Improved Direct Current Electrical Properties of Crosslinked Polyethylene Modified with the Polar Group Compound
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
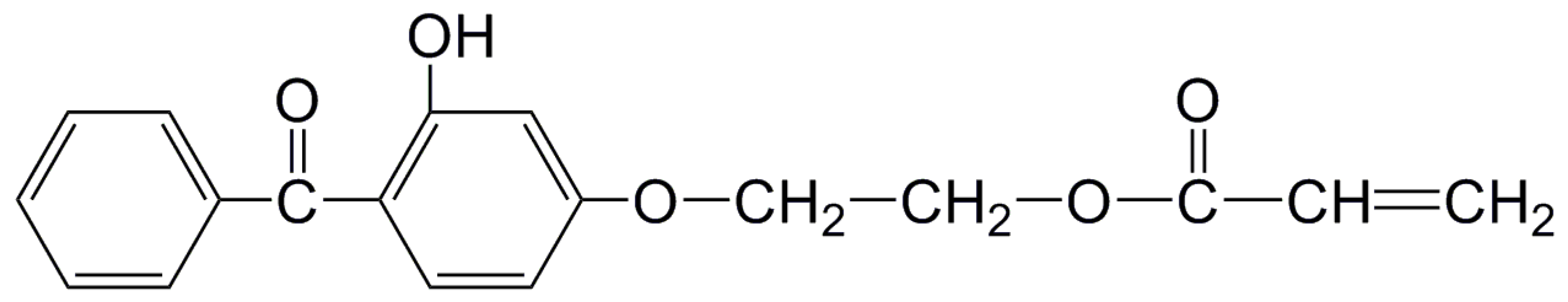
2.2. Preparation of Crosslinked Polyethylene (XLPE) Modified with 2-(4-Benzoyl-3-Hydroxyphenoxy) Ethyl Acrylate
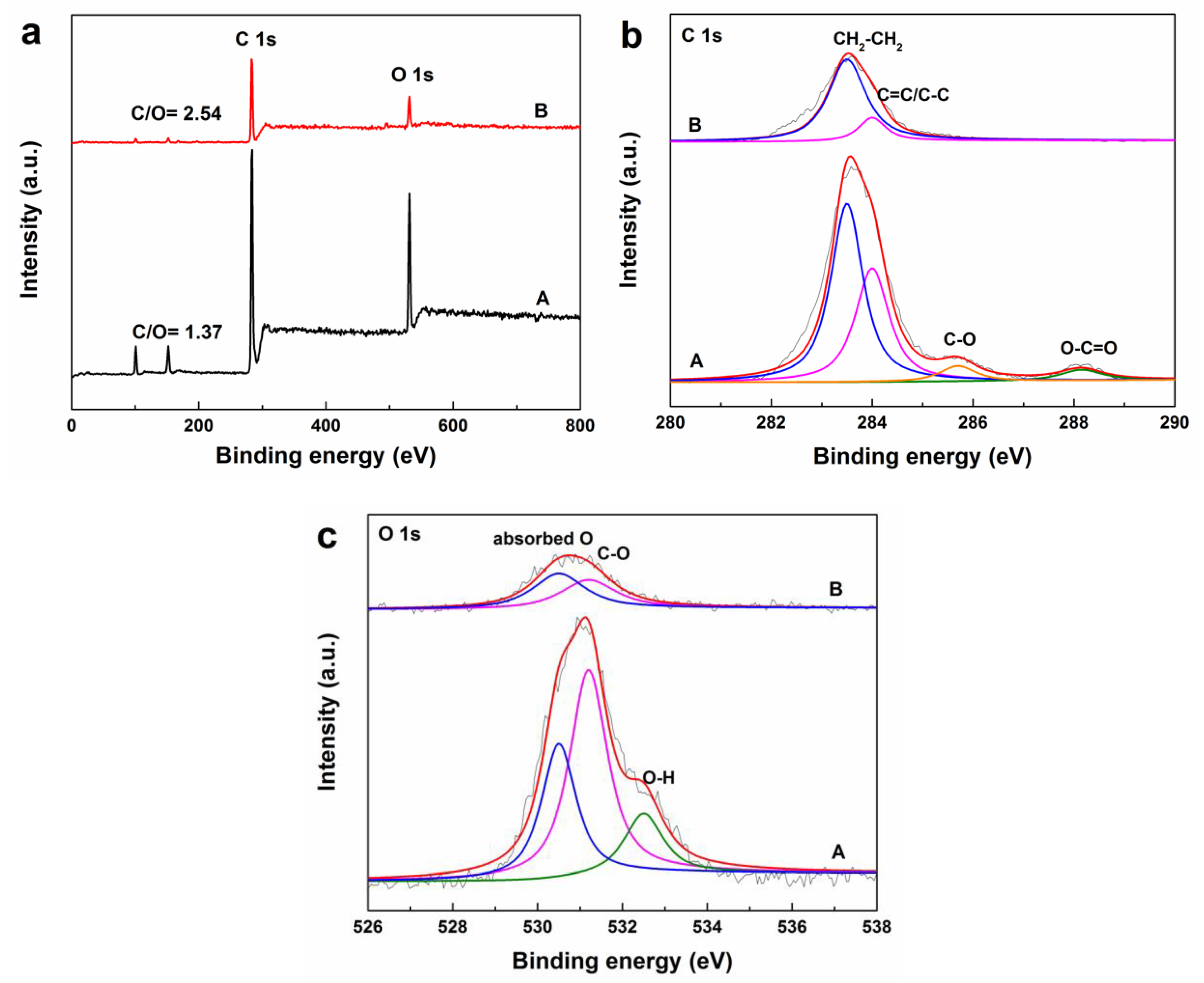
2.3. Characterization
2.4. Mechanical Properties Measurement
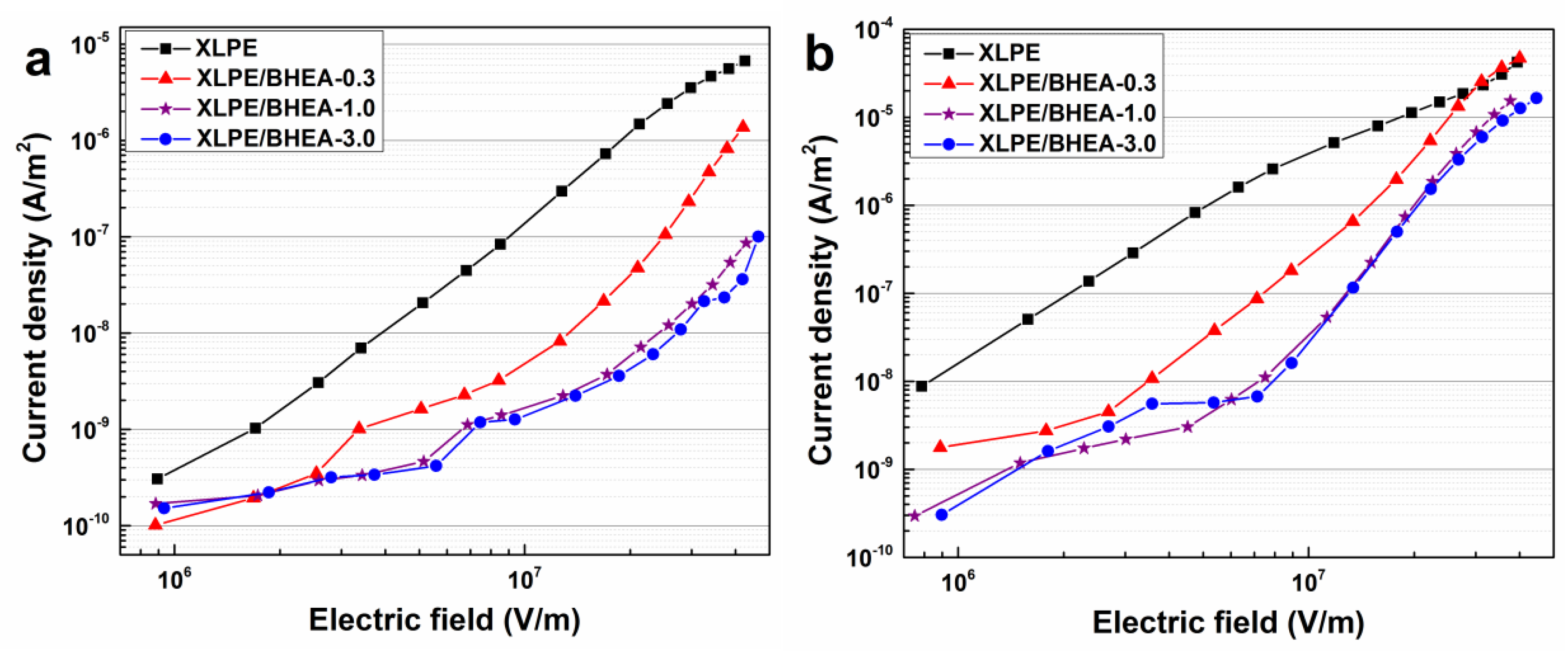
2.5. Electrical Performance Measurement
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tian, F.Q.; Yao, J.Y.; Li, P.; Wang, Y.; Wu, M.L.; Lei, Q.Q. Stepwise electric field induced charging current and its correlation with space charge formation in LDPE/ZnO nanocomposite. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 1232–1239. [Google Scholar] [CrossRef]
- Montanari, G.C.; Fabiani, D. Evaluation of dc insulation performance based on space-charge measurements and accelerated life tests. IEEE Trans. Dielectr. Electr. Insul. 2006, 13, 903–916. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Lewiner, J.; Alquie, C. Evidence of strong correlation between space-charge buildup and breakdown in cable insulation. IEEE Trans. Dielectr. Electr. Insul. 1996, 3, 778–783. [Google Scholar] [CrossRef]
- Rogti, F.; Mekhaldi, A.; Laurent, C. Space charge behavior at physical interfaces in cross-linked polyethylene under DC field. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 1478–1485. [Google Scholar] [CrossRef]
- Boggs, S. A rational consideration of space charge. IEEE Electr. Insul. M. 2004, 20, 22–27. [Google Scholar] [CrossRef]
- Gubanski, S.M. Insulating materials for next generations of HVAC and HVDC cables. Proceedings of 2016 IEEE International Conference on High Voltage Engineering and Application, Chengdu, China, 19–22 September 2016; pp. 1–6. [Google Scholar]
- Wang, Y.N.; Wu, J.D.; Yin, Y. Nanostructures and space charge characteristics of MgO/LDPE nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2390–2399. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Wang, C.; Chen, W.G.; Xiao, K. Effect of stretching on electrical properties of LDPE/MgO nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 1713–1722. [Google Scholar] [CrossRef]
