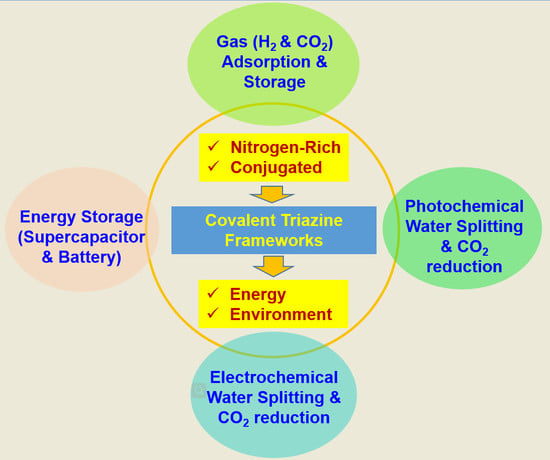
Recent Advancements in the Synthesis of Covalent Triazine Frameworks for Energy and Environmental Applications
Abstract
:1. Introduction
2. Structural Characteristic and Synthesis Routes
3. Energy- and Environment-Related Applications
3.1. Hydrogen Storage and CO2 Capture
3.1.1. Hydrogen Storage
3.1.2. Carbon Dioxide Capture
3.2. Photocatalytic Water Splitting and Carbon Dioxide Reduction
3.2.1. Photocatalytic Water Splitting
3.2.2. Photocatalytic Carbon Dioxide Reduction
3.3. Electrocatalysis for Energy Storage and Conversion
3.3.1. Oxygen Reduction and Methane Oxidation
3.3.2. Supercapactiors and Batteries
3.3.3. Electrocatalytic Carbon Dioxide Reduction
4. Outlook
Acknowledgments
Conflicts of Interest
References
- Das, S.; Heasman, P.; Ben, T.; Qiu, S.L. Porous organic materials: Strategic design and structure-function correlation. Chem. Rev. 2017, 117, 1515–1563. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.C.; Xu, F.; Sun, B.; Fu, R.W.; He, H.K.; Matyjaszewski, K. Design and preparation of porous polymers. Chem. Rev. 2012, 112, 3959–4015. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.H.; Jin, S.B.; Xu, H.; Nagai, A.; Jiang, D.L. Conjugated microporous polymers: Design, synthesis and application. Chem. Soc. Rev. 2013, 42, 8012–8031. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Ding, X.S.; Jiang, D.L. Covalent organic frameworks. Chem. Soc. Rev. 2012, 41, 6010–6022. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. 2008, 47, 3450–3453. [Google Scholar] [CrossRef] [PubMed]
- Ben, T.; Qiu, S.L. Porous aromatic frameworks: Synthesis, structure and functions. CrystEngComm 2013, 15, 17–26. [Google Scholar] [CrossRef]
- Tsyurupa, M.P.; Davankov, V.A. Hypercrosslinked polymers: basic principle of preparing the new class of polymeric materials. React. Funct. Polym. 2002, 53, 193–203. [Google Scholar] [CrossRef]
- McKeown, N.B.; Budd, P.M.; Msayib, K.J.; Ghanem, B.S.; Kingston, H.J.; Tattershall, C.E.; Makhseed, S.; Reynolds, K.J.; Fritsch, D. Polymers of intrinsic microporosity (PIMs): Bridging the void between microporous and polymeric materials. Chem. Eur. J. 2005, 11, 2610–2620. [Google Scholar] [CrossRef]
- Ren, S.; Bojdys, M.J.; Dawson, R.; Laybourn, A.; Khimyak, Y.Z.; Adams, D.J.; Cooper, A.I. Porous, fluorescent, covalent triazine-based frameworks via room-temperature and microwave-assisted synthesis. Adv. Mater. 2012, 24, 2357–2361. [Google Scholar] [CrossRef]
- Meier, C.B.; Sprick, R.S.; Monti, A.; Guiglion, P.; Lee, J.M.; Zwijnenburg, M.A.; Cooper, A.I. Structure-property relationships for covalent triazine-based frameworks: The effect of spacer length on photocatalytic hydrogen evolution from water. Polymer 2017, 126, 283–290. [Google Scholar] [CrossRef]
- Wang, K.W.; Yang, L.M.; Wang, X.; Guo, L.P.; Cheng, G.; Zhang, C.; Jin, S.B.; Tan, B.; Cooper, A. Covalent triazine frameworks via a low-temperature polycondensation approach. Angew. Chem. Int. Ed. 2017, 56, 14149–14153. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tian, C.; Mahurin, S.M.; Chai, S.H.; Wang, C.; Brown, S.; Veith, G.M.; Luo, H.; Liu, H.; Dai, S. A Superacid-catalyzed synthesis of porous membranes based on triazine frameworks for CO2 separation. J. Am. Chem. Soc. 2012, 134, 10478–10484. [Google Scholar] [CrossRef] [PubMed]
