An Industrial Scale Synthesis of Adipicdihydrazide (ADH)/Polyacrylate Hybrid with Excellent Formaldehyde Degradation Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Synthesis of Acrylic Emulsion
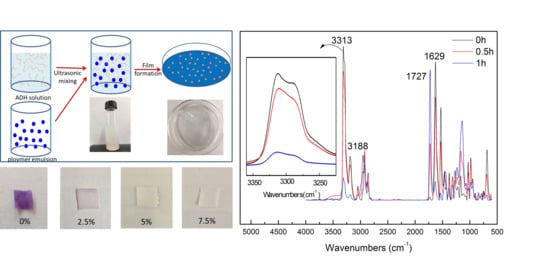
2.3. Preparation of ADH/P(MMA-BA-MAA) Hybrid Latex
2.4. Preparation of ADH/P(MMA-BA-MAA) Hybrid Films
2.5. Characterization and Instruments
2.5.1. Experimental Procedure of Formaldehyde Degradation
- (a)
- Dissolve 0.25 g AHMT in 50 mL, 0.1 mol/L hydrochloric acid solution (a.q.) to obtain AHMT solution;
- (b)
- Cut the qualitative filter paper into pieces of 1 cm × 1 cm and add a drop of 50 mg/kg formaldehyde solution using a pipetting gun;
- (c)
- Get the filter paper with clean tweezers, scrape the filter paper gently to remove the excess fluid, and paste it on the aldehyde removal hybrid film. The filter paper and hybrid film are placed in a large culture dish and encapsulated in a transparent seal bag. Let stand for one hour at room temperature;
- (d)
- Take out filter paper and drop 5 mol/L KOH solution and AHMT reagent on the filter paper. Let stand for 5 min and observe the color changes, and take photos with single lens reflex camera.
2.5.2. FTIR of Hybrid Films
2.5.3. Other Measurements
3. Results and Discussion
3.1. Characterization of ADH/P(MMA-BA-MAA) Hybrid Latex
3.2. Characterization of ADH/P(MMA/BA/MAA) Hybrid Films
3.3. Detection of Formaldehyde Degradation
3.3.1. Reaction Mechanism of Formaldehyde and ADH
3.3.2. Qualitative Test of the AHMT-RGB Method
3.3.3. Semi-Quantitative Measurement
3.4. Measurement of Thermodynamic Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salem, M.Z.M.; Böhm, M. Understanding of Formaldehyde Emissions from Solid Wood: An Overview. Bioresource 2013, 8, 4775–4790. [Google Scholar] [CrossRef]
- Nowshad, F.; Islam, M.N.; Khan, M.S. Concentration and Formation Behavior of Naturally Occurring Formaldehyde in Foods. Agric. Food Secur. 2018, 7, 17–25. [Google Scholar] [CrossRef]
- Wahed, P.; Razzaq, M.A.; Dharmapuri, S.; Corrales, M. Determination of Formaldehyde in Food and Feed by an In-house Validated HPLC Method. Food Chem. 2016, 202, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.F.; Duan, C.L.; Li, X.X.; Zhao, Y.; Cao, F.H.; Shang, S.; Ding, S.M.; Yue, X.P.; Gao, G.; Yang, H.; et al. Reduction of Endogenous Melatonin Accelerates Cognitive Decline in Mice in a Simulated Occupational Formaldehyde Exposure Environment. Int. J. Environ. Res. Public Health 2016, 13, 258. [Google Scholar] [CrossRef] [PubMed]
- Waydhas, C.; Weigl, K.; Sies, H. The Disposition of Formaldehyde and Formate Arising from Drug N-Demethylations Dependent on Cytochrome P-450 in Hepatocytes and in Perfused Rat Liver. Eur. J. Biochem. 1978, 89, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Norliana, S.; Abdulamir, A.S.; Bakar, F.A. The Health Risk of Formaldehyde to Human Beings. Am. J. Pharmacol. Toxicol. 2009, 4, 98–106. [Google Scholar] [CrossRef]
