Furfuryl Alcohol and Lactic Acid Blends: Homo- or Co-Polymerization?
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PFA–PLA Polymers
2.3. Bending Test
2.4. Scanning Electron Microscopy (SEM)
2.5. Solubility Tests
2.6. Thermogravimetric Analysis (TG)
2.7. Differential Scanning Calorimetry (DSC)
2.8. FT-IR Spectroscopy
2.9. Solid State 13C–NMR Spectroscopy
3. Results and Discussion
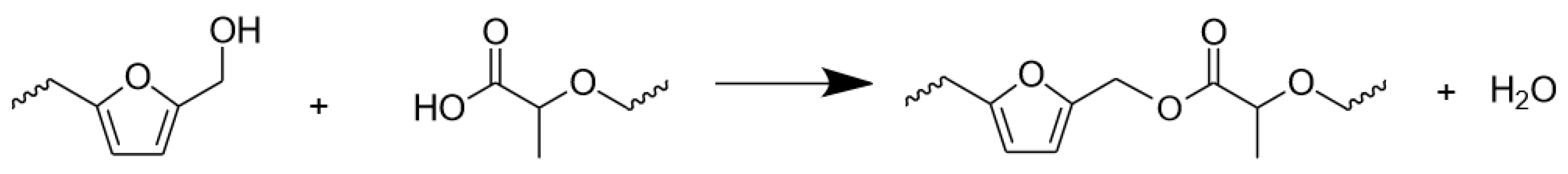
3.1. Preparation Method
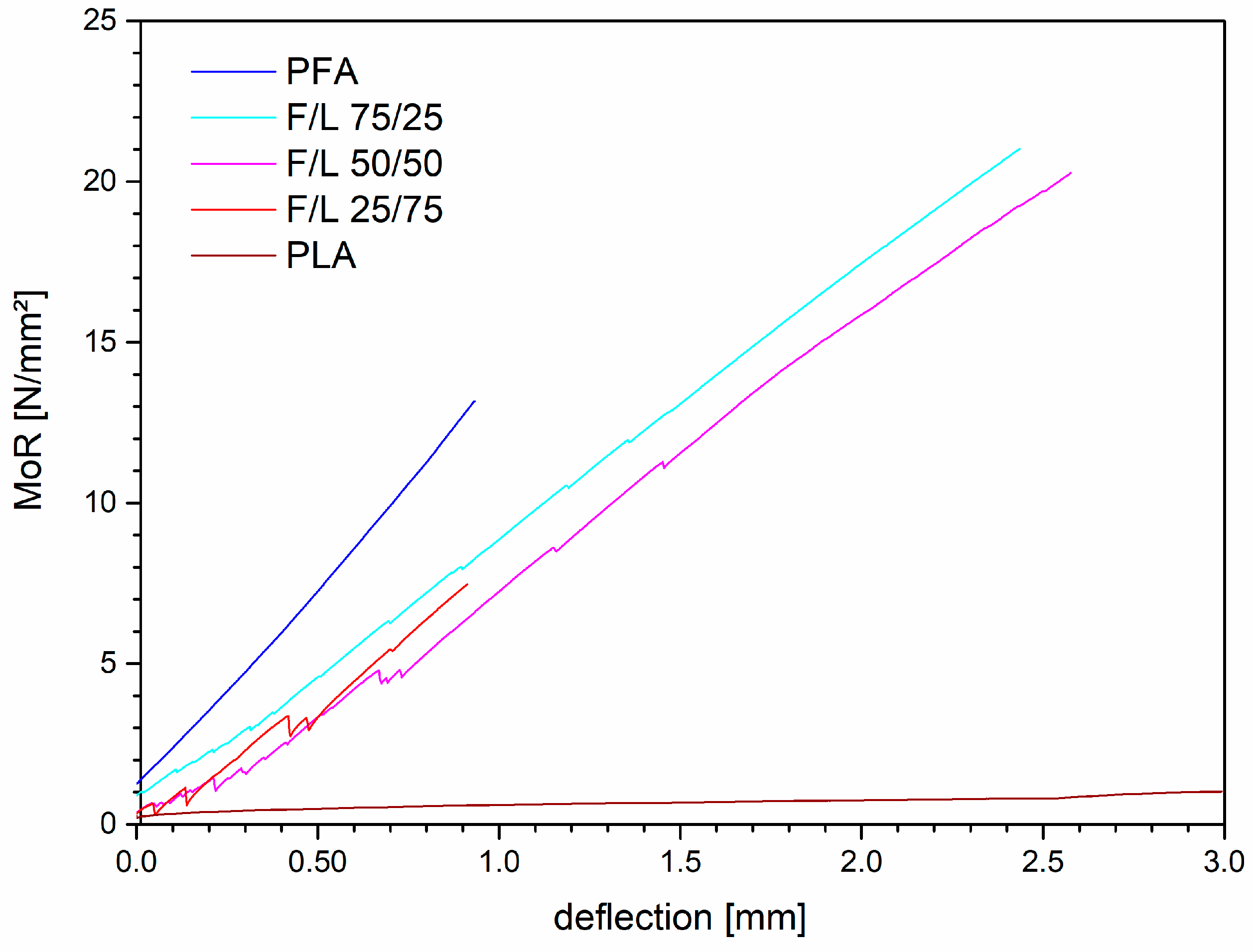
3.2. Bending Tests
3.3. SEM Analysis
3.4. Solubility Tests
3.5. Thermogravimetric Analysis
3.6. Differential Scanning Calorimetry
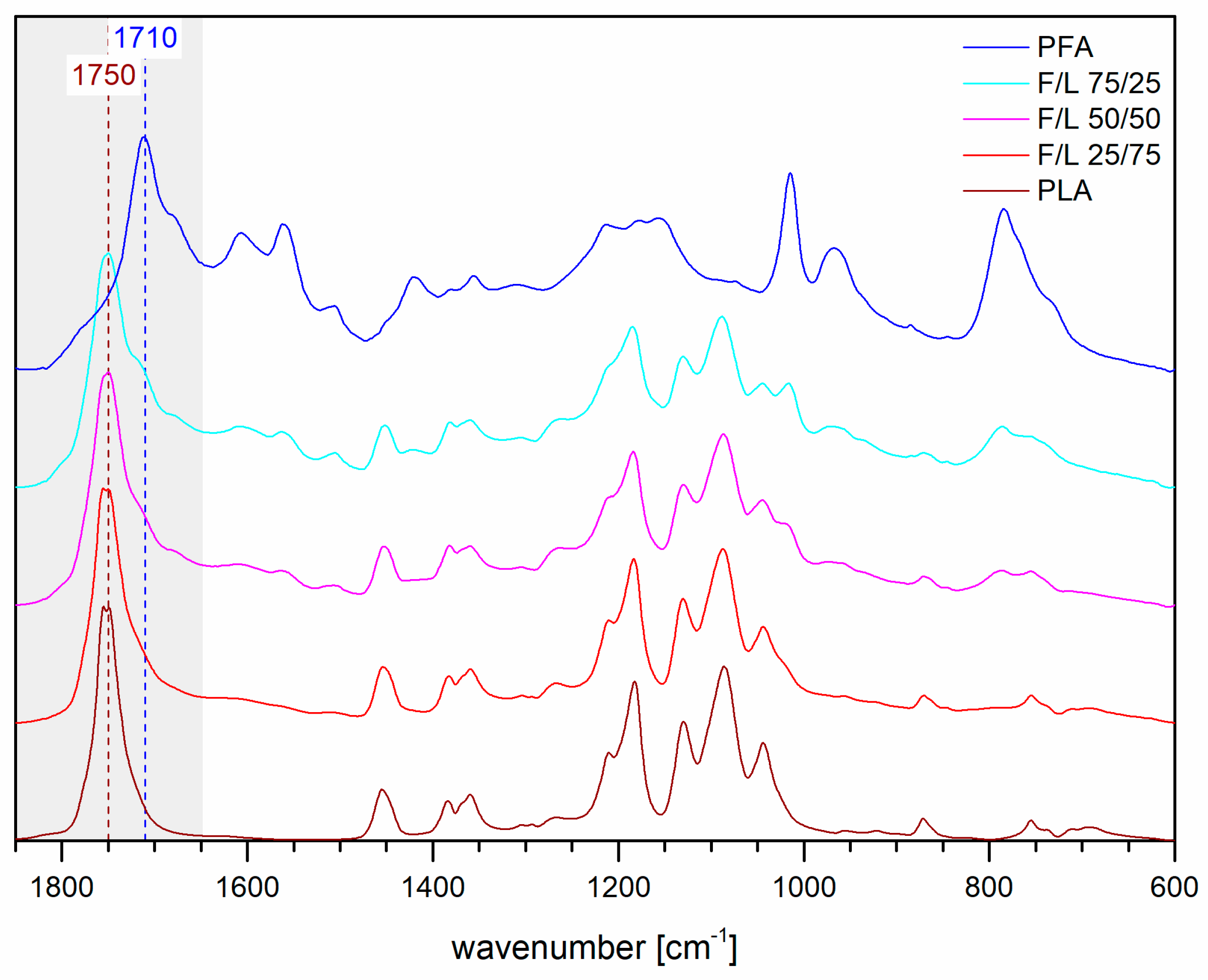
3.7. FT-IR Spectroscopy
3.8. Solid State 13C–NMR
4. Conclusions
- -
- Bending resistance of the formulations was enhanced and as soon as small amounts of FA were added, the formulation became rigid, producing sudden ruptures.
- -
- The SEM micrographs of the mixed formulations showed patterns of both homopolymers.
- -
- Higher furanic character then expected was observed for the formulations F/L 25/75 when exposed to water solubility test, TG and DSC analyses.
- -
- Sensibly higher resistance to chloroform then expected (>25%) was observed for all the mixed formulations.
- -
- No evidences of co-polymerization could be observed in FT-IR, while a small shoulder signal at 175 ppm in the 13C–NMR spectra of the mixed formulations reinforced the thesis of possible copolymerization occurrence.
Author Contributions
Funding
Conflicts of Interest
References
- Lichtenthaler, F.W. Carbohydrates as organic raw materials. Ullmann’s Encycl. Ind. Chem. 2000, 583. [Google Scholar]
- Choura, M.; Belgacem, N.M.; Gandini, A. Acid-catalyzed polycondensation of furfuryl alcohol: Mechanisms of chromophore formation and cross-linking. Macromolecules 1996, 29, 3839–3850. [Google Scholar] [CrossRef]
- Garlotta, D. A literature review of poly (lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Perez, R.F.; Fraga, M.A. Hemicellulose-derived chemicals: One-step production of furfuryl alcohol from xylose. Green Chem. 2014, 16, 3942–3950. [Google Scholar] [CrossRef]