- Katekemol, P.; Roeser, J.; Bojdys, M.J.; Weber, J.; Thomas, A. Covalent triazine frameworks prepared from 1,3,5-tricyanobenzene. Chem. Mater. 2013, 25, 1542–1548. [Google Scholar] [CrossRef]
- Gomes, R.; Bhanja, P.; Bhaumik, A. A triazine-based covalent organic polymer for efficient CO2 adsorption. Chem. Commun. 2015, 51, 10050–10053. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.Y.; Liu, D.Y.; Huang, W.; Liu, J.; Yang, R.Q. Synthesis of covalent triazine-based frameworks with high CO2 adsorption and selectivity. Polym. Chem. 2015, 6, 7410–7417. [Google Scholar] [CrossRef]
- Puthiaraj, P.; Cho, S.M.; Lee, Y.R.; Ahn, W.S. Microporous covalent triazine polymers: Efficient Friedel-Crafts synthesis and adsorption/storage of CO2 and CH4. J. Mater. Chem. A 2015, 3, 6792–6797. [Google Scholar] [CrossRef]
- Saleh, M.; Baek, S.B.; Lee, H.M.; Kim, K.S. Triazine-based microporous polymers for selective adsorption of CO2. J. Phys. Chem. C 2015, 119, 5395–5402. [Google Scholar] [CrossRef]
- Bhunia, A.; Esquivel, D.; Dey, S.; Fernandez-Teran, R.; Goto, Y.; Inagaki, S.; Voort, P.V.D.; Janiak, C. A photoluminescent covalent triazine framework: CO2 adsorption, light-driven hydrogen evolution and sensing of nitroaromatics. J. Mater. Chem. A 2016, 4, 13450–13457. [Google Scholar] [CrossRef]
- Dey, S.; Bhunia, A.; Esquivelb, D.; Janiak, C. Covalent triazine-based frameworks (CTFs) from triptycene and fluorene motifs for CO2 adsorption. J. Mater. Chem. A 2016, 4, 6259–6263. [Google Scholar] [CrossRef]
- Puthiaraj, P.; Kim, S.S.; Ahn, W.S. Covalent triazine polymers using a cyanuric chloride precursor via Friedel-Crafts reaction for CO2 adsorption/separation. Chem. Eng. J. 2016, 283, 184–192. [Google Scholar] [CrossRef]
- Tao, L.M.; Niu, F.; Liu, J.G.; Wang, T.M.; Wang, Q.H. Troger’s base functionalized covalent triazine frameworks for CO2 capture. RSC Adv. 2016, 6, 94365–94372. [Google Scholar] [CrossRef]
- Tao, L.M.; Niu, F.; Wang, C.; Liu, J.G.; Wang, T.M.; Wang, Q.H. Benzimidazole functionalized covalent triazine frameworks for CO2 capture. J. Mater. Chem. A 2016, 4, 11812–11820. [Google Scholar] [CrossRef]
- Wang, K.; Huang, H.; Liu, D.; Wang, C.; Li, J.; Zhong, C. Covalent triazine-based frameworks with ultramicropores and high nitrogen contents for highly selective CO2 capture. Environ. Sci. Technol. 2016, 50, 4869–4876. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tian, C.; Veith, G.M.; Abney, C.W.; Dehaudt, J.; Dai, S. In situ doping strategy for the preparation of conjugated triazine frameworks displaying efficient CO2 capture performance. J. Am. Chem. Soc. 2016, 138, 11497–11500. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Naidu, S.N.; Coskun, A. Chemically activated covalent triazine frameworks with enhanced textural properties for high capacity gas storage. ACS Appl. Mater. Interf. 2017, 9, 30679–30685. [Google Scholar] [CrossRef]
- Buyukcakir, O.; Je, S.H.; Talapaneni, S.N.; Kim, D.; Coskun, A. Charged covalent triazine frameworks for CO2 capture and conversion. ACS Appl. Mater. Interf. 2017, 9, 7209–7216. [Google Scholar] [CrossRef]
