholar] [CrossRef]
- Yuliwati, E.; Ismail, A.F.; Matsuura, T.; Kassim, M.A.; Abdullah, M.S. Characterization of surface-modified porous PVDF hollow fibers for refinery wastewater treatment using microscopic observation. Desalination 2011, 283, 206–213. [Google Scholar] [CrossRef]
- Zhou, S.; Xue, A.; Zhang, Y.; Li, M.; Li, K.; Zhao, Y.; Xing, W. Novel polyamidoamine dendrimer-functionalized palygorskite adsorbents with high adsorption capacity for Pb2+ and reactive dyes. Appl. Clay. Sci. 2015, 107, 220–229. [Google Scholar] [CrossRef]
- Salehi, E.; Madaeni, S.S.; Heidary, F. Dynamic adsorption of Ni(II) and Cd(II) ions from water using 8-hydroxyquinoline ligand immobilized PVDF membrane: Isotherms, thermodynamics and kinetics. Sep. Purif. Technol. 2012, 94, 1–8. [Google Scholar] [CrossRef]
- Ricordel, S.; Taha, S.; Cisse, I.; Dorange, G. Heavy metals removal by adsorption onto peanut husks carbon: Characterization, kinetic study and modeling. Sep. Purif. Technol. 2001, 24, 389–401. [Google Scholar] [CrossRef]
- Liu, Y. Is the Free Energy Change of Adsorption Correctly Calculated? J. Chem. Eng. Data 2009, 54, 1981–1985. [Google Scholar] [CrossRef]
- Li, Q.; Zhai, J.; Zhang, W.; Wang, M.; Zhou, J. Kinetic studies of adsorption of Pb(II), Cr(III) and Cu(II) from aqueous solution by sawdust and modified peanut husk. J. Hazard. Mater. 2007, 141, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Liu, D.; Tan, D.; Yuan, P.; Chen, M. FTIR spectroscopy study of the structure changes of palygorskite under heating. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 1052–1057. [Google Scholar] [CrossRef]
- Shang, X.; Zhu, Y.; Li, Z. Surface modification of silicon carbide with silane coupling agent and hexadecyl iodiele. Appl. Surf. Sci. 2017, 394, 169–177. [Google Scholar] [CrossRef]
- Ozturk, O.; Black, T.J.; Pizzolato, K.; Williams, C.T.; Parsons, F.W.; Ratliff, J.S.; Gao, J.; Murphy, C.J.; Xie, H.; Ploehn, H.J.; et al. Thermal Decomposition of Generation-4 Polyamidoamine Dendrimer Films: Decomposition Catalyzed by Dendrimer-Encapsulated Pt Particles. Langmuir ACS J. Surfaces Colloids 2005, 21, 3998–4006. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Kumar, N.; Prasad, P.; Shirbhate, S.; Sehrawat, S.; Lochab, B. Stable Dispersions of Covalently Tethered Polymer Improved Graphene Oxide Nanoconjugates as an Effective Vector for siRNA Delivery. ACS Appl. Mater. Interfaces 2018, 10, 14577–14593. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Fang, S.; Zhan, J.; Hu, Y.; Wu, J. Preparation and characterization of γ-irradiated crosslinked silicone rubber/organoattapulgite composites. Polym. Compos. 2010, 31, 405–410. [Google Scholar] [CrossRef]
- Kotte, M.R.; Kuvarega, A.T.; Cho, M.; Mamba, B.B.; Diallo, M.S. Mixed Matrix PVDF Membranes With in Situ Synthesized PAMAM Dendrimer-Like Particles: A New Class of Sorbents for Cu(II) Recovery from Aqueous Solutions by Ultrafiltration. Environ. Sci. Technol. 2015, 49, 9431–9442. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A.; Schmidt, E. Polymer-enhanced ultrafiltration process for heavy metals removal from industrial wastewater. Desalination 2010, 256, 90–93. [Google Scholar] [CrossRef]