- Gürbüz, E.I.; Gallo, J.M.R.; Alonso, D.M.; Wettstein, S.G.; Lim, W.Y.; Dumesic, J.A. Conversion of hemicellulose into furfural using solid acid catalysts in γ-valerolactone. Angew. Chem. Int. Ed. 2013, 52, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Chheda, J.N.; Román-Leshkov, Y.; Dumesic, J.A. Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono-and poly-saccharides. Green Chem. 2007, 9, 342–350. [Google Scholar] [CrossRef]
- Guigo, N.; Mija, A.; Zavaglia, R.; Vincent, L.; Sbirrazzuoli, N. New insights on the thermal degradation pathways of neat poly (furfuryl alcohol) and poly (furfuryl alcohol)/SiO2 hybrid materials. Polym. Degrad. Stab. 2009, 94, 908–913. [Google Scholar] [CrossRef]
- Coss, W.W. The Quakers Oats Company. Catalyst composition and method for curin furan-based foundry binders. U.S. Patent No. 4,451,577, 29 May 1984. [Google Scholar]
- de Miranda, L.F.; Vale, M.; Júnior, A.H.M.; Masson, T.J.; e Silva, L.G.D.A. Zinc Chloride Influence on The Resins Furan Polymerization to Foundry Moulds. In Characterization of Minerals, Metals, and Materials; Springer: Berlin, Germany, 2016; pp. 771–778. [Google Scholar]
- Trindade, W.G.; Hoareau, W.; Megiatto, J.D.; Razera, I.A.T.; Castellan, A.; Frollini, E. Thermoset phenolic matrices reinforced with unmodified and surface-grafted furfuryl alcohol sugar cane bagasse and curaua fibers: Properties of fibers and composites. Biomacromolecules 2005, 6, 2485–2496. [Google Scholar] [CrossRef] [PubMed]
- Luckeneder, P.; Gavino, J.; Kuchernig, R.; Petutschnigg, A.; Tondi, G. Sustainable phenolic fractions as basis for furfuryl alcohol-based co-polymers and their use as wood adhesives. Polymers 2016, 8, 396. [Google Scholar] [CrossRef]
- Tondi, G.; Pizzi, A. Tannin-based rigid foams: Characterization and modification. Ind. Crops Prod. 2009, 29, 356–363. [Google Scholar] [CrossRef]
- Link, M.; Kolbitsch, C.; Tondi, G.; Ebner, M.; Wieland, S.; Petutschnigg, A. Formaldehyde-free tannin based foams and their use as lightweight panels. BioResources 2011, 6, 4218–4228. [Google Scholar]
- Tondi, G.; Link, M.; Kolbitsch, C.; Lesacher, R.; Petutschnigg, A. Pilot plant up-scaling of tannin foams. Ind. Crops Prod. 2016, 79, 211–218. [Google Scholar] [CrossRef]