- Yuan, K.Y.; Liu, C.; Han, J.; Yu, G.P.; Wang, J.; Duan, H.; Wang, Z.; Jian, X.G. Phthalazinone structure-based covalent triazine frameworks and their gas adsorption and separation properties. RSC Adv. 2016, 6, 12009–12020. [Google Scholar] [CrossRef]
- Liebl, M.R.; Senker, J. Microporous functionalized triazine-based polyimides with high CO2 capture capacity. Chem. Mater. 2013, 25, 970–980. [Google Scholar] [CrossRef]
- Kamiya, K.; Kamai, R.; Hashimoto, K.; Nakanishi, S. Platinum-modified covalent triazine frameworks hybridized with carbon nanoparticles as methanol-tolerant oxygen reduction electrocatalysts. Nat. Commun. 2014, 5, 5040–5047. [Google Scholar] [CrossRef]
- Artz, J.; Mallmann, S.; Palkovits, R. Selective aerobic oxidation of HMF to 2,5-Diformylfuran on covalent triazine frameworks-supported Ru catalysts. ChemSusChem 2015, 8, 672–679. [Google Scholar]
- Chan-Thaw, C.E.; Villa, A.; Wang, D.; Santo, V.D.; Biroli, A.O.; Veith, G.M.; Thomas, M.; Prati, L. PdHx entrapped in a covalent triazine framework modulates selectivity in glycerol oxidation. ChemCatChem 2015, 7, 2149–2154. [Google Scholar] [CrossRef]
- Chan-Thaw, C.E.; Villa, A.; Katekomo, P.; Su, D.; Thomas, A.; Prati, L. Covalent Triazine Framework as Catalytic Support for Liquid Phase Reaction. Nano Lett. 2010, 10, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Puthiaraj, P.; Lee, Y.R.; Zhang, S.Q.; Ahn, W.S. Triazine-based covalent organic polymers: Design, synthesis and applications in heterogeneous catalysis. J. Mater. Chem. A 2016, 4, 16288–16311. [Google Scholar] [CrossRef]
- Roeser, J.; Kailasam, K.; Thomas, A. Covalent triazine frameworks as heterogeneous catalysts for the synthesis of cyclic and linear carbonates from carbon dioxide and epoxides. ChemSusChem 2012, 5, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Gunasekar, G.H.; Park, K.; Ganesan, V.; Lee, K.; Kim, N.K.; Jung, K.D.; Yoon, S. A covalent triazine framework, functionalized with Ir/N-heterocyclic carbene sites, for the efficient hydrogenation of CO2 to formate. Chem. Mater. 2017, 29, 6740–6748. [Google Scholar] [CrossRef]
- Artz, J. Covalent triazine-based frameworks tailor-made catalysts and catalyst supports for molecular and nanoparticulate species. ChemCatChem 2018, 10, 1753–1771. [Google Scholar] [CrossRef]
- Cui, Y.Z.; Du, J.F.; Liu, Y.C.; Yu, Y.; Wang, S.; Pang, H.; Liang, Z.Q.; Yu, G.H. Design and synthesis of a multifunctional porous N-rich polymer containing s-triazine and Troger’s base for CO2 adsorption, catalysis and sensing. Polym. Chem. 2018, 9, 2643–2649. [Google Scholar] [CrossRef]
- Iwase, K.; Kamiya, K.; Miyayama, M.; Hashimoto, K.; Nakanishi, S. Sulfur-linked covalent triazine frameworks doped with coordinatively unsaturated Cu(I) as electrocatalysts for oxygen reduction. ChemElectroChem. 2018, 5, 805–810. [Google Scholar] [CrossRef]
- Kann, A.; Hartmann, H.; Besmehn, A.; Hausoul, P.J.C.; Palkovits, R. Hydrogenation of CO2 to formate over ruthenium immobilized on solid molecular rhosphines. ChemSusChem 2018, 11, 1857–1865. [Google Scholar]
