Chemical Structure–Antioxidant Activity Relationship of Water–Based Enzymatic Polymerized Rutin and Its Wound Healing Potential
Abstract
:1. Introduction
2. Materials and Methods
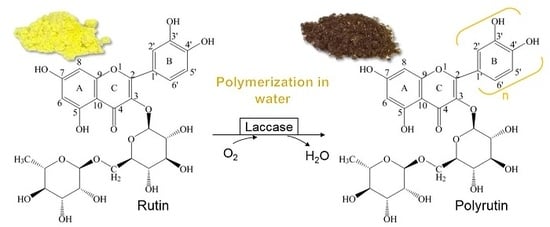
2.1. Synthesis of PR
2.2. Solubility Determination
2.3. 1H NMR Spectroscopy
2.4. ATR-FTIR Spectrometry
2.5. Size-Exclusion Chromatography (SEC)
2.6. Potentiometric Charge Titration
2.7. Antioxidant Activity
2.7.1. ABTS Radical Scavenging Assay
2.7.2. Superoxide Radical (O2•) Scavenging Assay
2.7.3. Nitric Oxide (NO•) Scavenging Assay
2.7.4. Hydroxyl Radical (OH•) Scavenging Assay
2.7.5. Fe2+ Chelating Activity
2.8. Cell Viability
2.9. Statistical Analysis
3. Results and Discussion
3.1. Chemical Structure of Polyrutin
3.1.1. Solubility of PR
3.1.2. 1H NMR Spectroscopy
3.1.3. ATR-FTIR Spectroscopy
3.1.4. SEC Analysis
3.1.5. Potentiometric Titration
3.2. Chemical Structure—Antioxidant Activity Relationship
3.3. Cell Viability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guardia, T.; Rotelli, A.E.; Juarez, A.O.; Pelzer, L.E. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Il Farmaco 2001, 56, 683–687. [Google Scholar] [CrossRef]
- Panhwar, Q.; Memon, S. Synthesis, characterisation, and antioxidant study of Cr(III)-rutin complex. Chem. Pap. 2014, 68, 614–623. [Google Scholar] [CrossRef]
- Tran, N.Q.; Joung, Y.K.; Lih, E.; Park, K.D. In Situ Forming and Rutin-Releasing Chitosan Hydrogels as Injectable Dressings for Dermal Wound Healing. Biomacromolecules 2011, 12, 2872–2880. [Google Scholar] [CrossRef] [PubMed]
- Banjare, L. Development of biocompatible nanoparticles for sustained topical delivery of Rutin. Int. J. Pharm. Biol. Arch. 2012, 3. [Google Scholar]
- Berlier, G.; Gastaldi, L.; Sapino, S.; Miletto, I.; Bottinelli, E.; Chirio, D.; Ugazio, E. MCM-41 as a useful vector for rutin topical formulations: Synthesis, characterization and testing. Int. J. Pharm. 2013, 457, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ku, S.-K.; Bae, J.-S. Barrier protective effects of rutin in LPS-induced inflammation in vitro and in vivo. Food Chem. Toxicol. 2012, 50, 3048–3055. [Google Scholar] [CrossRef]
- Chebil, L.; Rhouma, G.B.; Chekir-Ghedira, L.; Ghoul, M. Enzymatic Polymerization of Rutin and Esculin and Evaluation of the Antioxidant Capacity of Polyrutin and Polyesculin. In Biotechnology; Ekinci, D., Ed.; InTech: London, UK, 2015. [Google Scholar] [CrossRef] [Green Version]
- Kurisawa, M.; Chung, J.E.; Uyama, H.; Kobayashi, S. Enzymatic synthesis and antioxidant properties of poly(rutin). Biomacromolecules 2003, 4, 1394–1399. [Google Scholar] [CrossRef]
- Anthonia, J.; Lionnetonb, F.; Wieruszeskic, J.-M.; Magdaloub, J.; Engassera, J.-M.; Chebila, L.; Humeaua, C.; Ghoula, M. Investigation of enzymatic oligomerization of rutin. Rasayan J. Chem. 2008, 1, 718–731. [Google Scholar]
- Afanas’ev, I.B.; Dorozhko, A.I.; Brodskii, A.V.; Kostyuk, V.A.; Potapovitch, A.I. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem. Pharmacol. 1989, 38, 1763–1769. [Google Scholar] [CrossRef]
- Symonowicz, M.; Kolanek, M. Flavonoids and their properties to form chelate complexes. Biotechnol. Food Sci. 2012, 76, 6. [Google Scholar]
- More, S.S.; Renuka, P.S.; Pruthvi, K.; Swetha, M.; Malini, S.; Veena, S.M. Isolation, Purification, and Characterization of Fungal Laccase from Pleurotus sp. Enzyme Res. 2011, 2011, 7. [Google Scholar] [CrossRef]
- Wood, D.A. Production, Purification and Properties of Extracellular Laccase of Agaricus bisporus. Microbiology 1980, 117, 327–338. [Google Scholar] [CrossRef] [Green Version]
- Florey, K. Analytical Profiles of Drug Substances; Academic Press: San Diego, CA, USA; New York, NY, USA; Boston, MA, USA, 1990; Volume 19. [Google Scholar]
- Snyder, L.R. Classification off the Solvent Properties of Common Liquids. J. Chromatogr. Sci. 1978, 16, 223–234. [Google Scholar] [CrossRef]