- Tondi, G.; Link, M.; Kolbitsch, C.; Gavino, J.; Luckeneder, P.; Petutschnigg, A.; Van Doorslaer, C. Lignin-based foams: Production process and characterization. BioResources 2016, 11, 2972–2986. [Google Scholar] [CrossRef]
- Choura, M.; Belgacem, N.M.; Gandini, A. The acid-catalyzed polycondensation of furfuryl alcohol: Old puzzles unravelled. Macromol. Symp. 1997, 122, 263–268. [Google Scholar] [CrossRef]
- Guigo, N.; Mija, A.; Vincent, L.; Sbirrazzuoli, N. Chemorheological analysis and model-free kinetics of acid catalysed furfuryl alcohol polymerization. Phys. Chem. Chem. Phys. 2007, 9, 5359–5366. [Google Scholar] [CrossRef] [PubMed]
- Reyer, A.; Tondi, G.; Berger, R.J.F.; Petutschnigg, A.; Musso, M. Raman spectroscopic investigation of tannin-furanic rigid foams. Vib. Spectrosc. 2016, 84, 58–66. [Google Scholar] [CrossRef]
- Tondi, G.; Cefarin, N.; Sepperer, T.; D’Amico, F.; Berger, R.J.F.; Musso, M.; Birarda, G.; Reyer, A.; Schnabel, T.; Vaccari, L. Multi-technique investigation of polyfurfuryl alcohol. Polym. Chem. 2019. (under review). [Google Scholar]
- Conley, R.T.; Metil, I. An investigation of the structure of furfuryl alcohol polycondensates with infrared spectroscopy. J. Appl. Polym. Sci. 1963, 7, 37–52. [Google Scholar] [CrossRef]
- Barr, J.B.; Wallon, S.B. The chemistry of furfuryl alcohol resins. J. Appl. Polym. Sci. 1971, 15, 1079–1090. [Google Scholar] [CrossRef]
- Sharib, M.; Kumar, R.; Kumar, K.D. Polylactic acid incorporated polyfurfuryl alcohol bioplastics: Thermal, mechanical and curing studies. J. Therm. Anal. Calorim. 2018, 132, 1593–1600. [Google Scholar] [CrossRef]
- Matos, M.; Sousa, A.F.; Fonseca, A.C.; Freire, C.S.; Coelho, J.F.; Silvestre, A.J. A new Generation of furanic copolyesters with enhanced degradability: poly (ethylene 2, 5-furandicarboxylate)-co-poly (lactic acid) copolyesters. Macromol. Chem. Phys. 2014, 215, 2175–2184. [Google Scholar] [CrossRef]
