Functional Porous Carboxymethyl Cellulose/Cellulose Acetate Composite Microspheres: Preparation, Characterization, and Application in the Effective Removal of HCN from Cigarette Smoke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
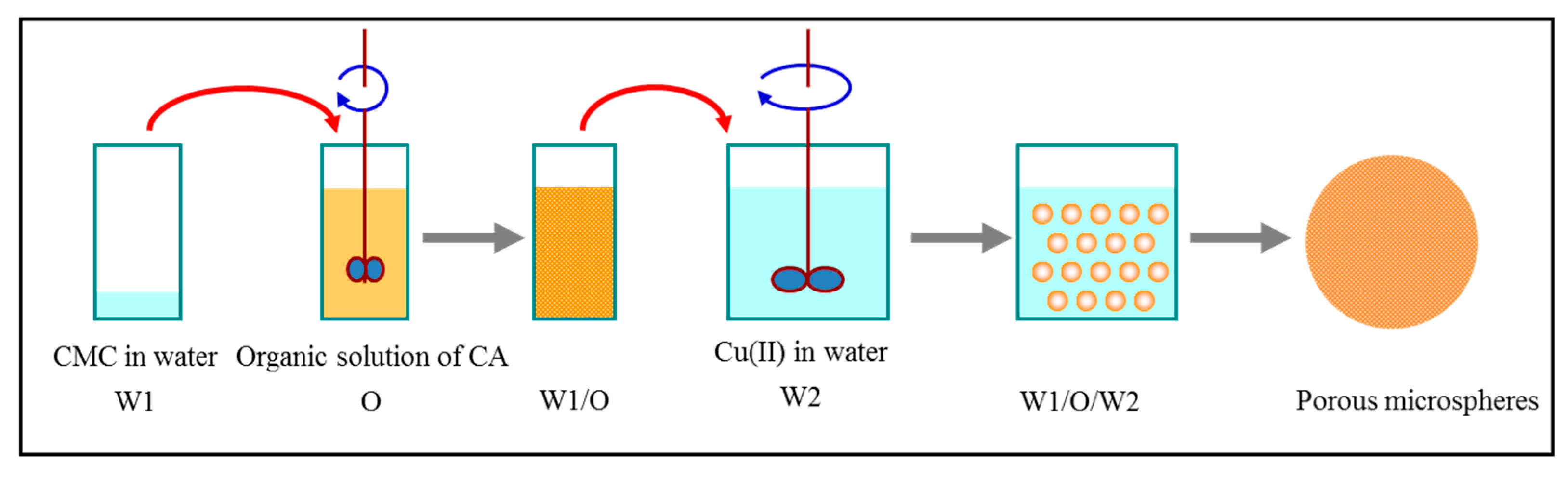
2.2. Preparation of CMC/CA Composite Microspheres
2.3. Characterization of Microspheres
2.4. Adsorption of HCN from Cigarette Smoke
3. Results and Discussion
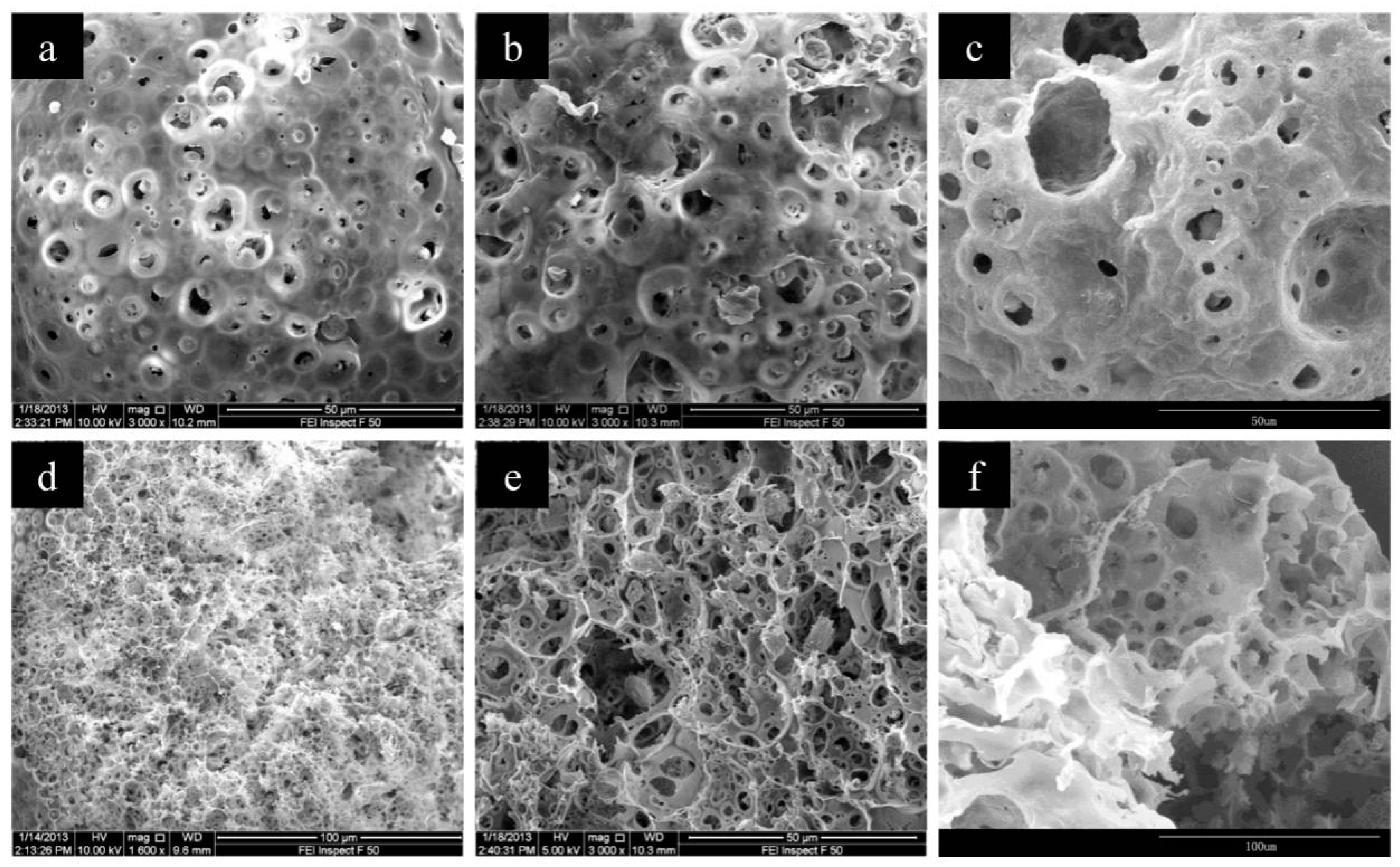
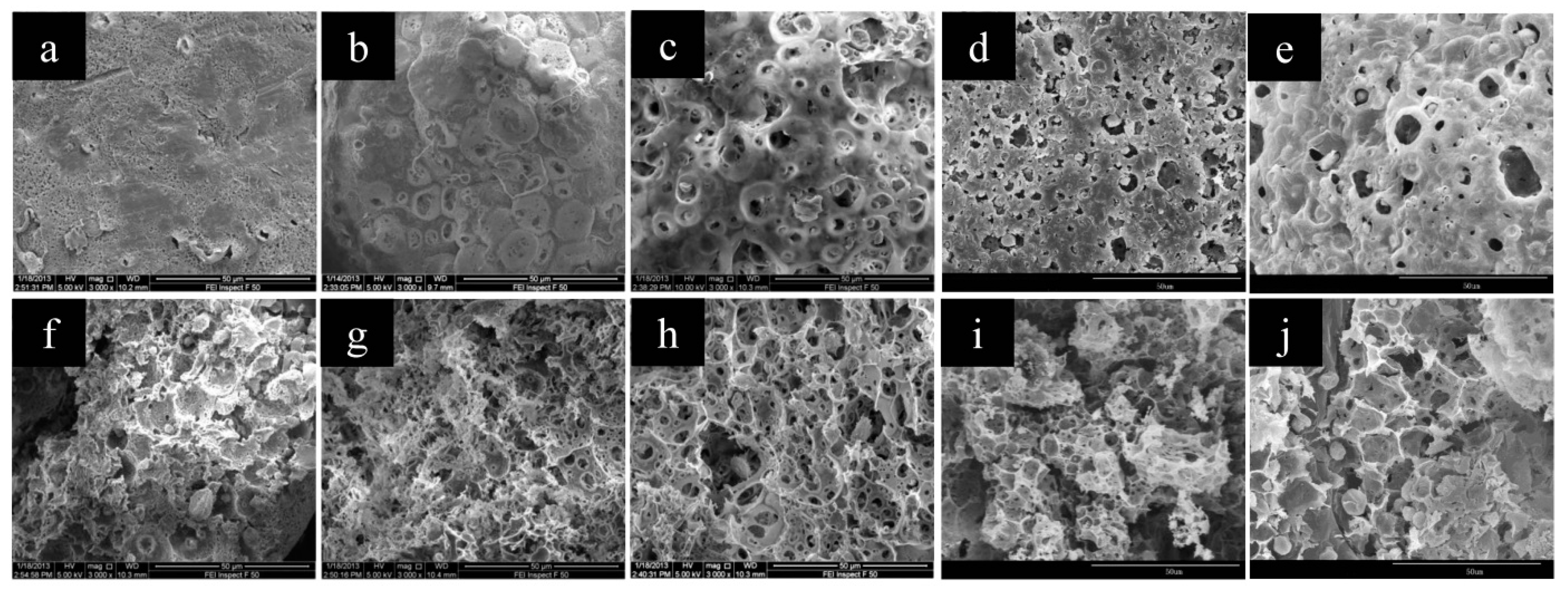
3.1. Preparation and Characterization of CMC/CA Composite Microspheres
3.2. Influence of Preparation Parameters on the Microsphere
3.2.1. CMC Concentration in the Inner Aqueous Solution
3.2.2. The W1/O Ratio
3.2.3. The Cu(II) Content in the Outer Aqueous Solution
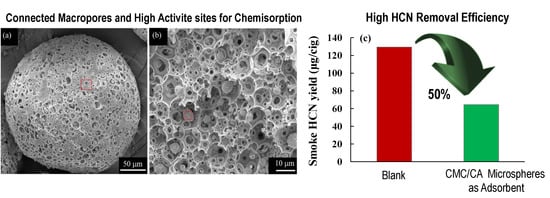
3.3. Evaluation of CMC/CA Microspheres as Absorbents in Cigarette Smoke
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rodgman, A.; Perfetti, T.A. The Chemical Components of Tobacco and Tobacco Smoke; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2008; pp. 1–928. [Google Scholar]
- WHO. The Scientific Basis of Tobacco Product Regulation, Second Report of a WHO Study Group; WHO Technical Report Series; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Piade, J.J.; Wajrock, S.; Jaccard, G.; Janeke, G. Formation of mainstream cigarette smoke constituents prioritized by the World Health Organization—Yield patterns observed in market surveys, clustering and inverse correlations. Food Chem. Toxicol. 