- Hui, L.; Schadler, L.S.; Nelson, J.K. The influence of moisture on the electrical properties of crosslinked polyethylene/silica nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 641–653. [Google Scholar] [CrossRef]
- Wang, S.J.; Zha, J.W.; Wu, Y.H.; Ren, L.; Dang, Z.M. Preparation, microstructure and properties of polyethylene/alumina nanocomposites for HVDC insulation. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 3350–3356. [Google Scholar] [CrossRef]
- Fleming, R.J.; Ammala, A.; Lang, S.B.; Casey, P.S. Conductivity and space charge in LDPE containing nano-and micro-sized ZnO particles. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 118–126. [Google Scholar] [CrossRef]
- Fleming, R.J.; Ammala, A.; Casey, P.S.; Lang, S.B. Conductivity and space charge in LDPE/BaSrTiO3 nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 15–23. [Google Scholar] [CrossRef]
- Du, B.X.; Han, C.L.; Li, Z.L.; Li, J. Effect of graphene oxide particles on space charge accumulation in LDPE/GO nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1479–1486. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Y.X.; Cui, X.Y.; Sha, Y.C.; Le, T.H. Effect of nanoparticle surface modification on breakdown and space charge behavior of XLPE/SiO2 nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1554–1564. [Google Scholar] [CrossRef]
- Takada, T.; Hayase, Y.; Tanaka, Y. Space charge trapping in electrical potential well caused by permanent and induced dipoles for LDPE/MgO nanocomposite. IEEE Trans. Dielect. Electr. Insul. 2008, 15, 152–160. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, J.; Dang, B.; He, J.L. Mechanism of highly improved electrical properties in polypropylene by chemical modification of grafting maleic anhydride. J. Phys. D Appl. Phys. 2016, 49, 415301. [Google Scholar] [CrossRef]
