Design and Characterization of New D–A Type Electrochromic Conjugated Copolymers Based on Indolo[3,2-b]Carbazole, Isoindigo and Thiophene Units
Abstract
:1. Introduction
2. Instrumentation and Materials
2.1. Instrumentation
2.2. Materials
2.3. Synthesis of Monomers
2.4. Synthesis of Copolymer PITID-1
2.5. Synthesis of Copolymer PITID-2
3. Results and Discussions
3.1. FT-IR Spectra of the Two Polymers
3.2. XPS Profiles of Polymer Films
3.3. Electrochemical Properties
3.4. Optical Behaviors of the Polymer Solutions and Films
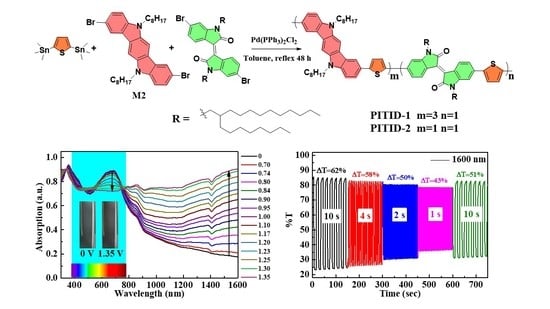
3.5. Spectroelectrochemistry
3.6. Electrochromic Switching Study
3.7. Colorimetric Analysis
3.8. Open Circuit Memory Experiments of the Two Polymers
3.9. Thermal Properties
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Eh, A.L.-S.; Tan, A.W.M.; Cheng, X.; Magdassi, S.; Lee, P.S. Recent advances in flexible electrochromic devices: Prerequisites, challenges, and prospects. Energy Technol. 2018, 6, 33–45. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Ellinger, S.; Reynolds, J.R. The donor-acceptor approach allows a black-to-transmissive switching polymeric electrochrome. Nat. Mater. 2008, 7, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Udum, Y.A.; Durmus, A.; Gunbas, G.E.; Toppare, L. Both p- and n-type dopable polymer toward electrochromic applications. Org. Electron. 2008, 9, 501–506. [Google Scholar] [CrossRef]
- Amb, C.M.; Dyer, A.L.; Reynolds, J.R. Navigating the color palette of solution-processable electrochromic polymers. Chem. Mater. 2011, 23, 397–415. [Google Scholar] [CrossRef]
- Neo, W.T.; Ye, Q.; Chua, S.-J.; Xu, J.W. Conjugated polymer-based electrochromics: Materials, device fabrication and application prospects. J. Mater. Chem. C 2016, 4, 7364–7376. [Google Scholar] [CrossRef]
- Teran, N.B.; Reynolds, J.R. Discrete donor-acceptor conjugated systems in neutral and oxidized states: Implications toward molecular design for high contrast electrochromics. Chem. Mater. 2017, 29, 1290–1301. [Google Scholar] [CrossRef]
- Liu, X.; Xie, Y.; Cai, X.; Li, Y.; Wu, H.; Su, S.-J.; Cao, Y. Synthesis and photovoltaic properties of A–D–A type non-fullerene acceptors containing isoindigo terminal units. RSC Adv. 2015, 5, 107566–107574. [Google Scholar] [CrossRef]
- Abraham, S.; Mangalath, S.; Sasikumar, D.; Joseph, J. Transmissive-to-black electrochromic devices based on cross-linkable tetraphenylethene-diphenylamine derivatives. Chem. Mater. 2017, 29, 9877–9881. [Google Scholar] [CrossRef]
- Groenendaal, L.; Zotti, G.; Aubert, P.H.; Waybright, S.M.; Reynolds, J.R. Electrochemistry of poly(3,4-alkylenedioxythiophene) derivatives. Adv. Mater. 2003, 15, 855–879. [Google Scholar] [CrossRef]
- Zhao, G.; Dong, H.; Zhao, H.; Jiang, L.; Zhang, X.; Tan, J.; Meng, Q.; Hu, W. Substitution effect on molecular packing and transistor performance of indolo[3,2-b]carbazole derivatives. J. Mater. Chem. 2012, 22, 4409–4417. [Google Scholar] [CrossRef]
- Lu, Y.; Ding, Y.; Wang, J.; Pei, J. Research progress in isoindigo-based polymer field-effect transistor materials. Chin. J. Org. Chem. 2016, 36, 2272–2283. [Google Scholar] [CrossRef]
- Cho, I.; Park, S.K.; Kang, B.; Chung, J.W.; Kim, J.H.; Cho, K.; Park, S.Y. Design, synthesis, and versatile processing of indolo[3,2-b]indole-based π-conjugated molecules for high-performance organic field-effect transistors. Adv. Funct. Mater. 2016, 26, 2966–2973. [Google Scholar] [CrossRef]
- Hwang, J.; Park, J.; Kim, Y.J.; Ha, Y.H.; Park, C.E.; Chung, D.S.; Kwon, S.-K.; Kim, Y.-H. Indolo[3,2-b]indole-containing donor–acceptor copolymers for high-efficiency organic solar cells. Chem. Mater. 2017, 29, 2135–2140. [Google Scholar] [CrossRef]