2013, 55, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Branton, P.; Bradley, R.H. Activated carbons for the adsorption of vapours from cigarette smoke. Adsorpt. Sci. Technol. 2010, 28, 3–21. [Google Scholar] [CrossRef]
- Branton, P.J.; McAdam, K.G.; Duke, M.G.; Liu, C.; Curle, M.; Mola, M.; Proctor, C.J.; Bradley, R.H. Use of classical adsorption theory to understand the dynamic filtration of volatile toxicants in cigarette smoke by active carbons. Adsorpt. Sci. Technol. 2011, 29, 117–138. [Google Scholar] [CrossRef]
- Barnes, P.A.; Chinn, M.J.; Dawson, E.A.; Norman, P.R. Preparation, characterisation and application of metal-doped carbons for hydrogen cyanide removal. Adsorpt. Sci. Technol. 2002, 20, 817–833. [Google Scholar] [CrossRef]
- Branton, P.J.; McAdam, K.G.; Winter, D.B.; Liu, C.; Duke, M.G.; Proctor, C.J. Reduction of aldehydes and hydrogen cyanide yields in mainstream cigarette smoke using an amine functionalised ion exchange resin. Chem. Cent. J. 2011, 5, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Branton, P.J.; Lu, A.H.; Schüth, F. The effect of carbon pore structure on the adsorption of cigarette smoke vapour phase compounds. Carbon 2009, 47, 1005–1011. [Google Scholar] [CrossRef]