- Meunier, M.; Quirke, N.; Aslanides, A. Molecular modeling of electron traps in polymer insulators: Chemical defects and impurities. J. Chem. Phys. 2001, 115, 2876–2881. [Google Scholar] [CrossRef]
- Teyssedre, G.; Laurent, C. Charge transport modeling in insulating polymers: From molecular to macroscopic scale. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 857–875. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, J.K.; Han, J.H.; Suh, K.S. Space charge and electrical conduction in maleic anhydride-grafted polyethylene. IEEE Trans. Dielectr. Electr. Insul. 1995, 2, 1132–1139. [Google Scholar]
- Suh, K.S.; Lee, C.R.; Zhu, Y.; Lim, J. Electrical properties of chemically modified polyethylenes. IEEE Trans. Dielectr. Electr. Insul. 1997, 4, 681–687. [Google Scholar] [CrossRef]
- Du, B.X.; Han, C.L.; Li, Z.L.; Li, J. Improved DC conductivity and space charge characteristics of XLPE for HVDC cable application: Effect of voltage stabilizers. IEEE Access 2019, 7, 66576–66583. [Google Scholar] [CrossRef]
- Khonakdara, H.A.; Morshediana, J.; Wagenknechtb, U.; Jafari, S.H. An investigation of chemical crosslinking effect on properties of high-density polyethylene. Polymer 2003, 44, 4301–4309. [Google Scholar] [CrossRef]
- Al-Malaika, S.; Riasat, S.; Lewucha, C. Reactive antioxidants for peroxide crosslinked polyethylene. Polym. Degrad. Stabil. 2017, 145, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.Q.; Zhao, H.; Xu, Z.Y.; Zhang, C.C.; Yang, J.M.; Zheng, C.J.; Lei, J.S. Accelerated water tree aging of crosslinked polyethylene with different degrees of crosslinking. Polym. Test. 2016, 56, 83–90. [Google Scholar] [CrossRef]
- Hirschl, Ch.; Biebl-Rydlo, M.; DeBiasio, M.; Mühleisen, W.; Neumaier, L.; Scherf, W.; Oreski, G.; Eder, G.; Chernev, B.; Schwab, W.; et al. Determining the degree of crosslinking of ethylene vinyl acetate photovoltaic module encapsulants–A comparative study. Sol. Energ. Mat. Sol. C 2013, 116, 203–218. [Google Scholar] [CrossRef]
- Yan, Q.; Xu, W.Y.; Bengt, R. Photoinitiated crosslinking of low density polyethylene I: Reaction and kinetics. Polym. Eng. Sci. 1991, 31, 1561–1566. [Google Scholar]
