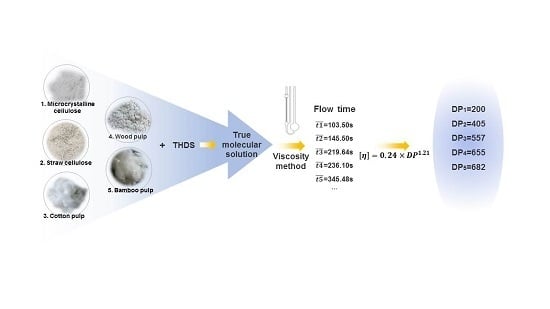
Elucidation of the Relationship between Intrinsic Viscosity and Molecular Weight of Cellulose Dissolved in Tetra-N-Butyl Ammonium Hydroxide/Dimethyl Sulfoxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Dilute Cellulose Solution
2.2.1. Preparation of Cellulose/Cuoxam Solution
2.2.2. Preparation of Cellulose/CED Solution
2.2.3. Preparation of Cellulose/THDS Solution
2.3. Viscosity Analysis of the Cellulose Solution by Ubbelohde Viscometer
2.3.1. Cellulose/Cuoxam Solution
2.3.2. Cellulose/CED Solution
2.3.3. Cellulose/THDS Solution
2.4. Determination of the Degree of Polymerization (DP) of Cellulose
2.4.1. Cuoxam Method
2.4.2. CED Method
2.4.3. THDS Method
2.4.4. Determination of the DP of the Derived Cellulose Acetate Samples by the GPC Method
2.5. The Analysis of Cellulose Molecules Dispersion Level in THDS Solution
2.5.1. Dynamic Light Scattering (DLS)
2.5.2. Atomic Force Microscope (AFM)
2.6. The Structure Analysis of Cellulose in THDS Solution
3. Results and Discussions
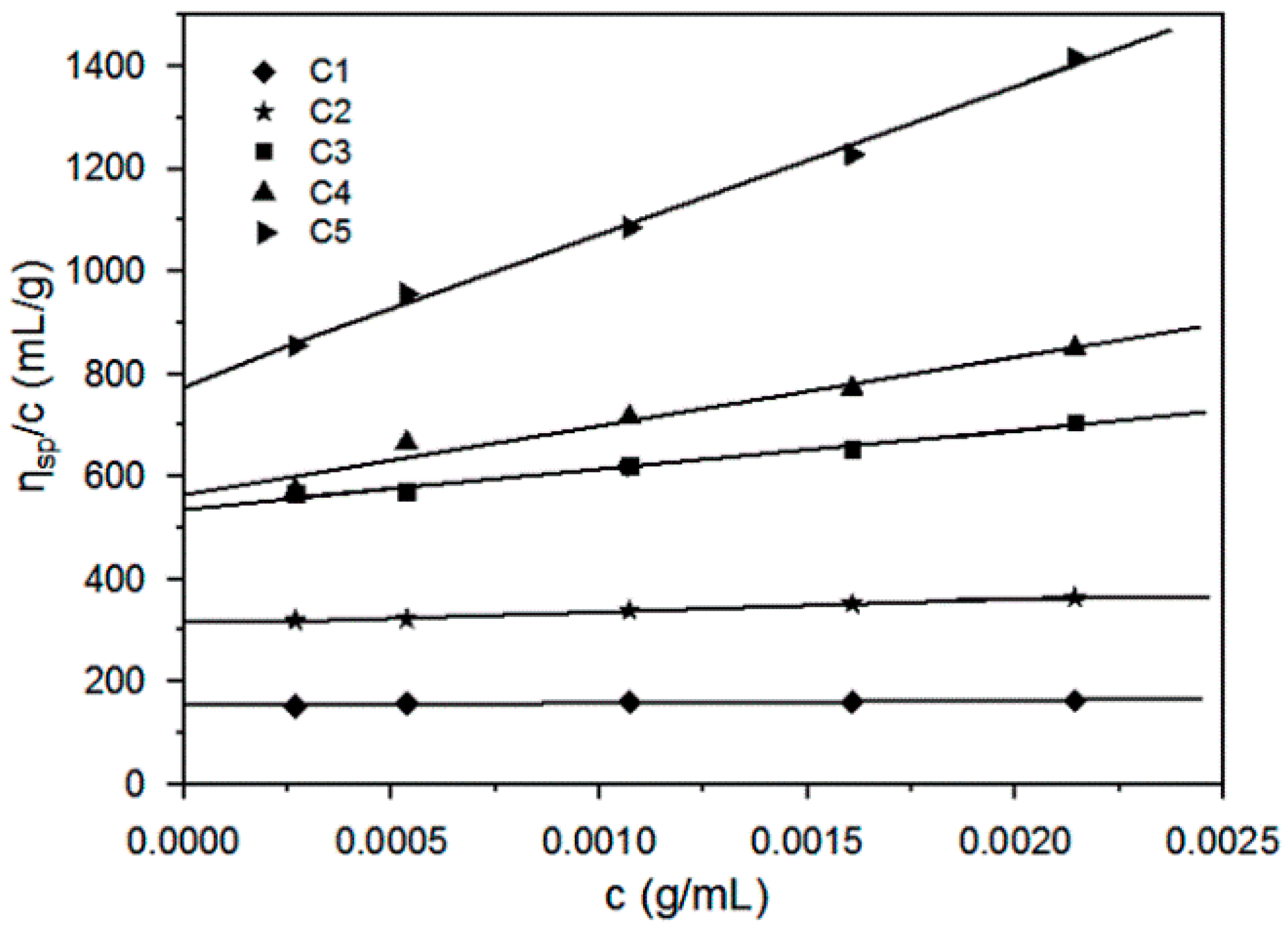
3.1. Preparation and Viscosity Analysis of the Cellulose in THDS Solution
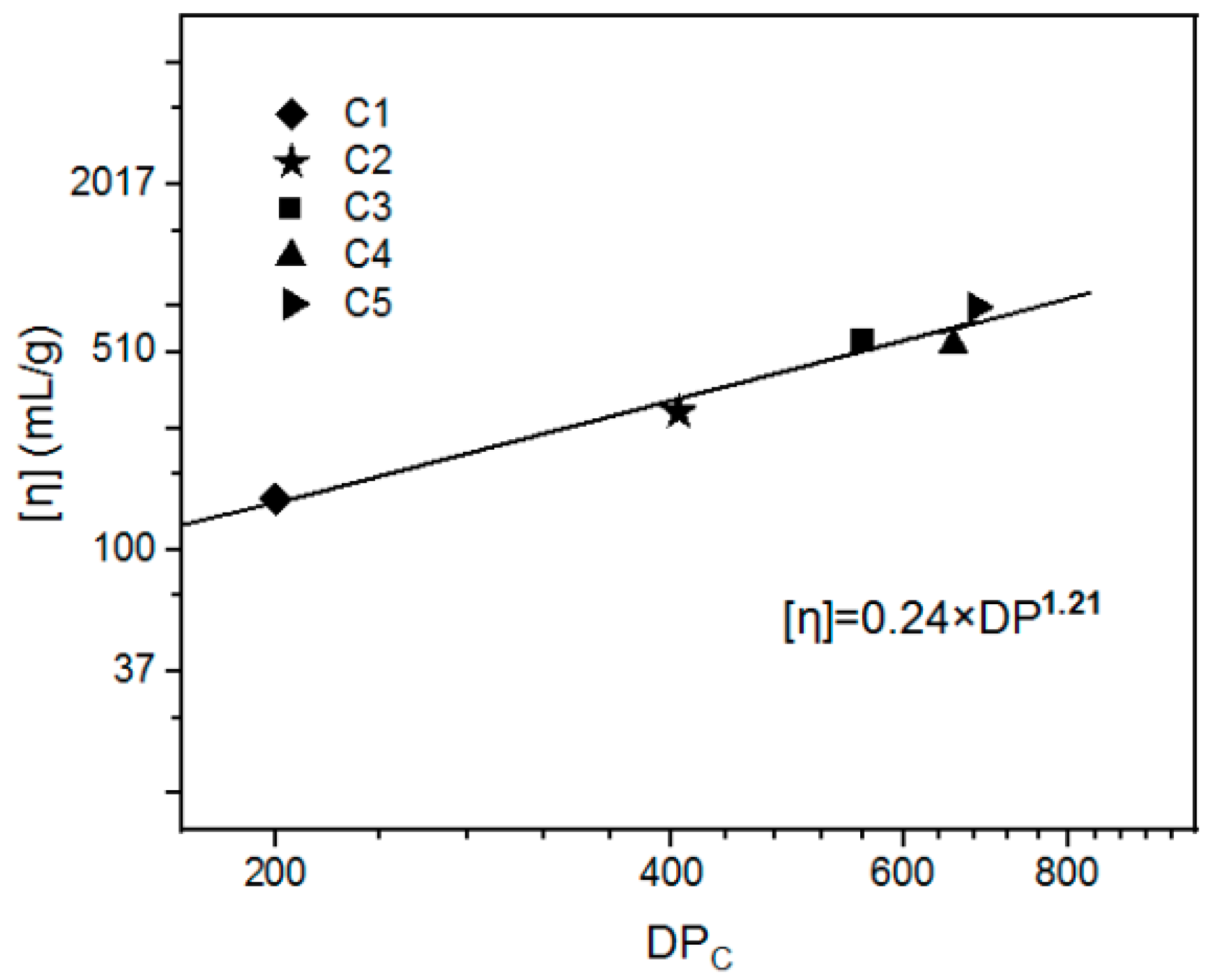
3.2. The Elucidation of the Mark–Houwink Equation for Cellulose/THDS Solution
3.3. Comparable Analysis of Cellulose DP
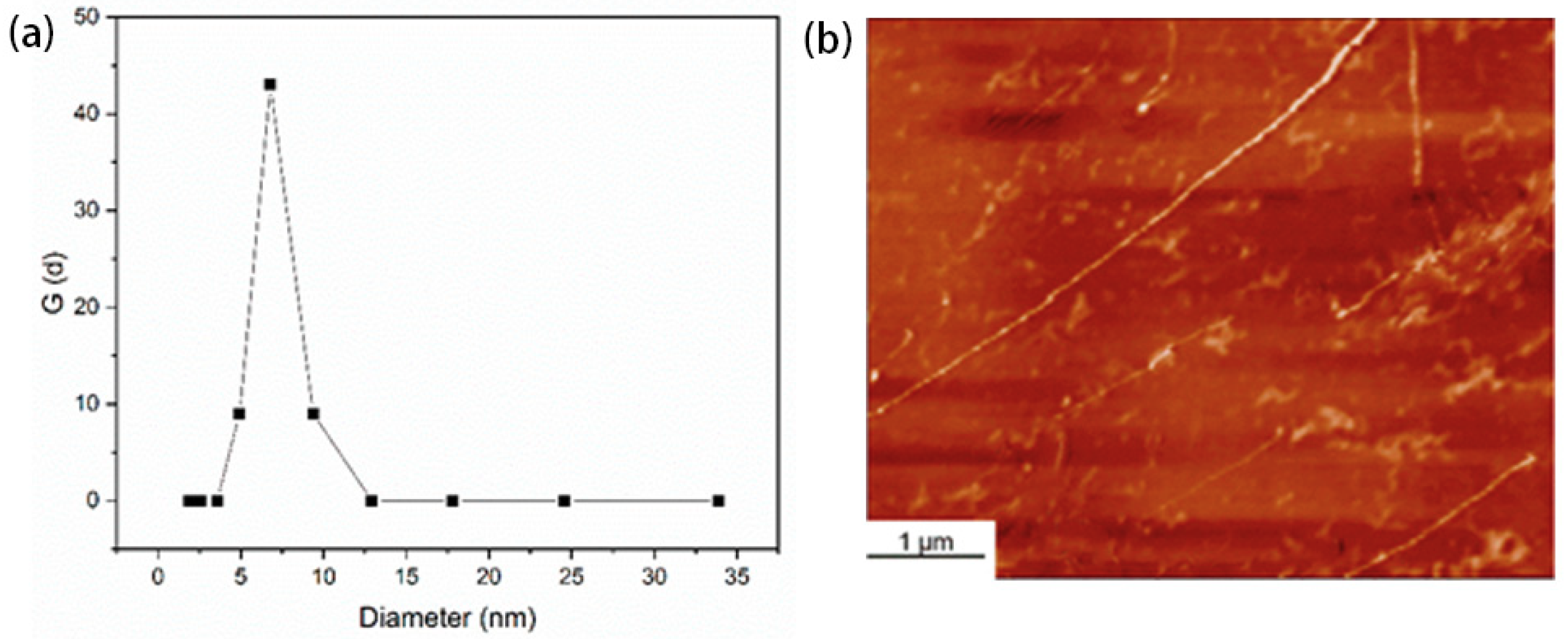
3.4. The Structure Analysis of Cellulose in THDS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P.; Ghasemi, M.; Furlani, E.P.; Tsianou, M. Solvent processing of cellulose for effective bioresource utilization. Curr. Opin. Green Sustain. Chem. 2018, 14, 40–52. [Google Scholar] [CrossRef]
- Kostag, M.; Jedvert, K.; Achtel, C.; Heinze, T.; El Seoud, O.A. Recent advances in solvents for the dissolution, shaping and derivatization of cellulose: Quaternary ammonium electrolytes and their solutions in water and molecular solvents. Molecules 2018, 23, 511. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wei, W.; Gou, G.; Jiang, M.; Cui, Y.; Zhang, S.; Wang, Y.; Zhou, Z. Cellulose films from the aqueous DMSO/TBAH-system. Cellulose 2018, 25, 1975–1986. [Google Scholar] [CrossRef]