- He, S.; Zhang, F.; Cheng, S.; Wang, W. Synthesis of sodium acrylate and acrylamide copolymer/GO hydrogels and their effective adsorption for Pb2+ and Cd2+. ACS Sustain. Chem. Eng. 2016, 4, 3948–3959. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Singh, P.; Pathania, D. Acrylic acid grafted cellulosic Luffa cylindrical fiber for the removal of dye and metal ions. Carbohydr. Polym. 2013, 98, 1214–1221. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Wang, A. Adsorption of lead ions from aqueous solution by using carboxymethyl cellulose-g-poly (acrylic acid)/attapulgite hydrogel composites. Desalination 2010, 259, 258–264. [Google Scholar] [CrossRef]
- Li, D.; Li, Q.; Mao, D.; Bai, N.; Dong, H. A versatile bio-based material for efficiently removing toxic dyes, heavy metal ions and emulsified oil droplets from water simultaneously. Bioresour. Technol. 2017, 245, 649–655. [Google Scholar] [CrossRef]
- Rustemeyer, P. 5.2 CA filter tow for cigarette filters. Macromol. Symp. 2004, 208, 267–292. [Google Scholar] [CrossRef]
- Sata, H.; Murayama, M.; Shimamoto, S. 5.4 Properties and applications of cellulose triacetate film. Macromol. Symp. 2004, 208, 323–334. [Google Scholar] [CrossRef]
- Liu, C.X.; Bai, R.B. Adsorptive removal of copper ions with highly porous chitosan/cellulose acetate blend hollow fiber membranes. J. Membr. Sci. 2006, 284, 313–322. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, M.; Liu, R.; Li, Y.; Wang, D.; Tan, J.; Wu, R.; Huang, Y. Electrospun membrane of cellulose acetate for heavy metal ion adsorption in water treatment. Carbohydr. Polym. 2011, 83, 743–748. [Google Scholar] [CrossRef]