- Ma, D.L.; Hugener, T.A.; Siegel, R.W.; Christerson, A.; Mårtensson, E.; Önneby, C.; Schadler, L.S. Influence of nanoparticle surface modification on the electrical behaviour of polyethylene nanocomposites. Nanotechnology 2005, 16, 724–731. [Google Scholar] [CrossRef]
- Lau, K.Y.; Vaughan, A.S.; Chen, G.; Hosier, I.L.; Holt, A.F.; Ching, K.Y. On the space charge and DC breakdown behavior of polyethylene/silica nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 340–351. [Google Scholar] [CrossRef]
- Feng, S.J.; Wang, Q.; Gao, Y.; Huang, Y.G.; Qing, F.L. Synthesis and characterization of a novel amphiphilic copolymer capable as anti-biofouling coating material. J. Appl. Polym. Sci. 2009, 114, 2071–2078. [Google Scholar] [CrossRef]
- Feng, S.J.; Huang, Y.G.; Wang, Q.; Gao, Y.; Qing, F.L. Synthesis and antibiofouling properties of novel crosslinkable terpolymers containing semifluoroalkyl substituted aromatic side chains. Polym. Eng. Sci. 2010, 50, 944–951. [Google Scholar] [CrossRef]
- Roy, S.; Das, T.; Ming, Y.; Chen, X.L.; Yue, C.Y.; Hu, X. Specific functionalization and polymer grafting on multiwalled carbon nanotubes to fabricate advanced nylon 12 composites. J. Mater. Chem. A 2014, 2, 3961–3970. [Google Scholar] [CrossRef]
- Teng, C.C.; Ma, C.C.M.; Lu, C.H.; Yang, S.Y.; Lee, S.H.; Hsiao, M.C.; Yen, M.Y.; Chiou, K.C.; Lee, T.M. Thermal conductivity and structure of non-covalent functionalized graphene/epoxy composites. Carbon 2011, 49, 5107–5116. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Q.H.; Cui, J.J. XPS study of surface absorbed oxygen of ABO3 mixed oxides. J. Rare Earth. 2008, 26, 511–514. [Google Scholar] [CrossRef]
- Lu, X.F.; Bian, X.J.; Nie, G.D.; Zhang, C.C.; Wang, C.; Wei, Y. Encapsulating conducting polypyrrole into electrospun TiO2 nanofibers: A new kind of nanoreactor for in situ loading Pd nanocatalysts towards p-nitrophenol hydrogenation. J. Mater. Chem. 2012, 22, 12723–12730. [Google Scholar] [CrossRef]
- Tian, F.Q.; Lei, Q.Q.; Wang, X.; Wang, Y. Effect of deep trapping states on space charge suppression in polyethylene/ZnO nanocomposite. Appl. Phys. Lett. 2011, 99, 142903. [Google Scholar] [CrossRef]