- Zhu, W.-P.; Gao, J.; Sun, S.-P.; Zhang, S.; Chung, T.-S. Poly(amidoamine) dendrimer (PAMAM) grafted on thin film composite (TFC) nanofiltration (NF) hollow fiber membranes for heavy metal removal. J. Membr. Sci. 2015, 487, 117–126. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, Y.; Liu, S.; Jiang, J.; Cao, C.; Yao, J. Simple fabrication of easy handling millimeter-sized porous attapulgite/polymer beads for heavy metal removal. J. Colloid Interface Sci. 2017, 502, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, H.; Wang, A. Adsorption characteristics of Cd(II) from aqueous solution onto activated palygorskite. Sep. Purif. Technol. 2007, 55, 157–164. [Google Scholar] [CrossRef]
- Zheng, Y.W.; Tao, W.W.; Zhang, G.F.; Lv, C.; Zhao, Y.P.; Chen, L. Adsorptive Removal of Ni(II) Ions from Aqueous Solution by Polyacrylic Acid/Attapulgite Composite Hydrogels. Key Eng. Mater. 2017, 727, 859–865. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Y.; Zhang, X.F.; Zhang, X.; Jiang, J.; Yao, J. Alginate-based attapulgite foams as efficient and recyclable adsorbents for the removal of heavy metals. J. Colloid Interface Sci. 2018, 514, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Tang, J.; Tan, L.; Wang, W.; Lu, H.; Guo, D. Synthesis and Adsorption of Ni(II) on Ni(II)-Imprinted Polyaniline Supported on Attapulgite Modified with 3-Methacryloxypropyltrimethoxysilane. Adsorpt. Sci. Technol. 2013, 31, 521–534. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Ma, J.; Xia, P.; Zhao, J. Removal of cadmium (II) from aqueous solution: A comparative study of raw attapulgite clay and a reusable waste-struvite/attapulgite obtained from nutrient-rich wastewater. J. Hazard. Mater. 2017, 329, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Mu, B.; Zheng, M.; Wang, A. One-Step Calcination of the Spent Bleaching Earth for the Efficient Removal of Heavy Metal Ions. ACS Sustain. Chem. Eng. 2015, 3, 1125–1135. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C. Chitosan-poly(vinyl alcohol)/attapulgite nanocomposites for copper(II) ions removal: pH dependence and adsorption mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2016, 500, 186–194. [Google Scholar] [CrossRef]
- Liu, G.; Li, Z.; Xu, L.; Xu, X.; Huang, Q.; Zeng, Y.; Wen, M. The dynamics and adsorption of Cd (II) onto hydroxyapatite attapulgite composites from aqueous solution. J. Sol-Gel Sci. Technol. 2018, 87, 269–284. [Google Scholar] [CrossRef]
- Bigui, W.; Xiaofei, Z.; Xiabing, C. Facile Preparation of Magnetic Graphene Oxide and Attapulgite Composite Adsorbent for the Adsorption of Ni (II). IOP Conf. Ser. Earth Environ. Sci. 2017, 104, 012019. [Google Scholar] [CrossRef]
- Kalaivani, S.S.; Muthukrishnaraj, A.; Sivanesan, S.; Ravikumar, L. Novel hyperbranched polyurethane resins for the removal of heavy metal ions from aqueous solution. Process. Saf. Environ. 2016, 104, 11–23. [Google Scholar] [CrossRef]









| Adsorbents | C0 a (mg·L−1) | Q (mg/g) | Reference | ||
|---|---|---|---|---|---|
| Cu(II) | Ni(II) | Cd(II) | |||
| porous palygorskite (Pal)/polymer beads | 500 | 25.3 | -- | 32.7 | [43] |
