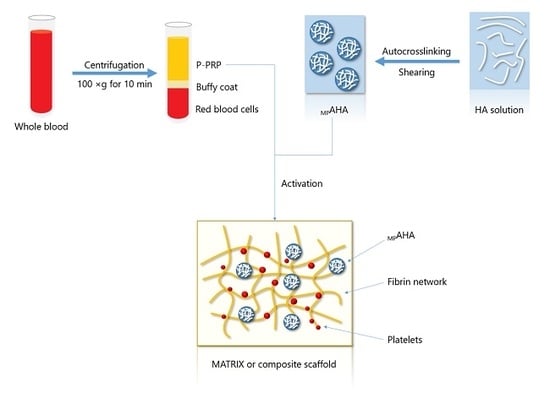
Association of Platelet-Rich Plasma and Auto-Crosslinked Hyaluronic Acid Microparticles: Approach for Orthopedic Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation and Characterization of Auto-Crosslinked Hyaluronic Acid (AHA)
2.2.2. Preparation and Characterization of Microparticles of AHA (MPAHA)
2.2.3. Attenuation of Negative Charge of AHA
2.2.4. Preparation and Characterization of Microparticles of AHA-CHT (MPAHA-CHT)
2.2.5. Preparation and Characterization of Activated P-PRP
2.2.6. Association between AP-PRP and MPAHA
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Structural Modifications of HA
3.2. Physicochemical and Mechanical Properties of MPs (MPAHA and MPAHA-CHT)
3.3. Rheological and Biological Properties of Associations AP-PRP/MPAHA and AP-PRP/MPAHA-CHT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rashid, H.; Kwoh, C.K. Should Platelet-Rich Plasma or Stem Cell Therapy Be Used to Treat Osteoarthritis? Rheum. Dis. Clin. 2019, 45, 3417–3438. [Google Scholar] [CrossRef] [PubMed]
- Vetrano, M.; Ranieri, D.; Nanni, M.; Pavan, A.; Malisan, F.; Vulpiani, M.C.; Visco, V. Hyaluronic Acid (HA), Platelet-Rich Plasm and Extracorporeal Shock Wave Therapy (ESWT) promote human chondrocyte regeneration in vitro and ESWT-mediated increase of CD44 expression enhances their susceptibility to HA treatment. PLoS ONE 2019, 14, 0218740. [Google Scholar] [CrossRef] [PubMed]
- McKay, J.; Franzen, K.; Vercruyssen, N.; Davis, A.; Hafsi, K.; Opitz, T.; Murrell, W. Rehabilitation following regenerative medicine treatment for knee osteoarthritis-current concept review. J. Clin. Orthop. Trauma 2018, 10, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Samadi, P.M.; Sheykhhasan, M.; Khoshinani, H. The Use of Platelet-Rich Plasma in Aesthetic and Regenerative Medicine: A Comprehensive Review. Aesthet. Plast Surg. 2019, 43, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Lana, J.F.; Weglein, A.; Sampson, S.E.; Vicente, E.F.; Huber, S.C.; Souza, C.V.; Annichino-Bizzacchi, J.M. Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J. Stem Cells Regen. Med. 2016, 12, 69–78. [Google Scholar]
- Araco, A. A prospective study comparing topic platelet-rich plasma vs. placebo on reducing superficial perioral wrinkles and restore dermal matrix. J. Cosmet. Laser Ther. 2019, 21, 309–315. [Google Scholar] [PubMed]
- Pereira, H.; Souza, D.A.; Cunha, A.; Andrade, R.; Espregueira-Mendes, J.; Oliveira, J.M.; Reis, R.L. Hyaluronic Acid. Adv. Exp. Med. Biol. 2018, 1059, 137–153. [Google Scholar]
- Zamani, M.; Yaghoubi, Y.; Movassaghpour, A.; Shakouri, K.; Mehdizadeh, A.; Pishgahi, A.; Yousefi, M. Novel therapeutic approaches in utilizing platelet lysate in regenerative medicine: Are we ready for clinical use? J. Cell. Physiol. 2019, 234, 17172–17186. [Google Scholar] [CrossRef]
- Iio, K.; Furukawa, K.; Tsuda, E.; Yamamoto, Y.; Maeda, S.; Naraoka, T.; Kimura, Y.; Ishibashi, Y. Hyaluronic acid induces the release of growth factors from platelet-rich plasma. Asia Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2016, 4, 27–32. [Google Scholar] [CrossRef]
- Bružauskait ė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—Different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef]
- Shimojo, A.A.M.; Galdames, S.E.M.; Duarte, A.S.S.; Pina, L.M.; Rodrigues, A.A.; Luzo, A.C.M.; Belangero, W.D.; Santana, M.H.A. The structuring of high molecular weight hyaluronic acid in microparticles or sponges improves its performance when associated with platelet-rich plasma. Trends Biomater. Artif. Organs 2014, 29, 160–169. [Google Scholar]