- Peng, S.M.; He, J.L.; Hu, J.; Huang, X.Y.; Jiang, P.K. Influence of functionalized MgO nanoparticles on electrical properties of polyethylene nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 1512–1519. [Google Scholar] [CrossRef]
- Unge, M.; Törnkvist, C.; Christen, T. Space charges and deep traps in polyethylene–ab initio simulations of chemical impurities and defects. Proceedings of 2013 IEEE International Conference on Solid Dielectrics, Bologna, Italy, 30 June–4 July 2013; pp. 935–939. [Google Scholar]
- Wang, W.W.; Min, D.M.; Li, S.T. Understanding the conduction and breakdown properties of polyethylene nanodielectrics: Effect of deep traps. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 564–572. [Google Scholar] [CrossRef]






| Specimen | XLPE | XLPE/BHEA-0.3 | XLPE/BHEA-1.0 | XLPE/BHEA-3.0 |
|---|---|---|---|---|
| Gel content | 85.2% | 89.6% | 88.3% | 91.1% |
| Thermal elongation | 75% | 60% | 68% | 52% |
| Tensile strength | 26.17 | 26.94 | 24.52 | 24.95 |
| Elongation at break | 512.99 | 502.78 | 487.85 | 480.15 |
| Elasticity modulus | 195.09 | 201.96 | 185.90 | 211.13 |
| Melting temperature | 102.9 | 103.3 | 102.5 | 103.9 |
| Degree of crystallinity | 41.07% | 40.61% | 40.07% | 40.06% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Chang, J.; Zhang, H.; Li, C.; Zhao, H. Improved Direct Current Electrical Properties of Crosslinked Polyethylene Modified with the Polar Group Compound. Polymers 2019, 11, 1624. https://doi.org/10.3390/polym11101624
Zhang C, Chang J, Zhang H, Li C, Zhao H. Improved Direct Current Electrical Properties of Crosslinked Polyethylene Modified with the Polar Group Compound. Polymers. 2019; 11(10):1624. https://doi.org/10.3390/polym11101624
Chicago/Turabian StyleZhang, Chengcheng, Jianxin Chang, Hongyu Zhang, Chunyang Li, and Hong Zhao. 2019. "Improved Direct Current Electrical Properties of Crosslinked Polyethylene Modified with the Polar Group Compound" Polymers 11, no. 10: 1624. https://doi.org/10.3390/polym11101624