| activated palygorskites | 500 | -- | -- | 52.99 | [44] |
| Polyacrylic Acid/palygorskite Composite Hydrogels | 200 | -- | 72.8 | -- | [45] |
| alginate-based palygorskite foams | 250 | 119 | -- | 160 | [46] |
| A novel Ni(II) ion-imprinted polymer | 100 | -- | 123.61 | -- | [47] |
| struvite/palygorskite | 100 | -- | -- | 121.14 | [48] |
| palygorskite/carbon nanocomposites | 1000 | 32.32 | -- | 46.72 | [49] |
| CTS-PVA/Pal | 210 | 35.79 | -- | -- | [50] |
| palygorskite hydroxyapatite composite | 120 | 126.45 | -- | 131.52 | [51] |
| a composite magnetic GO-Pal adsorbent | 100 | -- | 190.8 | -- | [52] |
| PVDF/hyperbranched-nano-palygorskite composite membrane | 200 | 155.19 | 124.28 | 125.55 | This work |
| Metalions | Samples | qe (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|---|
| qe1 (mg/g) | k1 (min−1) | r2 | qe2 (mg/g) | k2 (g/mg min) | r2 | |||
| Cu(II) | a | 24.8547 | 17.87316 | 0.0029 | 0.8834 | 27.1665 | 5.64 × 10−4 | 0.9964 |
| b | 37.5432 | 17.64072 | 0.0031 | 0.6403 | 38.1243 | 8.78 × 10−4 | 0.9989 | |
| c | 73.2668 | 50.61966 | 0.0047 | 0.9704 | 75.7002 | 2.89 × 10−4 | 0.9982 | |
| d | 112.7836 | 76.62627 | 0.0021 | 0.8211 | 115.2074 | 1.13 × 10−4 | 0.9912 | |
| e | 155.1950 | 115.2980 | 0.0024 | 0.9188 | 157.4803 | 7.35 × 10−5 | 0.9900 | |
| Ni(II) | a | 12.9696 | 8.136825 | 0.0077 | 0.8915 | 18.0115 | 3.05 × 10−3 | 0.9995 |
| b | 23.4972 | 13.01952 | 0.0033 | 0.7964 | 24.0211 | 1.09 × 10−3 | 0.9986 | |
| c | 52.1184 | 52.18734 | 0.0041 | 0.9870 | 58.5480 | 1.02 × 10−4 | 0.9688 | |
| d | 81.2291 | 65.37566 | 0.0044 | 0.9861 | 85.3242 | 1.63 × 10−4 | 0.9957 | |
| e | 124.2819 | 98.64721 | 0.0024 | 0.9503 | 132.6260 | 6.54 × 10−5 | 0.9853 | |
| Cd(II) | a | 17.1907 | 10.69846 | 0.0030 | 0.9238 | 15.5400 | 8.91 × 10−4 | 0.9958 |
| b | 29.7817 | 28.11463 | 0.0041 | 0.9986 | 32.7118 | 2.32 × 10−4 | 0.9876 | |
| c | 56.7012 | 63.24145 | 0.0043 | 0.9749 | 68.6342 | 5.21 × 10−5 | 0.9208 | |
| d | 85.3710 | 65.50392 | 0.0029 | 0.9355 | 90.2527 | 1.22 × 10−4 | 0.9939 | |
| e | 125.5581 | 97.09792 | 0.0034 | 0.9658 | 132.4503 | 9.39 × 10−5 | 0.9948 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Qin, Y.; Zhang, G.; Zhao, Y.; Lv, C.; Liu, X.; Chen, L. Preparation of PVDF/Hyperbranched-Nano-Palygorskite Composite Membrane for Efficient Removal of Heavy Metal Ions. Polymers 2019, 11, 156. https://doi.org/10.3390/polym11010156
Zhang X, Qin Y, Zhang G, Zhao Y, Lv C, Liu X, Chen L. Preparation of PVDF/Hyperbranched-Nano-Palygorskite Composite Membrane for Efficient Removal of Heavy Metal Ions. Polymers. 2019; 11(1):156. https://doi.org/10.3390/polym11010156
Chicago/Turabian StyleZhang, Xiaoye, Yingxi Qin, Guifang Zhang, Yiping Zhao, Chao Lv, Xingtian Liu, and Li Chen. 2019. "Preparation of PVDF/Hyperbranched-Nano-Palygorskite Composite Membrane for Efficient Removal of Heavy Metal Ions" Polymers 11, no. 1: 156. https://doi.org/10.3390/polym11010156
APA StyleZhang, X., Qin, Y., Zhang, G., Zhao, Y., Lv, C., Liu, X., & Chen, L. (2019). Preparation of PVDF/Hyperbranched-Nano-Palygorskite Composite Membrane for Efficient Removal of Heavy Metal Ions. Polymers, 11(1), 156. https://doi.org/10.3390/polym11010156