- Duan, Y.; Freyburger, A.; Kunz, W.; Zollfrank, C. Cellulose and chitin composite materials from an ionic liquid and a green co-solvent. Carbohydr. Polym. 2018, 192, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, F.; Shamspur, T.; Mostafavi, A.; Saljooqi, A.; Fathirad, F. Synthesis of cellulose acetate nanofibers and its application in the release of some drugs. Nanomed. Res. J. 2017, 2, 199–207. [Google Scholar]
- Mo, J.H.; Kim, J.Y.; Kang, Y.H.; Cho, S.Y.; Jang, K.S. Carbon Nanotube/Cellulose Acetate Thermoelectric Papers. ACS Sustain. Chem. Eng. 2018, 6, 15970–15975. [Google Scholar] [CrossRef]
- Zhou, R.; Li, J.; Jiang, H.; Li, H.; Wang, Y.; Briand, D.; Camara, M.; Zhou, G.; de Rooij, N.F. Highly transparent humidity sensor with thin cellulose acetate butyrate and hydrophobic AF1600X vapor permeating layers fabricated by screen printing. Sens. Actuators B 2019, 281, 212–220. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Zhang, B.; Zhang, X.; Xu, L.; Zhang, J.; He, J.; Liu, C.Y. Determination of intrinsic viscosity-molecular weight relationship for cellulose in BmimAc/DMSO solutions. Cellulose 2016, 23, 2341–2348. [Google Scholar] [CrossRef]
- El Nemr, A.; Ragab, S.; El Sikaily, A. Testing zinc chloride as a new catalyst for direct synthesis of cellulose di- and tri-acetate in a solvent free system under microwave irradiation. Carbohydr. Polym. 2016, 151, 1058–1067. [Google Scholar] [CrossRef]
- Cao, L.; Luo, G.; Tsang, D.C.W.; Chen, H.; Zhang, S.; Chen, J. A novel process for obtaining high quality cellulose acetate from green landscaping waste. J. Clean. Prod. 2018, 176, 338–347. [Google Scholar] [CrossRef]
- He, C.; Wang, Q. Viscometric study of cellulose in PF/DMSO solution. J. Macromol. Sci. Part A Pure Appl. Chem. 1999, 36, 105–114. [Google Scholar] [CrossRef]
- Gubitosi, M.; Duarte, H.; Gentile, L.; Olsson, U.; Medronho, B. On cellulose dissolution and aggregation in aqueous tetrabutylammonium hydroxide. Biomacromolecules 2016, 17, 2873–2881. [Google Scholar] [CrossRef] [PubMed]
- O’sullvian, A.C. Cellulose: The structure slowly unravels. Cellulose 1997, 4, 173–207. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Pinkert, A.; Marsh, K.N.; Pang, S.; Staiger, M.P. Ionic Liquids and Their Interaction with Cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef]
- Lindman, B.; Karlström, G.; Stigsson, L. On the mechanism of dissolution of cellulose. J. Mol. Liq. 2010, 156, 76–81. [Google Scholar] [CrossRef]