- Nanni, G.; Heredia-Guerrero, J.A.; Paul, U.C.; Dante, S.; Caputo, G.; Canale, C.; Bayer, I.S. Poly (furfuryl alcohol)-polycaprolactone blends. Polymers 2019, 11, 1069. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: Overview and limits. J. Biotechnol. 2011, 156, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Tay, A.; Yang, S.T. Production of L (+)-lactic acid from glucose and starch by immobilized cells of Rhizopus oryzae in a rotating fibrous bed bioreactor. Biotechnol. Bioeng. 2002, 80, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ilmén, M.; Koivuranta, K.; Ruohonen, L.; Suominen, P.; Penttilä, M. Efficient production of L-lactic acid from xylose by Pichia stipitis. Appl. Environ. Microbiol. 2007, 73, 117–123. [Google Scholar] [CrossRef]
- Filachione, E.M.; Fisher, C.H. Lactic acid condensation polymers. Ind. Eng. Chem. 1994, 36, 223–228. [Google Scholar] [CrossRef]
- Jandas, P.J.; Mohanty, S.; Nayak, S.K. Sustainability, compostability, and specific microbial activity on agricultural mulch films prepared from poly (lactic acid). Ind. Eng. Chem. Res. 2013, 52, 17714–17724. [Google Scholar] [CrossRef]
- Brodhagen, M.; Peyron, M.; Miles, C.; Inglis, D.A. Biodegradable plastic agricultural mulches and key features of microbial degradation. Appl. Microbiol. Biotechnol. 2015, 99, 1039–1056. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-lactic acid: Production, applications, nanocomposites, and release studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Webb, H.; Arnott, J.; Crawford, R.; Ivanova, E. Plastic degradation and its environmental implications with special reference to poly (ethylene terephthalate). Polymers 2013, 5, 1–18. [Google Scholar] [CrossRef]
- Noël, M.; Mougel, E.; Fredon, E.; Masson, D.; Masson, E. Lactic acid/wood-based composite material. Part 2: Physical and mechanical performance. Bioresour. Technol. 2009, 100, 4717–4722. [Google Scholar] [CrossRef] [PubMed]
- Grosse, C.; Noël, M.; Thévenon, M.F.; Rautkari, L.; Gérardin, P. Influence of water and humidity on wood modification with lactic acid. J. Renewable Mater. 2018, 6, 259–269. [Google Scholar] [CrossRef]
- Gao, S.; Tang, G.; Hua, D.; Xiong, R.; Han, J.; Jiang, S.; Zhang, Q.; Huang, C. Stimuli-responsive bio-based polymeric systems and their applications. J. Mater. Chem. B 2019, 7, 709–729. [Google Scholar] [CrossRef]