- Cai, Z.X.; Wu, J.; Du, B.Q.; Zhang, H.B. Impact of distribution of carboxymethyl substituents in the stabilizer of carboxymethyl cellulose on the stability of acidified milk drinks. Food Hydrocoll. 2018, 76, 150–157. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.; Roh, H.; Oh, S.; Kim, S.; Kim, M.; Kim, D.; Park, J. Preparation and characterization of superabsorbent polymers based on starch aldehydes and carboxymethyl cellulose. Polymers 2018, 10, 605. [Google Scholar] [CrossRef]
- Park, J.; An, S.J.; Jeong, S.I.; Gwon, H.J.; Lim, Y.M.; Nho, Y.C. Chestnut honey impregnated carboxymethyl cellulose hydrogel for diabetic ulcer healing. Polymers 2017, 9, 248. [Google Scholar] [CrossRef]
- Rasoulzadeh, M.; Namazi, H. Carboxymethyl cellulose/graphene oxide bionanocomposite hydrogel beads as anticancer drug carrier agent. Carbohydr. Polym. 2017, 168, 320–326. [Google Scholar] [CrossRef]
- Yang, J.; Li, J. Self-assembled cellulose materials for biomedicine: A review. Carbohydr. Polym. 2018, 181, 264–274. [Google Scholar] [CrossRef]
- Gasemloo, S.; Khosravi, M.; Sohrabi, M.R.; Dastmalchi, S.; Gharbani, P. Response surface methodology (RSM) modeling to improve removal of Cr (VI) ions from tannery wastewater using sulfated carboxymethyl cellulose nanofilter. J. Clean. Prod. 2019, 208, 736–742. [Google Scholar] [CrossRef]