- Glasser, W.G.; Atalla, R.H.; Blackwell, J.; Brown, M.M.; Burchard, W.; French, A.D.; Klemm, D.O.; Nishiyama, Y. About the structure of cellulose: Debating the Lindman hypothesis. Cellulose 2012, 19, 589–598. [Google Scholar] [CrossRef]
- Hettrich, K.; Pinnow, M.; Volkert, B.; Passauer, L.; Fischer, S. Novel aspects of nanocellulose. Cellulose 2014, 21, 2479–2488. [Google Scholar] [CrossRef]
- Rosenau, T.; Potthast, A.; Sixta, H.; Kosma, P. The chemistry of side reactions and byproduct formation in the system NMMO/cellulose (Lyocell process). Prog. Polym. Sci. 2001, 26, 1763–1837. [Google Scholar] [CrossRef]
- Rosenau, T.; Potthast, A.; Adorjan, I.; Hofinger, A.; Sixta, H.; Firgo, H.; Kosma, P. Cellulose solutions in N-methylmorpholine-N-oxide (NMMO)—degradation processes and stabilizers. Cellulose 2002, 9, 283–291. [Google Scholar] [CrossRef]
- Dogan, H.; Hilmioglu, N.D. Dissolution of cellulose with NMMO by microwave heating. Carbohydr. Polym. 2009, 75, 90–94. [Google Scholar] [CrossRef]
- Eckelt, J.; Eich, T.; Röder, T.; Rüf, H.; Sixta, H.; Wolf, B.A. Phase diagram of the ternary system NMMO/water/cellulose. Cellulose 2009, 16, 373–379. [Google Scholar] [CrossRef]
- Mccormick, C.L.; Callais, P.A.; Hutchinson, B.H. Solution Studies of Cellulose in Lithium Chloride and N, N-Dimethylacetamide. Macromolecules 1985, 18, 2394–2401. [Google Scholar] [CrossRef]
- Dupont, A.L. Cellulose in lithium chloride/N,N-dimethylacetamide, optimisation of a dissolution method using paper substrates and stability of the solutions. Polymer (Guildf.) 2003, 44, 4117–4126. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L. Unique gelation behavior of cellulose in NaOH/urea aqueous solution. Biomacromolecules 2006, 7, 183–189. [Google Scholar] [CrossRef]
- Lue, A.; Liu, Y.; Zhang, L.; Potthas, A. Light scattering study on the dynamic behaviour of cellulose inclusion complex in LiOH/urea aqueous solution. Polymer (Guildf.) 2011, 52, 3857–3864. [Google Scholar] [CrossRef]