- Xu, N.; Wang, R.L.; Li, D.P.; Meng, X.; Mu, J.L.; Zhou, Z.Y.; Su, Z.M. A new triazine-based covalent organic polymer for efficient photodegradation of both acidic and basic dyes under visible light. Dalton Trans. 2018, 47, 4191–4197. [Google Scholar] [CrossRef]
- Xu, R.; Wang, X.S.; Zhao, H.; Lin, H.; Huang, Y.B.; Cao, R. Rhenium-modified porous covalent triazine framework for highly efficient photocatalytic carbon dioxide reduction in a solid-gas system. Catal. Sci. Technol. 2018, 8, 2224–2230. [Google Scholar] [CrossRef]
- Zhu, G.; Shi, S.; Liu, M.; Zhao, L.; Wang, M.; Zheng, X.; Gao, J.; Xu, J. Formation of strong basicity on covalent triazine frameworks as catalysts for the oxidation of methylene compounds. ACS Appl. Mater. Interfaces 2018, 10, 12612–12617. [Google Scholar]
- Hao, L.; Li, X.L.; Zhi, L.J. Carbonaceous electrode materials for supercapacitors. Adv. Mater. 2013, 25, 3899–3904. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Ning, J.; Luo, B.; Wang, B.; Zhang, Y.; Tang, Z.; Yang, J.; Thomas, A.; Zhi, L. Structural evolution of 2D microporous covalent triazine-based framework toward the study of high-performance supercapacitors. J. Am. Chem. Soc. 2015, 137, 219–225. [Google Scholar]
- Zhu, J.H.; Zhuang, X.D.; Yang, J.; Feng, X.L.; Hiranod, S. Graphene-coupled nitrogen-enriched porous carbon nanosheets for energy storage. J. Mater. Chem. A 2017, 5, 16732–16739. [Google Scholar] [CrossRef]
- Bhanja, P.; Das, S.K.; Bhunia, K.; Pradhan, D.; Hayashi, T.; Hijikata, Y.; Irle, S.; Bhaumik, A. A new porous polymer for highly efficient capacitive energy storage. ACS Sustain. Chem. Eng. 2018, 6, 202–209. [Google Scholar] [CrossRef]
- Bi, J.H.; Fang, W.; Li, L.Y.; Wang, J.Y.; Liang, S.J.; He, Y.H.; Liu, M.H.; Wu, L. Covalent triazine-based frameworks as visible light photocatalysts for the splitting of water. Macromol. Rapid Commun. 2015, 36, 1799–1805. [Google Scholar] [CrossRef]
- Schwinghammer, K.; Hug, S.; Mesch, M.B.; Senkerd, J.; Lotsch, B.V. Phenyl-triazine oligomers for light-driven hydrogen evolution. Energy Environ. Sci. 2015, 8, 3345–3353. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Byun, J.; Rörich, I.; Ramanan, C.; Blom, P.W.M.; Lu, H.; Wang, D.; da Caire, S.L.; Li, R.; Wang, L.; et al. Asymmetric covalent triazine framework for enhanced visible-light photoredox catalysis via energy transfer cascade. Angew. Chem. Int. Ed. 2018, 57, 8316–8320. [Google Scholar] [CrossRef]
- Jiang, Q.; Sun, L.; Bi, J.; Liang, S.; Li, L.; Yu, Y.; Wu, L. MoS2 quantum dots-modified covalent triazine-based frameworks for enhanced photocatalytic hydrogen evolution. ChemSusChem. 2018, 11, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, Z.J.; Ma, B.C.; Ghasimi, S.; Gehrig, D.; Laquai, F.; Landfester, K.; Zhang, K. Hollow nanoporous covalent triazine frameworks via acid vapor-assisted solid phase synthesis for enhanced visible light photoactivity. J. Mater. Chem. A 2016, 4, 7555–7559. [Google Scholar] [CrossRef] [Green Version]
- Hug, S.; Tauchert, M.E.; Li, S.; Pachmayr, U.E.; Lotsch, B.V. A functional triazine framework based on N-heterocyclic building blocks. J. Mater. Chem. 2012, 22, 13956–13964. [Google Scholar] [CrossRef]