- Ren, Y.J.; Zhou, Z.Y.; Liu, B.F.; Xu, Q.Y.; Cui, F.Z. Preparation and characterization of fibroin/hyaluronic acid composite scaffold. Int. J. Biol. Macromol. 2009, 44, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Maroudas, N.G. Adhesion and Spreading of Cells on Charged Surfaces. J. Theor. Biol. 1975, 49, 417–424. [Google Scholar] [CrossRef]
- Andreani, T.; Kiill, C.P.; de Souza, A.L.; Fangueiro, J.F.; Fernandes, L.; Doktorovová, S.; Santos, D.L.; Garcia, M.L.; Gremião, M.P.; Souto, E.B.; et al. Surface engineering of silica nanoparticles for oral insulin delivery: Characterization and cell toxicity studies. Colloids Surf. B Biointerfaces 2014, 123, 916–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreani, T.; Miziara, L.; Lorenzón, E.N.; de Souza, A.L.; Kiill, C.P.; Fangueiro, J.F.; Garcia, M.L.; Gremião, P.D.; Silva, A.M.; Souto, E.B. Effect of mucoadhesive polymers on the in vitro performance of insulin-loaded silica nanoparticles: Interactions with mucin and biomembrane models. Eur. J. Pharm. Biopharm. 2015, 93, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Ferreira da Silva, C.; Severino, P.; Martins, F.; Santana, M.H.; Souto, E.B. Didanosine-loaded chitosan microspheres optimized by surface-response methodology: A modified “Maximum Likelihood Classification“ approach formulation for reverse transcriptase inhibitors. Biomed. Pharmacother. 2015, 70, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Ansa, C.R.; Cinu, T.A.; Chacko, A.J.; Aleykutty, N.A.; Ferreira, S.V.; Souto, E.B. Thermo-sensitive gels containing lorazepam microspheres for intranasal brain targeting. Int. J. Pharm. 2013, 441, 516–526. [Google Scholar] [CrossRef]
- Jose, S.; Fangueiro, J.F.; Smitha, J.; Cinu, T.A.; Chacko, A.J.; Premaletha, K.; Souto, E.B. Cross-linked chitosan microspheres for oral delivery of insulin: Taguchi design and in vivo testing. Colloids Surf. B Biointerfaces 2012, 92, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Fangueiro, J.F.; Smitha, J.; Cinu, T.A.; Chacko, A.J.; Premaletha, K.; Souto, E.B. Predictive modeling of insulin release profile from cross-linked chitosan microspheres. Eur. J. Med. Chem. 2013, 60, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Prema, M.T.; Chacko, A.J.; Thomas, A.C.; Souto, E.B. Colon specific chitosan microspheres for chronotherapy of chronic stable angina. Colloids Surf. B Biointerfaces 2011, 83, 277–283. [Google Scholar] [CrossRef]
- Severino, P.; Da Silva, C.F.; Dalla Costa, T.C.; Silva, H.; Chaud, M.V.; Santana, M.H.; Souto, E.B. In vivo absorption of didanosine formulated in pellets composed of chitosan microspheres. In Vivo 2014, 28, 1045–1050. [Google Scholar] [PubMed]
- Severino, P.; de Oliveira, G.G.G.; Ferraz, H.G.; Souto, E.B.; Santana, M.H.A. Preparation of gastro-resistant pellets containing chitosan microspheres for improvement of oral didanosine bioavailability. J. Pharm. Anal. 2012, 2, 188–192. [Google Scholar] [PubMed] [Green Version]
- Severino, P.; Souto, E.B.; Pinho, S.C.; Santana, M.H. Hydrophilic coating of mitotane-loaded lipid nanoparticles: Preliminary studies for mucosal adhesion. Pharm. Dev. Technol. 2013, 18, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Nasti, A.; Zaki, N.M.; de Leonardis, P.; Ungphaiboon, S.; Sansongsak, P.; Rimoli, M.G.; Tirelli, N. Chitosan/TPP and chitosan/TPP-hyaluronic acid nanoparticles: Systematic optimisation of the preparative process and preliminary biological evaluation. Pharm. Res. 2009, 26, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- Bellini, D.; Paparella, A.; O’Regan, M.; Callegaro, L. Autocross-linked Hyaluronic Acid and Related Pharmaceutical Compositions for the Treatment of Arthrophaties. U.S. Patent 6,251,876 B1, 26 June 2001. [Google Scholar]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Investigation of the swelling behavior of crosslinked hyaluronic acid films and hydrogels produced using homogeneous reactions. J. Appl. Polym. Sci. 2008, 109, 923–931. [Google Scholar] [CrossRef]