- Chen, Y.; Long, Y.; Li, Q.; Chen, X.; Xu, X. Synthesis of high-performance sodium carboxymethyl cellulose-based adsorbent for effective removal of methylene blue and Pb (II). Int. J. Biol. Macromol. 2019, 126, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B, Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]

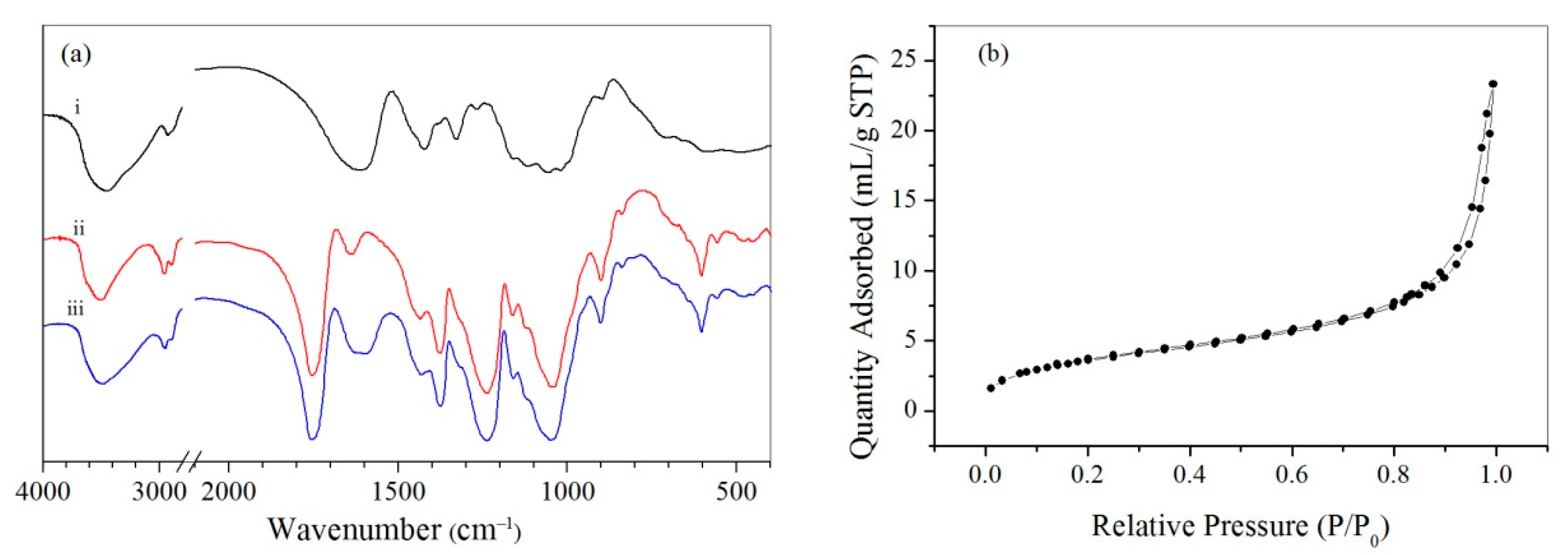





| Sample | Preparation Parameters | Characterization of the Microspheres. | |||||
|---|---|---|---|---|---|---|---|
| CMC (wt %) | W1/O (v/v) | Cu(II) in W2 (mM) | BET Surface Area (m2/g) | Pore Size (nm) | Pore Volume (mL/g) | The Cu(II) Loading Amount (wt %) | |
| A-1 | 2 | 50:100 | 50 | 5.06 | 952 | 3.23 | 1.8 |
| A-2 | 3 | 50:100 | 50 | 6.46 | 1780 | 2.97 | 2.6 |
| A-3 | 4 | 50:100 | 50 | 5.75 | 2264 | 2.45 | 3.3 |
| B-1 | 3 | 10:100 | 50 | 2.24 | 1058 | 1.04 | 0.8 |
| B-2 | 3 | 30:100 | 50 | 3.87 | 540 | 2.07 | 1.8 |
| B-3 | 3 | 50:100 | 50 | 6.46 | 1780 | 2.97 | 2.6 |
| B-4 | 3 | 70:100 | 50 | 13.50 | 1037 | 5.69 | 3.4 |
| B-5 | 3 | 90:100 | 50 | 7.42 | 1905 | 5.20 | 4.1 |
| C-1 | 3 | 50:100 | 25 | 5.86 | 2252 | 3.54 | 2.7 |
| C-2 | 3 | 50:100 | 50 | 6.46 | 1780 | 2.97 | 2.6 |
| C-3 | 3 | 50:100 | 100 | 3.86 | 898 | 2.12 | 2.6 |
| C-4 | 3 | 50:100 | 200 | 2.80 | 610 | 1.28 | 2.6 |
| Sample | HCN Yield (μg/cig) | HCN Removal Efficiency (%) |
|---|---|---|
| control | 129.5 | / |
| A1 | 89.6 | 30.9 |
| A2 | 64.6 | 50.1 |
| A3 | 69.8 | 46.1 |
| B1 | 94.1 | 27.3 |
| B2 | 82.7 | 36.1 |
| B3 | 64.6 | 50.1 |
| B4 | 75.4 | 41.8 |
| B5 | 71.5 | 44.8 |
| C1 | 69.6 | 46.3 |
| C2 | 64.6 | 50.1 |