- Alves, L.; Medronho, B.; Antunes, F.E.; Topgaard, D.; Lindman, B. Dissolution state of cellulose in aqueous systems. 1. Alkaline solvents. Cellulose 2016, 23, 247–258. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L. Solubility of Cellulose in NaOH/Urea Aqueous Solution. Polym. J. 2000, 32, 866–870. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Kuga, S.; Cheng, H.; Han, C.C.; Cai, J.; Chen, X.; Chu, B.; Liu, Y.; Xu, J.; Liu, S.; et al. Dynamic Self-Assembly Induced Rapid Dissolution of Cellulose at Low Temperatures. Macromolecules 2008, 41, 9345–9351. [Google Scholar]
- Shi, Y.; Kong, R.; Yang, F.; Ren, S.; Xie, J. Participation of sodium sulfamate as hydrogen bond-donating and hydrogen bond-accepting additive in the dissolution of cellulose in NaOH aqueous solution. Cellulose 2018, 25, 2785–2794. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Cai, J. Behavior of Cellulose in NaOH/Urea Aqueous Solution Characterized by Light Scattering and Viscometry. J. Polym. Sci. Part. B Polym. Phys. 2004, 42, 347–353. [Google Scholar] [CrossRef]
- Sánchez, P.B.; González, B.; Salgado, J.; Pádua, A.A.H.; García, J. Cosolvent effect on physical properties of 1,3-dimethyl imidazolium dimethyl phosphate and some theoretical insights on cellulose dissolution. J. Mol. Liq. 2018, 265, 114–120. [Google Scholar] [CrossRef]
- Satria, H.; Kuroda, K.; Tsuge, Y.; Ninomiya, K.; Takahashi, K. Dimethyl sulfoxide enhances both the cellulose dissolution ability and biocompatibility of a carboxylate-type liquid zwitterion. New J. Chem. 2018, 42, 13225–13228. [Google Scholar] [CrossRef]
- Tenhunen, T.M.; Lewandowska, A.E.; Orelma, H.; Johansson, L.S.; Virtanen, T.; Harlin, A.; Österberg, M.; Eichhorn, S.J.; Tammelin, T. Understanding the interactions of cellulose fibres and deep eutectic solvent of choline chloride and urea. Cellulose 2018, 25, 137–150. [Google Scholar] [CrossRef]
- Zhang, L.; Ruan, D.; Zhou, J. Structure and properties of regenerated cellulose films prepared from cotton linters in NaOH/urea aqueous solution. Ind. Eng. Chem. Res. 2001, 40, 5923–5928. [Google Scholar] [CrossRef]
- Lu, A.; Liu, Y.; Zhang, L.; Potthast, A. Investigation on metastable solution of cellulose dissolved in NaOH/urea aqueous system at low temperature. J. Phys. Chem. B 2011, 115, 12801–12808. [Google Scholar] [CrossRef]
- Östlund, Å.; Lundberg, D.; Nordstierna, L.; Holmberg, K.; Nydén, M. Dissolution and Gelation of Cellulose in TBAF/DMSO Solutions. Biomacromolecules 2009, 10, 2401–2407. [Google Scholar] [CrossRef]