- Tang, S.; Vickers, S.M.; Hsu, H.P.; Spector, M. Fabrication and characterization of porous hyaluronic acid–collagen composite scaffolds. J. Biomed. Mater. Res. Part A 2007, 82, 323–335. [Google Scholar] [CrossRef]
- Shu, X.Z.; Liu, Y.; Palumbo, F.S.; Luo, Y.; Prestwich, G.D. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials 2004, 25, 1339–1348. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Polexe, R.C.; Delair, T. Elaboration of stable and antibody functionalized positively charged colloids by polyelectrolyte complexation between chitosan and hyaluronic acid. Molecules 2013, 18, 8563–8578. [Google Scholar] [CrossRef]
- Rädler, J.O.; Koltover, I.; Jamieson, A.; Salditt, T.; Safinya, C.R. Structure and interfacial aspects of self-assembled cationic lipid-DNA gene carrier complexes. Langmuir 1998, 65, 4272–4283. [Google Scholar] [CrossRef]
- Perez, A.G.M.; Lana, J.F.S.D.; Rodrigues, A.A.; Luzo, A.C.M.; Belangero, W.D.; Santana, M.H.A. Relevant Aspects of Centrifugation Step in the Preparation of Platelet-Rich Plasma. ISRN Hematol. 2014, 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.G.M.; Lichy, R.; Lana, J.F.S.D.; Rodrigues, A.A.; Luzo, A.C.M.; Belangero, W.D.; Santana, M.H.A. Prediction and modulation of platelet recovery by discontinuous centrifugation of whole blood for the preparation of pure platelet-rich plasma. BioRes. Open Access 2013, 2, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, P.; Alves, P.; Valente, T.A.M.; Santos, R.; Correia, I.J.; Ferreira, P. Sodium hyaluronate/chitosan polyelectrolyte complex scaffolds for dental pulp regeneration: Synthesis and characterization. Int. J. Biol. Macromol. 2011, 49, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between Alginate and Chitosan Biopolymers Characterized Using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Kablik, J.; Monheit, G.D.; Yu, L.; Chang, G.; Gershkovich, J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol. Surg. 2009, 35, 302–312. [Google Scholar] [CrossRef]
- Tsay, R.C.; Vo, J.; Burke, A.; Eisig, S.B.; Lu, H.H.; Landesberg, R. Differential growth factor retention by platelet-rich plasma composites. J. Oral Maxillofac. Surg. 2005, 63, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Kawase, T.; Momose, M.; Murata, M.; Saito, Y.; Suzuki, H.; Wolff, L.F.; Yoshie, H. Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the proliferation of periodontally related cells in vitro. J. Periodontol. 2003, 74, 849–857. [Google Scholar] [CrossRef]
- King, W.J.; Krebsbach, P.H. Growth factor delivery: How surface interactions modulate release in vitro and in vivo. Adv. Drug Deliv. Rev. 2012, 64, 1239–1256. [Google Scholar] [CrossRef] [Green Version]


 ) AP-PRP, (♦) AP-PRP/MPAHA, (▲) AP-PRP/MPAHA-CHT90:10, (●) AP-PRP/MPAHA-CHT75:25, and (■) AP-PRP/MPAHA-CHT50:50. G’ (closed symbol), G” (open symbol).
) AP-PRP, (♦) AP-PRP/MPAHA, (▲) AP-PRP/MPAHA-CHT90:10, (●) AP-PRP/MPAHA-CHT75:25, and (■) AP-PRP/MPAHA-CHT50:50. G’ (closed symbol), G” (open symbol).
 ) AP-PRP, (♦) AP-PRP/MPAHA, (▲) AP-PRP/MPAHA-CHT90:10, (●) AP-PRP/MPAHA-CHT75:25, and (■) AP-PRP/MPAHA-CHT50:50. G’ (closed symbol), G” (open symbol).
) AP-PRP, (♦) AP-PRP/MPAHA, (▲) AP-PRP/MPAHA-CHT90:10, (●) AP-PRP/MPAHA-CHT75:25, and (■) AP-PRP/MPAHA-CHT50:50. G’ (closed symbol), G” (open symbol).