- Ma, H.; Ren, H.; Meng, S.; Sun, F.; Zhu, G. Novel porphyrinic porous organic frameworks for high performance separation of small hydrocarbons. Sci. Rep. 2013, 3, 2611. [Google Scholar] [CrossRef] [PubMed]
- Jena, H.S.; Krishnara, C.; Wang, G.; Leus, K.; Schmidt, J.; Chaoui, N.; Voort, P.V.D. Acetylacetone covalent triazine framework: An efficient carbon capture and storage material and a highly stable heterogeneous catalyst. Chem. Mater. 2018, 30, 4102–4111. [Google Scholar] [CrossRef]
- Liu, J.J.; Zan, Z.; Li, L.; Yang, Y.; Bu, F.X.; Xu, Y.X. Solution synthesis of semiconducting two-dimensional polymer via trimerization of carbonitrile. J. Am. Chem. Soc. 2017, 139, 11666–11669. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Mahmood, J.; Noh, H.J.; Seo, J.M.; Jung, S.M.; Shin, S.H.; Im, Y.K.; Jeon, I.Y.; Baek, J.B. Direct synthesis of a covalent triazine-based framework from aromatic amides. Angew. Chem. Int. Ed. 2018, 57, 8438–8442. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Zheng, S.H.; Liu, X.; Li, P.; Sun, L.; Yang, R.X.; Wang, S.; Wu, Z.S.; Bao, X.H.; Deng, W.Q. Conductive microporous covalent triazine-Based framework for high-performance electrochemical capacitive energy storage. Angew. Chem. Int. Ed. 2018, 57, 7992–7996. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.J.; Shevlin, S.A.; Ruan, Q.S.; Moniz, S.J.A.; Liu, Y.R.; Liu, X.; Li, Y.M.; Lau, C.C.; Guo, Z.X.; Tang, J.W. Efficient visible light-driven water oxidation and proton reduction by an ordered covalent triazine-based framework. Energy Environ. Sci. 2018, 11, 1617–1624. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.M.; Chen, Q.; Zhao, Y.C.; Laursen, B.W.; Han, B.H. Facile synthesis of hierarchical triazine-based porous carbons for hydrogen storage. Microporous Microporous Mater. 2016, 224, 129–134. [Google Scholar] [CrossRef]
- Bhunia, A.; Vasylyeva, V.; Janiak, C. From a supramolecular tetranitrile to a porous covalent triazine-based framework with high gas uptake capacities. Chem. Commun. 2013, 49, 3961–3963. [Google Scholar] [CrossRef]
- Hug, S.; Mesch, M.B.; Oh, H.; Popp, N.; Hirscher, M.; Senker, J.; Lotsch, B.V. A fluorene based covalent triazine framework with high CO2 and H2 capture and storage capacities. J. Mater. Chem. A 2014, 2, 5928–5936. [Google Scholar] [CrossRef]
- Chen, X.W.; Yuan, F.; Gu, Q.F.; Yu, X.B. Light metals decorated covalent triazine-based frameworks as a high capacity hydrogen storage medium. J. Mater. Chem. A 2013, 1, 11705–11710. [Google Scholar] [CrossRef]
- He, H.; Chen, X.; Zou, W.; Li, R. Transition metal decorated covalent triazine-based frameworks as a capacity hydrogen storage medium. Int. J. Hydrogen. Energ. 2018, 43, 2823–2830. [Google Scholar] [CrossRef]
- Bhunia, A.; Boldog, I.; Moller, A.; Janiak, C. Highly stable nanoporous covalent triazine-based frameworks with an adamantane core for carbon dioxide sorption and separation. J. Mater. Chem. A 2013, 1, 14990–14999. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Yao, K.X.; Teng, B.Y.; Zhang, T.; Han, Y. A perfluorinated covalent triazine-based framework for highly selective and water-tolerant CO2 capture. Energy Environ. Sci. 2013, 6, 3684–3692. [Google Scholar] [CrossRef]