- Rinaldi, R. Instantaneous dissolution of cellulose in organic electrolyte solutions. Chem. Commun. 2011, 47, 511–513. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Cai, X.M.; Huang, S.; Wang, F. Cellulose Dissolution in a Mixed Solvent of Tetra(n-butyl)ammonium Hydroxide/Dimethyl Sulfoxide via Radical Reactions. ACS Sustain. Chem. Eng. 2018, 6, 2898–2904. [Google Scholar] [CrossRef]
- Abe, M.; Fukaya, Y.; Ohno, H. Fast and facile dissolution of cellulose with tetrabutylphosphonium hydroxide containing 40 wt % water. Chem. Commun. 2012, 48, 1808–1810. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Meng, F.; Cui, Y.; Jiang, M.; Zhou, Z. Room temperature dissolution of cellulose in tetra-butylammonium hydroxide aqueous solvent through adjustment of solvent amphiphilicity. Cellulose 2017, 24, 49–59. [Google Scholar] [CrossRef]
- Mu, C.; Jiang, M.; Zhu, J.; Zhao, M.; Zhu, S.; Zhou, Z. Isolation of cellulose from steam-exploded rice straw with aniline catalyzing dimethyl formamide aqueous solution. Renew. Energy 2014, 63, 324–329. [Google Scholar] [CrossRef]
- Ass, B.A.P.; Frollini, E.; Heinze, T. Studies on the homogeneous acetylation of cellulose in the novel solvent dimethyl sulfoxide/tetrabutylammonium fluoride trihydrate. Macromol. Biosci. 2004, 4, 1008–1013. [Google Scholar] [CrossRef]
- Hussain, M.A.; Liebert, T.; Heinze, T. Acylation of cellulose with N,N′-carbonyldiimidazole-activated acids in the novel solvent dimethyl sulfoxide/tetrabutylammomnium fluoride. Macromol. Rapid Commun. 2004, 25, 916–920. [Google Scholar] [CrossRef]
- Argyropoulos, D.S.; Kilpelainen, I.; Granstrom, M.; King, A.; Xie, H. Thorough Chemical Modification of Wood-Based Lignocellulosic Materials in Ionic Liquids. Biomacromolecules 2007, 8, 3740–3748. [Google Scholar]
- Zhang, J.; Wu, J.; Cao, Y.; Sang, S.; Zhang, J.; He, J. Synthesis of cellulose benzoates under homogeneous conditions in an ionic liquid. Cellulose 2009, 16, 299–308. [Google Scholar] [CrossRef]
- Abe, M.; Sugimura, K.; Nishiyama, Y.; Nishio, Y. Rapid Benzylation of Cellulose in Tetra-n-butylphosphonium Hydroxide Aqueous Solution at Room Temperature. ACS Sustain. Chem. Eng. 2017, 5, 4505–4510. [Google Scholar] [CrossRef]