- Giordano, R.A.; Wu, B.M.; Borland, S.W.; Cima, L.G.; Sachs, E.M.; Cima, M.J. Mechanical properties of dense polylactic acid structures fabricated by three dimensional printing. J. Biomater. Sci. Polym. Ed. 1997, 8, 63–75. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, H.; Li, Z.; Li, P.; Shi, S.Q. Development and application of wood flour-filled polylactic acid composite filament for 3D printing. Materials 2017, 10, 339. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, V.; Solonin, A.; Urzhumtsev, O.; Schilling, R.; Tavitov, A. Strength of PLA components fabricated with fused deposition technology using a desktop 3D printer as a function of geometrical parameters of the process. Polymers 2018, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.Z.; Zeng, Z.W.; Zhou, G.L.; Wang, J.J.; Li, F.Z.; Wang, A.M. Recent advances in PEG–PLA block copolymer nanoparticles. Int. J. Nanomed. 2010, 5, 1057. [Google Scholar]
- Ovitt, T.M.; Coates, G.W. Stereoselective ring-opening polymerization of rac-lactide with a single-site, racemic aluminum alkoxide catalyst: Synthesis of stereoblock poly (lactic acid). J. Polym. Sci. Part. A Polym. Chem. 2000, 38, 4686–4692. [Google Scholar] [CrossRef]
- Moon, S.I.; Lee, C.W.; Miyamoto, M.; Kimura, Y. Melt polycondensation of L-lactic acid with Sn (II) catalysts activated by various proton acids: A direct manufacturing route to high molecular weight Poly (L-lactic acid). J. Polym. Sci. Part. A Polym. Chem. 2000, 38, 1673–1679. [Google Scholar] [CrossRef]
- Wewerka, E.M. Study of the γ-alumina polymerization of furfuryl alcohol. J. Polym. Sci. Part. A Polym. Chem. 1971, 9, 2703–2715. [Google Scholar] [CrossRef]
- Dachs, E.; Benisek, A. A sample-saving method for heat capacity measurements on powders using relaxation calorimetry. Cryogenics 2011, 51, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Ditmars, D.A.; Ishihara, S.; Chang, S.S.; Bernstein, G.; West, E.D. Measurements of the relative enthalpy of pure α-Al2O3 (NBS heat capacity and enthalpy standard reference material no. 720) from 10 to 1950 K. J. Res. Natl. Bur. Stand. 1982, 87, 5–9. [Google Scholar]
- Tondi, G.; Petutschnigg, A. Middle infrared (ATR FT-MIR) characterization of industrial tannin extracts. Ind. Crops Prod. 2015, 65, 422–428. [Google Scholar] [CrossRef]