- Napolitano, J.G.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Complete 1H NMR spectral analysis of ten chemical markers of Ginkgo biloba. Magn. Reason. Chem. 2012, 50, 569–575. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Gutteridge, J.M.; Aruoma, O.I. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem. 1987, 165, 215–219. [Google Scholar] [CrossRef]
- Parwani, L.; Bhatnagar, M.; Bhatnagar, A.; Sharma, V. Antioxidant and iron-chelating activities of cyanobacterial exopolymers with potential for wound healing. J. Appl. Phycol. 2013, 26, 1473–1482. [Google Scholar] [CrossRef]
- Jeon, J.K.; Lee, J.; Imm, J.-Y. Effects of laccase-catalyzed rutin polymer fraction on adipogenesis inhibition in 3T3-L1 adipocytes. Process Biochem. 2014, 49, 1189–1195. [Google Scholar] [CrossRef]
- Desentis-Mendoza, R.M.; Hernandez-Sanchez, H.; Moreno, A.; Rojas del C., E.; Chel-Guerrero, L.; Tamariz, J.; Jaramillo-Flores, M.E. Enzymatic polymerization of phenolic compounds using laccase and tyrosinase from Ustilago maydis. Biomacromolecules 2006, 7, 1845–1854. [Google Scholar] [CrossRef]
- Jeon, J.R.; Baldrian, P.; Murugesan, K.; Chang, Y.S. Laccase-catalysed oxidations of naturally occurring phenols: From in vivo biosynthetic pathways to green synthetic applications. Microb. Biotechnol. 2012, 5, 318–332. [Google Scholar] [CrossRef]
- Muñiz-Mouro, A.; Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Laccase Activity as an Essential Factor in the Oligomerization of Rutin. Catalysts 2018, 8, 321. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal laccases—Occurrence and properties. FEMS Microb. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef]
- Madhavi, V.; Lele, S.S. Laccase: Properties and Applications. Bioresources 2009, 4, 24. [Google Scholar]
- Božič, M.; Gorgieva, S.; Kokol, V. Homogeneous and heterogeneous methods for laccase-mediated functionalization of chitosan by tannic acid and quercetin. Carbohydr. Polym. 2012, 89, 854–864. [Google Scholar] [CrossRef]
- Uzan, E.; Portet, B.; Lubrano, C.; Milesi, S.; Favel, A.; Lesage-Meessen, L.; Lomascolo, A. Pycnoporus laccase-mediated bioconversion of rutin to oligomers suitable for biotechnology applications. Appl. Microbiol. Biotechnol. 2011, 90, 97–105. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S.; Tosic, M.; Marjanovic, B.; Simic, M.G. Flavonoids as Antioxidants. J. Am. Chem. Soc. 1994, 116, 4846–4851. [Google Scholar] [CrossRef]
- Mohammadpour, M.; Behjati, M.; Sadeghi, A.; Fassihi, A. Wound healing by topical application of antioxidant iron chelators: Kojic acid and deferiprone. Int. Wound J. 2013, 10, 260–264. [Google Scholar] [CrossRef]
- Han, R.M.; Zhang, J.P.; Skibsted, L.H. Reaction dynamics of flavonoids and carotenoids as antioxidants. Molecules (Basel, Switzerland) 2012, 17, 2140–2160. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Chobot, V.; Kubicova, L.; Bachmann, G.; Hadacek, F. Versatile redox chemistry complicates antioxidant capacity assessment: Flavonoids as milieu-dependent anti- and pro-oxidants. Int. J. Mol. Sci. 2013, 14, 11830–11841. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S.; Hara, Y.; Simic, M.G. Reduction potentials of flavonoid and model phenoxyl radicals. Which ring in flavonoids is responsible for antioxidant activity? J. Chem. Soc. Perkin Trans. 2 1996, 2497–2504. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Gramss, G. Reappraising a Controversy: Formation and Role of the Azodication (ABTS2+) in the Laccase-ABTS Catalyzed Breakdown of Lignin. Fermentation 2017, 3, 27. [Google Scholar] [CrossRef]
- Buettner, G.R. The pecking order of free radicals and antioxidants: Lipid peroxidation, alpha-tocopherol, and ascorbate. Arch. Biochem. Biophys. 1993, 300, 535–543. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Talorete, T.P.; Bouaziz, M.; Sayadi, S.; Isoda, H. Influence of medium type and serum on MTT reduction by flavonoids in the absence of cells. Cytotechnology 2006, 52, 189–198. [Google Scholar] [CrossRef]