- Kasaai, M.R. Comparison of various solvents for determination of intrinsic viscosity and viscometric constants for cellulose. J. Appl. Polym. Sci. 2002, 86, 2189–2193. [Google Scholar] [CrossRef]
- Valtasaari, L.E.A. The Configuration of Cellulose Dissolved in Iron-sodiumtartrate. Macromol. Chem. Phys. 1971, 150, 117–126. [Google Scholar] [CrossRef]
- Kamide, K.; Saito, M. Light Scattering and Viscometric Study of Cellulose in Aqueous Lithium Hydroxide. Polym. J. 2006, 18, 569–579. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Tayeb, P.; Joyce, M.; Tyagi, P.; Kehoe, M.; Dimic-Misic, K.; Pal, L. Rheology of nanocellulose-rich aqueous suspensions: A review. BioResources 2017, 12, 9556–9661. [Google Scholar]
- Wan, Z.; Li, L.; Cui, S. Capturing the portrait of isolated individual natural cellulose molecules. Biopolymers 2008, 89, 1170–1173. [Google Scholar] [CrossRef]
- Yokota, S.; Ueno, T.; Kitaoka, T.; Wariishi, H. Molecular imaging of single cellulose chains aligned on a highly oriented pyrolytic graphite surface. Carbohydr. Res. 2007, 342, 2593–2598. [Google Scholar] [CrossRef]
- El Nemr, A.; Ragab, S.; El Sikaily, A.; Khaled, A. Synthesis of cellulose triacetate from cotton cellulose by using NIS as a catalyst under mild reaction conditions. Carbohydr. Polym. 2015, 130, 41–48. [Google Scholar] [CrossRef]
- Kono, H.; Hashimoto, H.; Shimizu, Y. NMR characterization of cellulose acetate: Chemical shift assignments, substituent effects, and chemical shift additivity. Carbohydr. Polym. 2015, 118, 91–100. [Google Scholar] [CrossRef]
- Meehan, E. Characterisation of hydroxypropylmethylcellulose phthalate (HPMCP) by GPC using a modified organic solvent. Anal. Chim. Acta 2006, 557, 2–6. [Google Scholar] [CrossRef]
- Nelson, M.L.; O’Connor, R.T. Relation of certain infrared bands to cellulose crystallinity and crystal latticed type. Part, I. Spectra of lattice types I, II, III and of amorphous cellulose. J. Appl. Polym. Sci. 1964, 8, 1311–1324. [Google Scholar] [CrossRef]
- Colom, X.; Carrillo, F. Crystallinity changes in lyocell and viscose-type fibres by caustic treatment. Eur. Polym. J. 2002, 38, 2225–2230. [Google Scholar] [CrossRef]
- Atalla, R.H.; Gast, J.C.; Sindorf, D.W.; Bartuska, V.J.; Maciel, G.E. 13C NMR Spectra of Cellulose Polymorphs. J. Am. Chem. Soc. 1980, 102, 3249–3251. [Google Scholar] [CrossRef]