- Salthammer, T.; Mentese, S.; Marutzky, R. Formaldehyde in Indoor Environment. Chem. Rev. 2010, 110, 2536–2572. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Lim, D.; Lee, Y.K.; Kim, J.H. Effects of Indoor Air Pollutants on Atopic Dermatitis. Int. J. Environ. Res. Public Health 2016, 13, 1220. [Google Scholar] [CrossRef]
- Chen, X.Y.; Li, F.; Liu, C.Y.; Yang, J.; Zhang, J.D.; Peng, C.L. Monitoring, Human Health Risk Assessment and Optimized Management for Typical Pollutants in Indoor Air from Random Families of University Staff, Wuhan City, China. Sustainability 2017, 9, 1115. [Google Scholar] [CrossRef]
- Spengler, J.; Sexton, K. Indoor air pollution: A public health perspective. Science 1983, 221, 9–17. [Google Scholar] [CrossRef]
- Wen, Q.B.; Li, C.T.; Cai, Z.H.; Zhang, W.; Gao, H.L.; Chen, L.J.; Zeng, G.M.; Shu, X.; Zhao, Y.P. Study on Activated Carbon Derived from Sewage Sludge for Absorption of Gaseous Formaldehyde. Bioresour. Technol. 2011, 102, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Sekine, Y. Oxidative Decomposition of Dormaldehyde by Metal Oxides at Room Temperature. Atmos. Environ. 2002, 36, 5543–5547. [Google Scholar] [CrossRef]
- Liu, Y. Study on Properties and Application of Amine Formaldehyde Fixative; Chang’an University: Xi’an, China, 2009. [Google Scholar]
- Rengga, W.D.P.; Chafidz, A.; Sudibandriyo, M.; Nasikin, M.; Abasaeed, A.E. Silver Nano-particles Deposited on Bamboo-based Activated Carbon for Removal of Formaldehyde. J. Environ. Chem. Eng. 2017, 5, 1657–1665. [Google Scholar] [CrossRef]
- He, B.J. Preparation and Properties Investigation of Formaldehyde-Eliminative Wood Coatings; South China University of Technology: Guangzhou, China, 2016. [Google Scholar]
- Wang, B.; Wilkes, G.L.; Hedrick, J.C.; Liptak, S.C.; Mcgrath, J.E. New High Refractive Index Organic/Inorganic Hybrid Materials from Sol-Gel Processing. Macromolecules 1991, 24, 3449–3450. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.T.; Wu, X.Y.; Zhang, G.K. Pt-TiO2, microspheres with exposed {001 facets for degradation of formaldehyde in air: Formation mechanism and enhanced visible light photocatalytic activity. Mater. Res. Bull. 2017, 96, 262–269. [Google Scholar] [CrossRef]
- Hu, M.; Yao, Z.; Liu, X.; Ma, L.; He, Z.; Wang, X. Enhancement mechanism of hydroxyapatite for photocatalytic degradation of gaseous formaldehyde over TiO2/hydroxyapatite. J. Taiwan Inst. Chem. Eng. 2018, 85, 91–97. [Google Scholar] [CrossRef]
- McMurry, J. Fundamentals of Organic Chemistry; Mechanical Industry Press: Beijing, China, 2005; pp. 276–397. [Google Scholar]
- Robert, D. Introduction to Chemical Analysis. J. Chem. Educ. 1983, 60, 283. [Google Scholar]
- Minyaev, R.M.; Starikov, A.G.; Lepin, E.A. Pathways of Reactions of Nucleophilic Addition of H2O and HF Molecules to Formaldehyde in Gas Phase and in Complex with Formic Acid: Ab initio, Calculations. Russ. Chem. Bull. 1998, 47, 2078–2086. [Google Scholar] [CrossRef]
- Wang, S.; Iglesia, E. Entropy-Driven High Reactivity of Formaldehyde in Nucleophilic Attacked by Enolates on Oxide Surfaces. J. Am. Chem. Soc. 2018, 140, 775–782. [Google Scholar] [CrossRef]