- Peng, L.; Wang, B.; Ren, P. Reduction of MTT by flavonoids in the absence of cells. Colloids Surf. B Biointerfaces 2005, 45, 108–111. [Google Scholar] [CrossRef]
- Zemljič, L.; Čakara, D.; Michaelis, N.; Heinze, T.; Stana Kleinschek, K. Protonation behavior of 6-deoxy-6-(2-aminoethyl)amino cellulose: A potentiometric titration study. Cellulose 2011, 18, 33–43. [Google Scholar] [CrossRef]
- Kessler, M.; Ubeaud, G.; Jung, L. Anti- and pro-oxidant activity of rutin and quercetin derivatives. J. Pharm. Pharmacol. 2003, 55, 131–142. [Google Scholar] [CrossRef]
- Mansuri, M.L.; Parihar, P.; Solanki, I.; Parihar, M.S. Flavonoids in modulation of cell survival signalling pathways. Genes Nutr. 2014, 9, 400. [Google Scholar] [CrossRef]
- Ferrari, M.; Fornasiero, M.C.; Isetta, A.M. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J. Immunol. Methods 1990, 131, 165–172. [Google Scholar] [CrossRef]
- Van de Loosdrecht, A.A.; Beelen, R.H.; Ossenkoppele, G.J.; Broekhoven, M.G.; Langenhuijsen, M.M. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J. Immunol. Methods 1994, 174, 311–320. [Google Scholar] [CrossRef]
- Su, J.; Fu, J.; Wang, Q.; Silva, C.; Cavaco-Paulo, A. Laccase: A green catalyst for the biosynthesis of poly-phenols. Crit. Rev. Biotechnol. 2018, 38, 294–307. [Google Scholar] [CrossRef]
- Vanella, R.; Bazin, A.; Ta, D.T.; Nash, M.A. Genetically Encoded Stimuli-Responsive Cytoprotective Hydrogel Capsules for Single Cells Provide Novel Genotype–Phenotype Linkage. Chem. Mater. 2019, 31, 1899–1907. [Google Scholar] [CrossRef]
- Vanella, R.; Ta, D.T.; Nash, M.A. Enzyme-mediated hydrogel encapsulation of single cells for high-throughput screening and directed evolution of oxidoreductases. Biotechnol. Bioeng. 2019, 116, 1878–1886. [Google Scholar] [CrossRef]







| Solvent | Water | DMSO | DMA | DMF | Pyridine | Methanol | Acetone | THF | Toluene | |
|---|---|---|---|---|---|---|---|---|---|---|
| Polarity index | 10.2 | 7.2 | 6.5 | 6.4 | 5.3 | 5.1 | 5.1 | 4.0 | 2.4 | |
| Solubility Determined Photometrically (mg/mL) | ||||||||||
| Reaction media | Aqueous | 753 ± 27 | 465 ± 15 | 92 ± 14 | 104 ± 24 | 2.4 ± 1 | insoluble | insoluble | insoluble | insoluble |
| Solubility Determined by Visual Observation by Kurisawa et al. [8] | ||||||||||
| Reaction media | Aqueous organic | ++ | ++ | ++ | +/− | + | − | − | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pivec, T.; Kargl, R.; Maver, U.; Bračič, M.; Elschner, T.; Žagar, E.; Gradišnik, L.; Stana Kleinschek, K. Chemical Structure–Antioxidant Activity Relationship of Water–Based Enzymatic Polymerized Rutin and Its Wound Healing Potential. Polymers 2019, 11, 1566. https://doi.org/10.3390/polym11101566
Pivec T, Kargl R, Maver U, Bračič M, Elschner T, Žagar E, Gradišnik L, Stana Kleinschek K. Chemical Structure–Antioxidant Activity Relationship of Water–Based Enzymatic Polymerized Rutin and Its Wound Healing Potential. Polymers. 2019; 11(10):1566. https://doi.org/10.3390/polym11101566
Chicago/Turabian StylePivec, Tanja, Rupert Kargl, Uroš Maver, Matej Bračič, Thomas Elschner, Ema Žagar, Lidija Gradišnik, and Karin Stana Kleinschek. 2019. "Chemical Structure–Antioxidant Activity Relationship of Water–Based Enzymatic Polymerized Rutin and Its Wound Healing Potential" Polymers 11, no. 10: 1566. https://doi.org/10.3390/polym11101566
APA StylePivec, T., Kargl, R., Maver, U., Bračič, M., Elschner, T., Žagar, E., Gradišnik, L., & Stana Kleinschek, K. (2019). Chemical Structure–Antioxidant Activity Relationship of Water–Based Enzymatic Polymerized Rutin and Its Wound Healing Potential. Polymers, 11(10), 1566. https://doi.org/10.3390/polym11101566