- Earl, W.L.; VanderHart, D.L. High Resolution, Magic Angle Sample Spinning 13C NMR of Solid Cellulose I. J. Am. Chem. Soc. 1980, 102, 3251–3252. [Google Scholar] [CrossRef]
- Earl, W.L.; VanderHart, D.L. Observations by High-Resolution Carbon-13 Nuclear Magnetic Resonance of Cellulose I Related to Morphology and Crystal Structure. Macromolecules 1981, 14, 570–574. [Google Scholar] [CrossRef]
- Atalla, R.H.; VanderHart, D.L. Native cellulose: A composite of two distinct crystalline forms. Science 1984, 223, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Horii, F.; Hirai, A.; Kitamaru, R. CP/MAS carbon-13 NMR study of spin relaxation phenomena of cellulose containing crystalline and noncrystalline components. J. Carbohydr. Chem. 1984, 3, 641–662. [Google Scholar] [CrossRef]
- Bertran, M.S.; Dale, B.E. Enzymatic hydrolysis and recrystallization behavior of initially amorphous cellulose. Biotechnol. Bioeng. 1985, 27, 177–181. [Google Scholar] [CrossRef] [PubMed]
- HORII, F.; HIRAI, A.; KITAMARU, R. Cross-polarization/magic angle spinning 13C-NMR study: Molecular chain conformations of native and regenerated cellulose. ACS Symp. Ser. 1984, 260, 27–42. [Google Scholar]
- Gubitosi, M.; Nosrati, P.; Hamid, M.K.; Kuczera, S.; Behrens, M.A.; Johansson, E.G.; Olsson, U. Stable, metastable and unstable cellulose solutions. R. Soc. Open Sci. 2017, 4, 1–11. [Google Scholar] [CrossRef]

| Concentration (wt %) | Mother Liquor (mL) | TBAH (50% w/w, mL) | DMSO (mL) |
|---|---|---|---|
| 0.05 | 6 | 5.34 | 18.66 |
| 0.10 | 12 | 4.00 | 14.00 |
| 0.15 | 18 | 2.67 | 9.33 |
| 0.20 | 24 | 1.34 | 4.66 |
| Parameters | Samples | ||||||
|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | |
| [η] (mL/g) | 152.8 | 310.6 | 538.8 | 564.5 | 738.6 | 550.6 | 693.4 |
| K1 | 0.2 | 0.3 | 0.3 | 0.4 | 0.5 | 0.2 | 0.4 |
| Intrinsic Viscosity (mL/g) * | Sample | ||||
|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | |
| [η]Cuoxam | 29.5 | 68.1 | 137.2 | 277.4 | 279.9 |
| [η]CED | - | 281.6 | 335.8 | 419.9 | 458.1 |
| [η]THDS | 152.8 | 310.6 | 538.8 | 564.5 | 738.6 |
| DP | Sample | ||||||
|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | |
| DPC | 200 | 405 | 557 | 655 | 682 | 486 | 600 |