- Hug, S.; Stegbauer, L.; Oh, H.; Hirscher, M.; Lotsch, B.V. Nitrogen-rich covalent triazine frameworks as high-performance platforms for selective carbon capture and storage. Chem. Mater. 2015, 27, 8001–8010. [Google Scholar] [CrossRef]
- Dey, S.; Bhunia, A.; Boldog, I.; Janiak, C. A mixed-linker approach towards improving covalent triazine-based frameworks for CO2 capture and separation. Microporous Microporous Mater. 2017, 241, 303–315. [Google Scholar] [CrossRef]
- Dey, S.; Bhunia, A.; Breitzke, H.; Groszewicz, P.B.; Buntkowskyb, G.; Janiak, C. Two linkers are better than one: Enhancing CO2 capture and separation with porous covalent triazine-based frameworks from mixed nitrile linkers. J. Mater. Chem. A. 2017, 5, 3609–3620. [Google Scholar] [CrossRef]
- Tuci, G.; Pilaski, M.; Ba, H.; Rossin, A.; Luconi, L.; Caporali, S.; Pham-Huu, C.; Palkovits, R.; Giambastiani, G. Unraveling surface basicity and bulk morphology relationship on covalent triazine frameworks with unique catalytic and gas adsorption properties. Adv. Funct. Mater. 2017, 27, 1605672. [Google Scholar] [CrossRef]
- Wang, G.; Leus, K.; Zhao, S.; Voort, P.V.D. Newly designed covalent triazine framework based on novel N-heteroaromatic building blocks for efficient CO2 and H2 capture and storage. ACS Appl. Mater. Interfaces 2018, 10, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Gu, S.; Fu, Y.; Xiong, S.; Pan, C.; Liu, Y.; Yu, G. Carbazole-decorated covalent triazine frameworks: Novel nonmetal catalysts for carbon dioxide fixation and oxygen reduction reaction. J. Catal. 2018, 362, 1–9. [Google Scholar] [CrossRef]
- Hu, X.M.; Chen, Q.; Zhao, Y.C.; Laursen, B.W.; Han, B.H. Straightforward synthesis of a triazine-based porous carbon with high gas-uptake capacities. J. Mater. Chem. A 2014, 2, 14201–14208. [Google Scholar] [CrossRef]
- Gomes, R.; Bhaumik, A. A new triazine functionalized luminescent covalent organic framework for nitroaromatic sensing and CO2 storage. RSC Adv. 2016, 6, 28047–28054. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, P.; Zhao, J.J. 2D covalent triazine framework: A new class of organic photocatalyst for water splitting. J. Mater. Chem. A 2015, 3, 7750–7758. [Google Scholar] [CrossRef]
- Li, L.; Fang, W.; Zhang, P.; Bi, J.; He, Y.; Wang, J.; Su, W. Sulfur-doped covalent triazine-based frameworks for enhanced photocatalytic hydrogen evolution from water under visible light. J. Mater. Chem. A 2016, 4, 12402–12406. [Google Scholar] [CrossRef]
- Kuecken, S.; Acharjya, A.; Zhi, L.; Schwarze, M.; Schomäcker, R.; Thomas, A. Fast tuning of covalent triazine frameworks for photocatalytic hydrogen evolution. Chem. Commun. 2017, 53, 5854–5857. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.A.; Fang, Y.; Zhang, Y.; Wang, X. Photocatalytic oxygen evolution from functional triazine-based polymers with tunable band structures. Angew. Chem. Int. Ed. 2018, 130, 479–483. [Google Scholar] [CrossRef]
- Liu, M.Y.; Huang, Q.; Wang, S.L.; Li, Z.Y.; Li, B.Y.; Jin, S.B.; Tan, B. Crystalline covalent triazine frameworks by in situ oxidation of alcohols to aldehyde monomers. Angew. Chem. Int. Ed. 2018, 57, 11968–11972. [Google Scholar] [CrossRef]