| Physicochemical Properties | ||||||
| Microparticles | Swelling Ratio | Ve | Mc | Particle Size (µm) | Weight Loss in 24 h (%) | |
| MPAHA | 61 ± 8 | 1.28 × 10−6 | 1,015,979 | 190 ± 6 | 34 ± 6 | |
| MPAHA-CHT90:10 | 47 ± 1 | 2.79 × 10−5 | 155,067 | 273 ± 4 | 46 ± 3 | |
| MPAHA-CHT75:25 | 38 ± 2 | 3.72 × 10−5 | 116,635 | 280 ± 4 | 18 ± 7 | |
| MPAHA-CHT50:50 | 35 ± 1 | 4.18 × 10−5 | 103,614 | 263 ± 4 | 14 ± 3 | |
| Mechanical Properties | ||||||
| Microparticles | G’ (Pa) | G” (Pa) | G* (Pa) | tan δ (=G”/G’) in 1 Hz | n ƞ = K·γn−1 | Extrusion Force (N) |
| MPAHA | 335.8 | 63.8 | 341.8 | 0.19 | 0.39 | 20 ± 1 |
| MPAHA-CHT90:10 | 954.1 | 156.4 | 966.8 | 0.16 | 0.24 | 14 ± 2 |
| MPAHA-CHT75:25 | 3211 | 436.4 | 3240.5 | 0.14 | 0.27 | 19.9 ± 0.6 |
| MPAHA-CHT50:50 | 4933 | 560.2 | 4964.7 | 0.11 | 0.32 | 26.7 ± 0.4 |
| Microparticles | G’ (Pa) | G” (Pa) | G* (Pa) | tan δ (=G”/G’) in 1 Hz | n ƞ = K·γn−1 |
|---|---|---|---|---|---|
| AP-PRP | 20.17 | 6.94 | 21.3 | 0.34 | 0.1953 |
| AP-PRP/MPAHA | 45.03 | 12.54 | 46.7 | 0.28 | 0.2456 |
| AP-PRP/MPAHA-CHT90:10 | 94.66 | 18.03 | 96.4 | 0.19 | 0.3381 |
| AP-PRP/MPAHA-CHT75:25 | 142.8 | 26.1 | 145.2 | 0.18 | 0.2412 |
| AP-PRP/MPAHA-CHT50:50 | 181.8 | 36.86 | 185.5 | 0.20 | 0.1173 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins Shimojo, A.A.; Santos Duarte, A.d.S.; Santos Duarte Lana, J.F.; Malheiros Luzo, Â.C.; Fernandes, A.R.; Sanchez-Lopez, E.; Barbosa Souto, E.; Andrade Santana, M.H. Association of Platelet-Rich Plasma and Auto-Crosslinked Hyaluronic Acid Microparticles: Approach for Orthopedic Application. Polymers 2019, 11, 1568. https://doi.org/10.3390/polym11101568
Martins Shimojo AA, Santos Duarte AdS, Santos Duarte Lana JF, Malheiros Luzo ÂC, Fernandes AR, Sanchez-Lopez E, Barbosa Souto E, Andrade Santana MH. Association of Platelet-Rich Plasma and Auto-Crosslinked Hyaluronic Acid Microparticles: Approach for Orthopedic Application. Polymers. 2019; 11(10):1568. https://doi.org/10.3390/polym11101568
Chicago/Turabian StyleMartins Shimojo, Andréa Arruda, Adriana da Silva Santos Duarte, José Fábio Santos Duarte Lana, Ângela Cristina Malheiros Luzo, Ana Rita Fernandes, Elena Sanchez-Lopez, Eliana Barbosa Souto, and Maria Helena Andrade Santana. 2019. "Association of Platelet-Rich Plasma and Auto-Crosslinked Hyaluronic Acid Microparticles: Approach for Orthopedic Application" Polymers 11, no. 10: 1568. https://doi.org/10.3390/polym11101568
APA StyleMartins Shimojo, A. A., Santos Duarte, A. d. S., Santos Duarte Lana, J. F., Malheiros Luzo, Â. C., Fernandes, A. R., Sanchez-Lopez, E., Barbosa Souto, E., & Andrade Santana, M. H. (2019). Association of Platelet-Rich Plasma and Auto-Crosslinked Hyaluronic Acid Microparticles: Approach for Orthopedic Application. Polymers, 11(10), 1568. https://doi.org/10.3390/polym11101568