| DPCED | 275 | 563 | 713 | 958 | 1073 | - | - |
| DPGPC a | 232 | 556 | 731 | 983 | - | - | - |
| DPTHDS | - | - | - | - | - | 616 | 752 |
| (DPCED-DPC)/DPCED (%) | 27 | 28 | 21 | 31 | 36 | - | - |
| (DPGPC-DPC)/DPGPC (%) | 13 | 27 | 23 | 33 | - | - | - |
| (DPTHDS-DPC)/DPTHDS (%) | - | - | - | - | - | 21 | 22 |
| Samples | Molecular Weight | |||||
|---|---|---|---|---|---|---|
| Mn | Mw | Mz | Mη | Mp | Mw/Mn | |
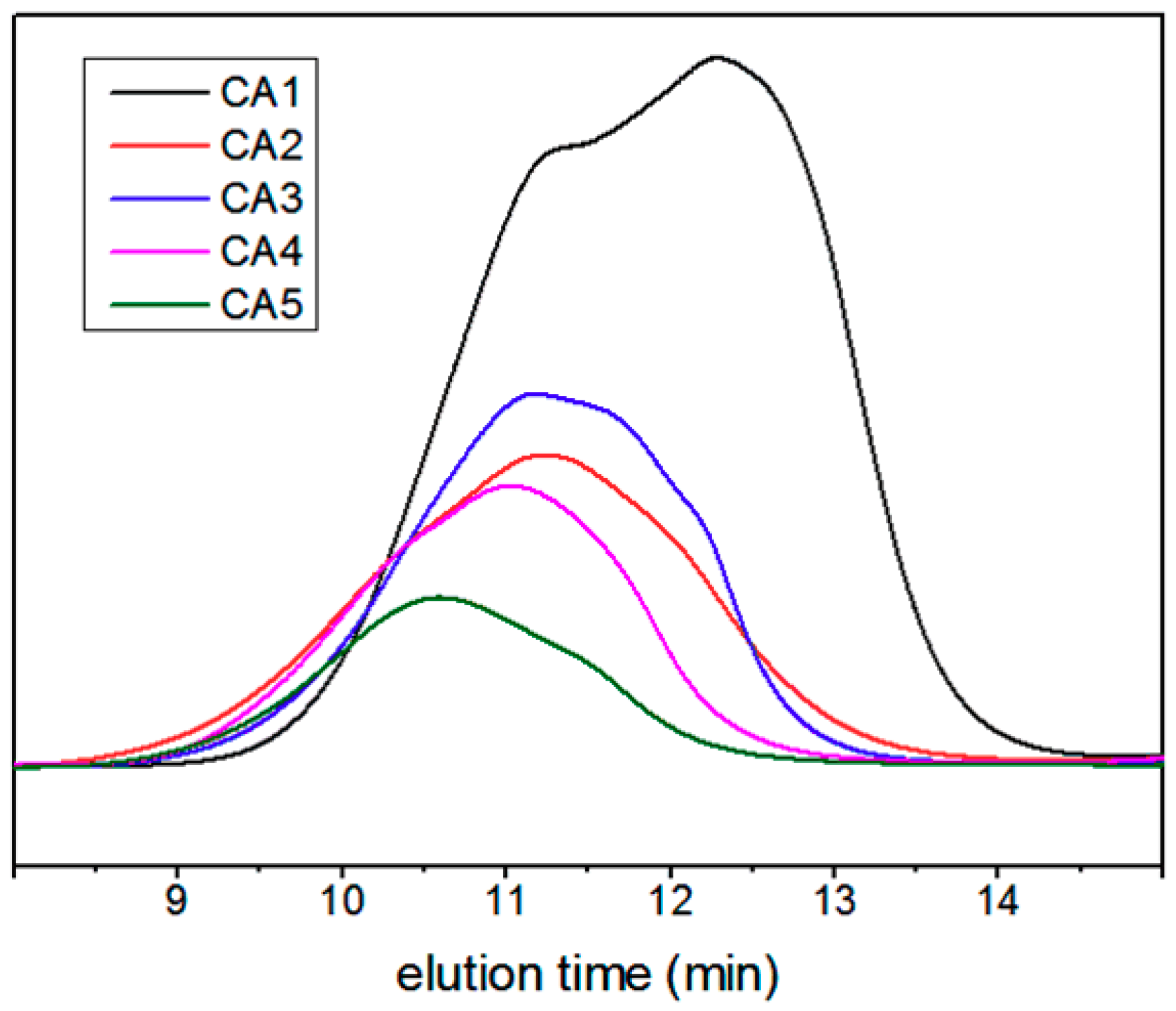
| CA1 | 11784 | 47898 | 252932 | 47898 | 15527 | 4.1 |
| CA2 | 32411 | 111016 | 1039096 | 111016 | 45753 | 3.4 |
| CA3 | 49199 | 165543 | 980068 | 165543 | 57839 | 3.4 |
| CA4 | 29147 | 200420 | 4247791 | 200420 | 44473 | 4.9 |
| CA5 | 75664 | 373374 | 4805672 | 373374 | 98679 | 6.9 |
| Sample | Parameter | |||
|---|---|---|---|---|
| [η] (mL/g) | ([η]Cn − [η]RCn)/[η]Cn (%) | DP | (DPCn − DPRCn)/DPCn (%) | |
| C6 | 550.6 | 16 | 616 | 14 |
| RC6 | 459.8 | 528 | ||
| C7 | 693.4 | 16 | 752 | 14 |
| RC7 | 579.9 | 644 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, D.; Hu, X.; Yang, Z.; Yang, X.; Wei, W.; Jiang, M.; Zhou, Z.; Zaman, A. Elucidation of the Relationship between Intrinsic Viscosity and Molecular Weight of Cellulose Dissolved in Tetra-N-Butyl Ammonium Hydroxide/Dimethyl Sulfoxide. Polymers 2019, 11, 1605. https://doi.org/10.3390/polym11101605
Bu D, Hu X, Yang Z, Yang X, Wei W, Jiang M, Zhou Z, Zaman A. Elucidation of the Relationship between Intrinsic Viscosity and Molecular Weight of Cellulose Dissolved in Tetra-N-Butyl Ammonium Hydroxide/Dimethyl Sulfoxide. Polymers. 2019; 11(10):1605. https://doi.org/10.3390/polym11101605
Chicago/Turabian StyleBu, Daqin, Xiangzhou Hu, Zhijie Yang, Xue Yang, Wei Wei, Man Jiang, Zuowan Zhou, and Ahsan Zaman. 2019. "Elucidation of the Relationship between Intrinsic Viscosity and Molecular Weight of Cellulose Dissolved in Tetra-N-Butyl Ammonium Hydroxide/Dimethyl Sulfoxide" Polymers 11, no. 10: 1605. https://doi.org/10.3390/polym11101605
APA StyleBu, D., Hu, X., Yang, Z., Yang, X., Wei, W., Jiang, M., Zhou, Z., & Zaman, A. (2019). Elucidation of the Relationship between Intrinsic Viscosity and Molecular Weight of Cellulose Dissolved in Tetra-N-Butyl Ammonium Hydroxide/Dimethyl Sulfoxide. Polymers, 11(10), 1605. https://doi.org/10.3390/polym11101605