- Zhong, W.; Xu, C.; Xiao, B.; Fan, L.; Wu, H.; Zhang, B.; Yang, W. High molecular weight broad band-gap polymers based on indolo[3,2-b]carbazole and thiazolo[5,4-d]thiazole derivatives for solar cells. Polym. Sci. Ser. B 2016, 58, 587–593. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.; Kong, L.; Tian, Y.; Yang, J. A novel indolo[3,2- b ]carbazole derivative with D-π-A structure exhibiting aggregation-enhanced emission and mechanofluorochromic properties. Dyes Pigm. 2018, 159, 314–321. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.; Wang, X.-Y.; Guo, S.-Z.; Dong, Y.-B.; Yu, G.-A.; Yin, J.; Liu, S.-H. Redox-modulated near-infrared electrochromism, electroluminochromism, and aggregation-induced fluorescence change in an indolo[3,2-b]carbazole-bridged diamine system. Sens. Actuators B 2017, 246, 570–577. [Google Scholar] [CrossRef]
- Shi, H.; Yuan, J.; Wu, X.; Dong, X.; Fang, L.; Miao, Y.; Wang, H.; Cheng, F. Two novel indolo[3,2-b]carbazole derivatives containing dimesitylboron moieties: Synthesis, photoluminescent and electroluminescent properties. New J. Chem. 2014, 38, 2368–2378. [Google Scholar] [CrossRef]
- Mula, S.; Leclerc, N.; Leveque, P.; Retailleau, P.; Ulrich, G. Synthesis of indolo[3,2- b]carbazole-based boron complexes with tunable photophysical and electrochemical properties. J. Org. Chem. 2018, 83, 14406–14418. [Google Scholar] [CrossRef]
- Qian, X.; Shao, L.; Li, H.; Yan, R.; Wang, X.; Hou, L. Indolo[3,2-b]carbazole-based multi-donor–π–acceptor type organic dyes for highly efficient dye-sensitized solar cells. J. Power Sources. 2016, 319, 39–47. [Google Scholar] [CrossRef]
- Reig, M.; Puigdollers, J.; Velasco, D. Molecular order of air-stable p-type organic thin-film transistors by tuning the extension of the π-conjugated core: The cases of indolo[3,2-b]carbazole and triindole semiconductors. J. Mater. Chem. C 2015, 3, 506–513. [Google Scholar] [CrossRef]
- Lengvinaite, S.; Grazulevicius, J.V.; Grigalevicius, S.; Gu, R.; Dehaen, W.; Jankauskas, V.; Zhang, B.; Xie, Z. Indolo[3,2-b]carbazole-based functional derivatives as materials for light emitting diodes. Dyes Pigm. 2010, 85, 183–188. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.; Wang, B.; Liu, Z.; Zhao, J.; Xie, Y. Yellow-to-blue switching of indole[3,2-b]carbazole-based electrochromic polymers and the corresponding electrochromic devices with outstanding photopic contrast, fast switching speed, and satisfactory cycling stability. Electrochim. Acta 2019, 302, 373–384. [Google Scholar] [CrossRef]
- Wang, E.; Ma, Z.; Zhang, Z.; Vandewal, K.; Henriksson, P.; Inganas, O.; Zhang, F.; Andersson, M.R. An easily accessible isoindigo-based polymer for high-performance polymer solar cells. J. Am. Chem. Soc. 2011, 133, 14244–14247. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, M.; Kong, L.; Zhang, Y.; Ju, X.; Zhao, J. The optimization of donor-to-acceptor feed ratios with the aim of obtaining black-to-transmissive switching polymers based on isoindigo as the electron-deficient moiety. RSC Adv. 2017, 7, 11840–11851. [Google Scholar] [CrossRef] [Green Version]
- Gu, H.; Ming, S.L.; Lin, K.W.; Chen, S.; Liu, X.M.; Lu, B.Y.; Xu, J.K. Isoindigo as an electron-deficient unit for high-performance polymeric electrochromics. Electrochim. Acta 2018, 260, 772–782. [Google Scholar] [CrossRef]
- Deng, P.; Zhang, Q. Recent developments on isoindigo-based conjugated polymers. Polym. Chem. 2014, 5, 3298–3305. [Google Scholar] [CrossRef]
- Stalder, R.; Puniredd, S.R.; Hansen, M.R.; Koldemir, U.; Grand, C.; Zajaczkowski, W.; Mullen, K.; Pisula, W.; Reynolds, J.R. Ambipolar charge transport in isoindigo-based donor-acceptor polymers. Chem. Mater. 2016, 28, 1286–1297. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Zhao, J.; Cui, C.; Liu, J. Effects of alkyl or alkoxy side chains on the electrochromic properties of four ambipolar donor–acceptor type polymers. RSC Adv. 2014, 4, 52712–52726. [Google Scholar] [CrossRef]