- Valentin, C.D.; Rosa, M.; Pacchioni, G. Radical Versus Nucleophilic Mechanism of Formaldehyde Polymerization Catalyzed by (WO3)3 Clusters on Reduced or Stoichiometric TiO2(110). J. Am. Chem. Soc. 2012, 134, 14086–14098. [Google Scholar] [CrossRef]
- Photong, S.; Boonamnuayvitaya, V. Enhancement of Formaldehyde Degradation by Amine Functionalized Silica/Titania films. J. Environ. Sci. 2009, 21, 1741–1746. [Google Scholar] [CrossRef]
- Photong, S.; Boonamnuayvitaya, V. Preparation and Characterization of Amine-functionalized SiO2/TiO2 Films for Formaldehyde Degradation. Appl. Surf. Sci. 2009, 255, 9311–9315. [Google Scholar] [CrossRef]
- Saeung, S.; Boonamnuayvitaya, V. Absorption of Formaldehyde Vapor by Amine-functionalized Mesoporous Silica Materials. J. Environ. Sci. 2008, 20, 379–384. [Google Scholar]
- Sprung, M. A Summary of the Reactions of Aldehydes with Amines. Chem. Rev. 1940, 26, 297–338. [Google Scholar] [CrossRef]
- Xu, Z.J.; Wang, L.; Hou, H.P. Formaldehyde Removal by Potted Plant-soil Systems. J. Hazard. Mater. 2011, 192, 314–318. [Google Scholar] [CrossRef]
- Hanson, A.D.; Roje, S. One-carbon metabolism in higher plants. Annu. Rev. Plant Biol. 2001, 52, 119–137. [Google Scholar] [CrossRef]
- Haslam, R.; Rust, S.; Pallett, K.; Cole, D.; Coleman, J. Cloning and Characterisation of S-formylglutathione Hydrolase from Arabidopsisthaliana: A Pathway for Formaldehyde Detoxification. Plant Physiol. Biochem. 2002, 40, 281–288. [Google Scholar] [CrossRef]
- Guo, Z.Q.; Hua, Y.P.; Tong, H.B.; Wei, X.H. Synthesis and Crystal Structure Analysis of 3,5,5,7-Tetraacetylnonane-2,8-Dione. J. Chem. Crystallogr. 2011, 41, 1501–1504. [Google Scholar] [CrossRef]
- Photong, S.; Boonamnuayvitaya, V. Synthesis of APTMS-Functionalized SiO2/TiO2, Transparent Film Using Peroxo Titanic Acid Refluxed Solution for Formaldehyde Removal. Water Air Soil Pollut. 2010, 210, 453–461. [Google Scholar] [CrossRef]
- Ma, C.J.; Li, X.H.; Zhu, T.L. Removal of Low-concentration Formaldehyde in Air by Absorption on Activated Carbon Modified by Hexamethylenediamine. Carbon 2011, 49, 2873–2875. [Google Scholar] [CrossRef]
- Li, J.R.; Yu, Q.L.; Hu, Z.H.; Zhu, J.L.; Jia, J. Study on the Reaction Characteristics of Tea Polyphenols and Formaldehyde. J. Chin. Inst. Food Sci. Technol. 2008, 8, 52–57. [Google Scholar]
- Sun, H.Y.; Fang, W.J.; Guo, Y.S.; Zhao, Z.R.; Lin, R.S. Effects of Anti oxidation on Thermal-oxidation stability of Fuel NNJ-150. J. Chem. Eng. Chin.Univ. 2006, 20, 455–459. [Google Scholar]
- Gallardo, V.; Morales, M.E.; Ruiz, M.A.; Delgado, A.V. An Experimental Investigation of Stability of Ethylcellulose Latex Correlation between Zeta Potential and Sedimentation. Eur. J. Pharm. Sci. 2005, 26, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Patze, C.; Broedner, K.; Rominger, F.; Trapp, O.; Bunz, U.H. Aldehyde cruciforms: Dosimeters for Primary and Secondary Amines. Chemistry 2011, 17, 13720–13725. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Jia, F.; Ma, L.N.; Li, Z.P. FeCl2-Catalyzed Oxidative Amidation of Aldehydes with Primary and Secondary Amines. Acta Chim. Sin. 2015, 73, 1–4. [Google Scholar] [CrossRef]