- Yadav, R.K.; Kumar, A.; Park, N.J.; Kong, K.J.; Baeg, J.O. A highly efficient covalent organic framework film photocatalyst for selective solar fuel production from CO2. J. Mater. Chem. A 2016, 4, 9413–9418. [Google Scholar] [CrossRef]
- Iwase, K.; Yoshioka, T.; Nakanishi, S.; Hashimoto, K.; Kamiya, K. Copper-modified covalent triazine frameworks as non-noble-metal electrocatalysts for oxygen reduction. Angew. Chem. Int. Ed. 2015, 54, 11068–11072. [Google Scholar]
- Hao, L.; Zhang, S.S.; Liu, R.J.; Ning, J.; Zhang, G.J.; Zhi, L.J. Bottom-up construction of triazine-based frameworks as metal-free electrocatalysts for oxygen reduction reaction. Adv. Mater. 2015, 27, 3190–3195. [Google Scholar] [CrossRef] [PubMed]
- Palkovits, R.; Antonietti, M.; Kuhn, P.; Thomas, A.; Schüth, F. Solid catalysts for the selective low-temperature oxidation of methane to methanol. Angew. Chem. Int. Ed. 2009, 48, 6909–6912. [Google Scholar] [CrossRef] [PubMed]
- Soorholtz, M.; Jones, L.C.; Samuelis, D.; Weidenthaler, C.; White, R.J.; Titirici, M.M.; Cullen, D.A.; Zimmermann, T.; Antonietti, M.; Maier, J.; et al. Local platinum environments in a solid analogue of the molecular periana catalyst. ACS Catal. 2016, 6, 2332–2340. [Google Scholar] [CrossRef]
- Hao, L.; Luo, B.; Li, X.L.; Jin, M.H.; Fang, Y.; Tang, Z.H.; Jia, Y.Y.; Liang, M.H.; Thomas, A.; Yang, J.H.; et al. Terephthalonitrile-derived nitrogen-rich networks for high performance supercapacitors. Energy Environ. Sci. 2012, 5, 9747–9751. [Google Scholar]
- Liao, H.; Ding, H.; Li, B.; Ai, X.; Wang, C. Covalent-organic frameworks: Potential host materials for sulfur impregnation in lithium–sulfur batteries. J. Mater. Chem. A 2014, 2, 8854–8858. [Google Scholar] [CrossRef]
- Talapaneni, S.N.; Hwang, T.H.; Je, S.H.; Buyukcakir, O.; Choi, J.W.; Coskun, A. elemental-sulfur-mediated facile synthesis of a covalent triazine framework for high-performance lithium–sulfur batteries. Angew. Chem. Int. Ed. 2016, 55, 3106–3111. [Google Scholar] [CrossRef]
- Shan, J.; Liu, Y.; Su, Y.; Liu, P.; Zhuang, X.; Wu, D.; Zhang, F.; Feng, X. Graphene-directed two-dimensional porous carbon frameworks for high-performance lithium–sulfur battery cathodes. J. Mater. Chem. A 2016, 4, 314–320. [Google Scholar] [CrossRef]
- Stoeck, U.; Balach, J.; Klose, M.; Wadewitz, D.; Ahrens, E.; Eckert, J.; Giebeler, L. Reconfiguration of lithium sulphur batteries: “Enhancement of Li–S cell performance by employing a highly porous conductive separator coating”. J. Power Sources 2016, 309, 76–81. [Google Scholar] [CrossRef]
- Xu, F.; Yang, S.; Jiang, G.; Ye, Q.; Wei, B.; Wang, H. Fluorinated, sulfur-rich, covalent triazine frameworks for enhanced confinement of polysulfides in lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2017, 9, 37731–37738. [Google Scholar] [CrossRef]