- Randell, N.M.; Radford, C.L.; Yang, J.; Quinn, J.; Hou, D.; Li, Y.; Kelly, T.L. Effect of acceptor unit length and planarity on the optoelectronic properties of isoindigo–thiophene donor–acceptor polymers. Chem. Mater. 2018, 30, 4864–4873. [Google Scholar] [CrossRef]
- Gross, Y.M.; Trefz, D.; Tkachov, R.; Untilova, V.; Brinkmann, M.; Schulz, G.L.; Ludwigs, S. Tuning aggregation by regioregularity for high-performance n-type P(NDI2OD-T2) donor–acceptor copolymers. Macromolecules 2017, 50, 5353–5366. [Google Scholar] [CrossRef]
- Liu, X.; Kong, L.; Du, H.; Zhang, Y.; Zhao, J.; Xie, Y. Synthesis and electrochromic properties of electrochromic polymers based on propylenedioxythiophene, diketopyrrolopyrrole and benzodithiophene units. Org. Electron. 2019, 64, 223–235. [Google Scholar] [CrossRef]
- Mery, A.; Bernard, P.; Valero, A.; Alper, J.P.; Herlin-Boime, N.; Haon, C.; Duclairoir, F.; Sadki, S. A polyisoindigo derivative as novel n-type conductive binder inside Si@C nanoparticle electrodes for Li-ion battery applications. J. Power Sources 2019, 420, 9–14. [Google Scholar] [CrossRef]
- Dante, R.C.; Chamorro-Posada, P.; Vázquez-Cabo, J.; Rubiños-López, Ó.; Sánchez-Árevalo, F.M.; Huerta, L.; Martín-Ramos, P.; Lartundo-Rojas, L.; Ávila-Vega, C.F.; Rivera-Tapia, E.D.; et al. Nitrogen-carbon graphite-like semiconductor synthesized from uric acid. Carbon 2017, 121, 368–379. [Google Scholar] [CrossRef] [Green Version]
- Ming, S.; Zhen, S.; Lin, K.; Zhao, L.; Xu, J.; Lu, B. Thiadiazolo[3,4-c]pyridine as an acceptor toward fast switching green donor acceptor type electrochromic polymer with low bandgap. ACS Appl. Mater. Interfaces 2015, 7, 11089–11098. [Google Scholar] [CrossRef] [PubMed]













| Copolymer | Mn | Mw | PDI | Yield | Eonset,ox | Redox Peak |
|---|---|---|---|---|---|---|
| /kDa | /kDa | /V | /V | |||
| PITID-1 | 20.1 | 27.5 | 1.37 | 74 | 0.79 | 1.43/0.86 |
| PITID-2 | 17.7 | 23.7 | 1.34 | 73 | 0.68 | 1.20/0.97 |
| Compound | In Solution | In Film State | aEgopt | bEonset | c HOMO (eV) | d LUMO (eV) | ||
|---|---|---|---|---|---|---|---|---|
| λmax (nm) | λonset (nm) | λmax(nm) | λonset (nm) | (eV) | (V) | |||
| PTDDB-1 | 354; 658 | 744 | 355; 674 | 795 | 1.56 | 0.79 | −5.04 | −3.48 |
| PTDDB-2 | 354; 664 | 748 | 353; 672 | 786 | 1.58 | 0.68 | −4.93 | −3.35 |
| Polymer | λmax | %∆T | a Response Time | b ∆OD | c ∆Qd | dCE | |
|---|---|---|---|---|---|---|---|
| nm | tc(s) | tb(s) | (mC cm−2) | cm2 C−1 | |||
| PITID-1 | 670 | 12 | 2.89 | 0.39 | 0.09 | 1.08 | 52.94 |
| 1500 | 33 | 2.36 | 2.23 | 0.25 | 2.71 | 92.92 | |
| PITID-2 | 675 | 18 | 2.04 | 0.33 | 0.19 | 1.13 | 171.52 |
| 1600 | 58 | 1.5 | 1.35 | 0.51 | 3.37 | 153.08 | |
| Compound | Tei (°C) | Tef (°C) | Tp (°C) | Td (°C) | % char |
|---|---|---|---|---|---|
| PITID-1 | 427 | 495 | 464 | 422 | 20 |
| PITID-2 | 419 | 496 | 465 | 399 | 41 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, S.; Zhang, Y.; Du, H.; Zhao, J. Design and Characterization of New D–A Type Electrochromic Conjugated Copolymers Based on Indolo[3,2-b]Carbazole, Isoindigo and Thiophene Units. Polymers 2019, 11, 1626. https://doi.org/10.3390/polym11101626
Zhang Y, Chen S, Zhang Y, Du H, Zhao J. Design and Characterization of New D–A Type Electrochromic Conjugated Copolymers Based on Indolo[3,2-b]Carbazole, Isoindigo and Thiophene Units. Polymers. 2019; 11(10):1626. https://doi.org/10.3390/polym11101626
Chicago/Turabian StyleZhang, Yuling, Shuang Chen, Yan Zhang, Hongmei Du, and Jinsheng Zhao. 2019. "Design and Characterization of New D–A Type Electrochromic Conjugated Copolymers Based on Indolo[3,2-b]Carbazole, Isoindigo and Thiophene Units" Polymers 11, no. 10: 1626. https://doi.org/10.3390/polym11101626