- Kawamura, K.; Kermana, K.; Fujiharab, M.; Nagatania, N.; Hashibab, T.; Tamiya, E. Development of a novel hand-held formaldehyde gas sensor for the rapid detection of sick building syndrome. Sens. Actuators B Chem. 2005, 105, 495–501. [Google Scholar] [CrossRef]
- Liu, X.; Bayer, A.K.; Xue, G. Infrared Quantitative Study of Acetylation of Microspheres of Polystyrene. Spectrosc. Lett. 1997, 30, 17–23. [Google Scholar] [CrossRef]
- Court, R.W.; Sephton, M.A. Quantitative flash pyrolysis Fourier transform infrared spectroscopy of organic materials. Anal. Chim. Acta 2009, 639, 62–66. [Google Scholar] [CrossRef]
- Liu, K.Z.; Jia, L.; Kelsey, S.M.; Newland, A.C.; Mantsch, H.H. Quantitative determination of apoptosis on leukemia cells by infrared spectroscopy. Apoptosis 2001, 6, 269–278. [Google Scholar] [CrossRef]
- Christy, A.A.; Hopland, A.L.; Barth, T.; Kvalheim, O.M. Quantitative determination of thermal maturity in sedimentary organic matter by diffuse reflectance infrared spectroscopy of asphaltenes. Org. Geochem. 1989, 14, 77–81. [Google Scholar] [CrossRef]
- Matsukawa, S.; Ando, I. Study of Self-Diffusion of Molecules in a Polymer Gel by Pulsed-Gradient Spin-Echo 1H NMR. 2. Intermolecular Hydrogen-Bond Interaction between the Probe Polymer and Network Polymer in N,N-Dimethylacrylamide−Acrylic Acid Copolymer Gel Systems. Macromolecules 1999, 30, 1865–1871. [Google Scholar] [CrossRef]











| Ingredient | Weight (g) | |
|---|---|---|
| monomer | MMA | 50 |
| BA | 48 | |
| MAA | 2 | |
| co-stabilizer | HD | 2 |
| emulsifier | SDS | 2 |
| Initiator | KPS | 0.5 |
| Deionized water | DI water | 150 |
| Time | S1(0 h) | S2(0.5 h) | S3(1 h) | |
|---|---|---|---|---|
| Parameter | ||||
| Peak heights | 0.63727 | 0.53486 | 0.09174 | |
| Sample | TGA Data | DSC Data (°C) | |||
|---|---|---|---|---|---|
| T0.5 (°C) | Residual Carbon (%) | Tonset | Tend | Tg | |
| Neat polymer | 395.8 | 4.8 | 6.2 | 18.4 | 15 |
| ADH/polymer hybrid | 403.2 | 6.5 | 14 | 25.1 | 18.9 |
| Hybrid film after adsorbing HCHO | 404.1 | 6.8 | 15.4 | 26.8 | 20.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, R.; Chen, R.; Duo, Y.; Zhang, S.; Xie, D.; Mei, Y. An Industrial Scale Synthesis of Adipicdihydrazide (ADH)/Polyacrylate Hybrid with Excellent Formaldehyde Degradation Performance. Polymers 2019, 11, 86. https://doi.org/10.3390/polym11010086
Zhu R, Chen R, Duo Y, Zhang S, Xie D, Mei Y. An Industrial Scale Synthesis of Adipicdihydrazide (ADH)/Polyacrylate Hybrid with Excellent Formaldehyde Degradation Performance. Polymers. 2019; 11(1):86. https://doi.org/10.3390/polym11010086
Chicago/Turabian StyleZhu, Rui, Renjie Chen, Yunxia Duo, Saigang Zhang, Delong Xie, and Yi Mei. 2019. "An Industrial Scale Synthesis of Adipicdihydrazide (ADH)/Polyacrylate Hybrid with Excellent Formaldehyde Degradation Performance" Polymers 11, no. 1: 86. https://doi.org/10.3390/polym11010086
APA StyleZhu, R., Chen, R., Duo, Y., Zhang, S., Xie, D., & Mei, Y. (2019). An Industrial Scale Synthesis of Adipicdihydrazide (ADH)/Polyacrylate Hybrid with Excellent Formaldehyde Degradation Performance. Polymers, 11(1), 86. https://doi.org/10.3390/polym11010086