| C3 | 88.8 | 31.4 |
| C4 | 99.2 | 23.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, P.; Yang, S.; Sun, X.; Wang, Y.; Pan, L.; Wang, H.; Wang, X.; Guo, J.; Nie, C. Functional Porous Carboxymethyl Cellulose/Cellulose Acetate Composite Microspheres: Preparation, Characterization, and Application in the Effective Removal of HCN from Cigarette Smoke. Polymers 2019, 11, 181. https://doi.org/10.3390/polym11010181
Sun P, Yang S, Sun X, Wang Y, Pan L, Wang H, Wang X, Guo J, Nie C. Functional Porous Carboxymethyl Cellulose/Cellulose Acetate Composite Microspheres: Preparation, Characterization, and Application in the Effective Removal of HCN from Cigarette Smoke. Polymers. 2019; 11(1):181. https://doi.org/10.3390/polym11010181
Chicago/Turabian StyleSun, Peijian, Song Yang, Xuehui Sun, Yipeng Wang, Lining Pan, Hongbo Wang, Xiaoyu Wang, Jizhao Guo, and Cong Nie. 2019. "Functional Porous Carboxymethyl Cellulose/Cellulose Acetate Composite Microspheres: Preparation, Characterization, and Application in the Effective Removal of HCN from Cigarette Smoke" Polymers 11, no. 1: 181. https://doi.org/10.3390/polym11010181
APA StyleSun, P., Yang, S., Sun, X., Wang, Y., Pan, L., Wang, H., Wang, X., Guo, J., & Nie, C. (2019). Functional Porous Carboxymethyl Cellulose/Cellulose Acetate Composite Microspheres: Preparation, Characterization, and Application in the Effective Removal of HCN from Cigarette Smoke. Polymers, 11(1), 181. https://doi.org/10.3390/polym11010181