- Su, P.; Iwase, K.; Harada, T.; Kamiya, K.; Nakanishi, S. Covalent triazine framework modified with coordinatively-unsaturated Co or Ni atoms for CO2 electrochemical reduction. Chem. Sci. 2018, 9, 3941–3947. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Chen, J.X.; Wang, G.X.; Li, Y.; Wen, Z.H. Perfluorinated covalent triazine framework derived hybrids for the highly selective electroconversion of carbon dioxide into methane. Angew. Chem. Int. Ed. 2018, 57, 13120–13124. [Google Scholar] [CrossRef] [PubMed]








| CTFs | Surface Area (m2 g−1) | Condition | Hydrogen Uptake (wt %) | References |
|---|---|---|---|---|
| DCBP network | 2475 | 1.00 bar, 77 K | 1.55 | [6] |
| TPC-2 | 1250 | 1.00 bar, 77 K | 2.34 | [60] |
| TPC-3 | 1530 | 1.00 bar, 77 K | 1.84 | [60] |
| PCTF-1 | 2235 | 1.0 bar, 77 K | 1.86 | [61] |
| fl-CTF400 | 2862 | 1.0 bar, 77 K | 1.95 | [62] |
| fl-CTF400 | 2862 | 20 bar, 77 K | 4.36 | [62] |
| caCTF-1-700 | 2367 | 77, 1 bar | 2.46 | [26] |
| caCTF-1-700 | 2367 | 87 K, 1 bar | 1.66 | [26] |
| CTF–Li6 | - | - | 12.3 | [63] |
| CTF–Na6 | - | - | 10.3 | [63] |
| CTF–Ca6 | - | - | 8.8 | [63] |
| CTFs | Surface Area (m2 g−1) | Condition | CO2 Uptake | References |
|---|---|---|---|---|
| P6M | 947 | 273 K and 1 bar | 4.17 mmol g−1 | [10] |
| PCTF-1 | 2235 | 273 K, 1 bar | 73.0 cm g−1 | [65] |
| PCTF-5 | 1183 | 273 K, 1 bar | 58.1 cm g−1 | [65] |
| PCTF-7 | 613 | 273 K, 1 bar | 48.9 cm g−1 | [65] |
| CTF-1-600 | 1553 | 273 K, 1 bar | 3.83 mmol g−1 | [66] |
| F-CTF-1-600 | 1535 | 273 K, 1 bar | 5.53 mmol g−1 | [66] |
| pym-CTF-500 | 208 | 273 K, 1 bar | 2.75 mmol g−1 | [67] |
| Bipy-CTF600 | 2479 | 273 K, 1 bar | 5.58 mmol g−1 | [67] |
| Ad4L1 | 1617 | 273 K, 1 bar | 76.33 cm3 g−1 | [68] |
| Ad4L3 | 1341 | 273 K, 1 bar | 74.58 cm3 g−1 | [68] |
| MM1 | 1800 | 273 K, 1 bar | 83.5 cm3 g−1 (3.68 mmol g−1) | [69] |
| MM2 | 1360 | 273 K, 1 bar | 106.8 cm3 g−1 (4.70 mmol g−1) | [69] |
| CTF-Ph | 1991 | 273 K, 1 bar | 3.05 mmol g−1 | [70] |
| CTF-Py | 1239 | 273 K, 1 bar | 3.79 mmol g−1 | [70] |
| CTF-20-400 | 1458 | 273 K, 1 bar | 3.48 mmol g−1 | [71] |
| CTF-5-500 | 853 | 273 K, 1 bar | 3.02 mmol g−1 | [71] |
| CTF-CSU1 | 685 | 273 K, 1 bar | 15.1 wt % | [72] |
| CTF-CSU19 | 982 | 273 K, 1 bar | 12.9 wt % | [72] |
| Acac-CTF-10-500 | 1556 | 273 K, 1 bar | 3.30 mmol g−1 | [55] |
| TPC-1 | 1940 | 273 K, 1 bar | 4.90 mmol g−1 | [73] |
| cCTF-400 | 744 | 273 K, 1 bar | 126 mg g−1 | [26] |
| cCTF-500 | 1247 | 273 K, 1 bar | 133 mg g−1 | [26] |
| TRIPTA-COF | 609 | 273 K, 5 bar | 12.97 mmol g−1 | [74] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Jin, S. Recent Advancements in the Synthesis of Covalent Triazine Frameworks for Energy and Environmental Applications. Polymers 2019, 11, 31. https://doi.org/10.3390/polym11010031
Zhang Y, Jin S. Recent Advancements in the Synthesis of Covalent Triazine Frameworks for Energy and Environmental Applications. Polymers. 2019; 11(1):31. https://doi.org/10.3390/polym11010031
Chicago/Turabian StyleZhang, Ying, and Shangbin Jin. 2019. "Recent Advancements in the Synthesis of Covalent Triazine Frameworks for Energy and Environmental Applications" Polymers 11, no. 1: 31. https://doi.org/10.3390/polym11010031
APA StyleZhang, Y., & Jin, S. (2019). Recent Advancements in the Synthesis of Covalent Triazine Frameworks for Energy and Environmental Applications. Polymers, 11(1), 31. https://doi.org/10.3390/polym11010031