- Steinbeck, C.; Krause, S.; Kuhn, S. NMRShiftDB constructing a free chemical information system with open-source components. J. Chem. Inf. Comput. Sci. 2003, 43, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Banfi, D.; Patiny, L. www. nmrdb. org: Resurrecting and processing NMR spectra on-line. Chimia Int. J. Chem. 2008, 62, 280–281. [Google Scholar] [CrossRef]
- Castillo, A.M.; Patiny, L.; Wist, J. Fast and accurate algorithm for the simulation of NMR spectra of large spin systems. J. Magn. Reson. 2011, 209, 123–130. [Google Scholar] [CrossRef] [PubMed]





| Label | Furfuryl Alcohol (%) | Lactic Acid (%) | Bending Resistance (MoR) 1 (N/mm2) | Solubility in Water (%) 1 | Solubility in Chloroform (%) 1 |
|---|---|---|---|---|---|
| PFA−2 | 100 | 0 | 13.7 (5.6) | 2.36 (1.08) | 1.23 (0.20) |
| F/L 75/25 | 75 | 25 | 28.1 (6.3) | 1.55 (0.89) | 18.46 (0.21) |
| F/L 50/50 | 50 | 50 | 25.4 (1.9) | 2.05 (1.04) | 31.15 (0.83) |
| F/L 25/75 | 25 | 75 | 7.2 (0.3) | 2.33 (1.44) | 55.45 (3.20) |
| PLA | 0 | 100 | Elastic | 9.12 (13.42) | 98.29 (2.37) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommerauer, L.; Grzybek, J.; Elsaesser, M.S.; Benisek, A.; Sepperer, T.; Dachs, E.; Hüsing, N.; Petutschnigg, A.; Tondi, G. Furfuryl Alcohol and Lactic Acid Blends: Homo- or Co-Polymerization? Polymers 2019, 11, 1533. https://doi.org/10.3390/polym11101533
Sommerauer L, Grzybek J, Elsaesser MS, Benisek A, Sepperer T, Dachs E, Hüsing N, Petutschnigg A, Tondi G. Furfuryl Alcohol and Lactic Acid Blends: Homo- or Co-Polymerization? Polymers. 2019; 11(10):1533. https://doi.org/10.3390/polym11101533
Chicago/Turabian StyleSommerauer, Lukas, Jakub Grzybek, Michael S. Elsaesser, Artur Benisek, Thomas Sepperer, Edgar Dachs, Nicola Hüsing, Alexander Petutschnigg, and Gianluca Tondi. 2019. "Furfuryl Alcohol and Lactic Acid Blends: Homo- or Co-Polymerization?" Polymers 11, no. 10: 1533. https://doi.org/10.3390/polym11101533
APA StyleSommerauer, L., Grzybek, J., Elsaesser, M. S., Benisek, A., Sepperer, T., Dachs, E., Hüsing, N., Petutschnigg, A., & Tondi, G. (2019). Furfuryl Alcohol and Lactic Acid Blends: Homo- or Co-Polymerization? Polymers, 11(10), 1533. https://doi.org/10.3390/polym11101533








