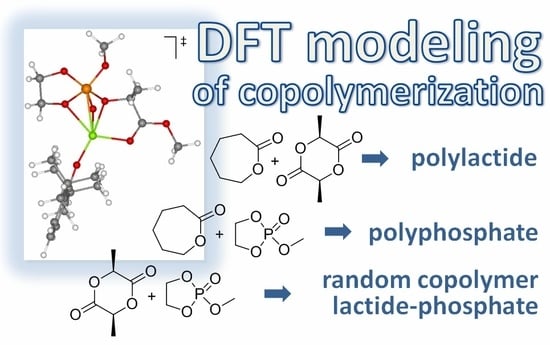
DFT Visualization and Experimental Evidence of BHT-Mg-Catalyzed Copolymerization of Lactides, Lactones and Ethylene Phosphates
Abstract
Share and Cite
Nifant’ev, I.; Shlyakhtin, A.; Kosarev, M.; Gavrilov, D.; Karchevsky, S.; Ivchenko, P. DFT Visualization and Experimental Evidence of BHT-Mg-Catalyzed Copolymerization of Lactides, Lactones and Ethylene Phosphates. Polymers 2019, 11, 1641. https://doi.org/10.3390/polym11101641
Nifant’ev I, Shlyakhtin A, Kosarev M, Gavrilov D, Karchevsky S, Ivchenko P. DFT Visualization and Experimental Evidence of BHT-Mg-Catalyzed Copolymerization of Lactides, Lactones and Ethylene Phosphates. Polymers. 2019; 11(10):1641. https://doi.org/10.3390/polym11101641
Chicago/Turabian StyleNifant’ev, Ilya, Andrey Shlyakhtin, Maxim Kosarev, Dmitry Gavrilov, Stanislav Karchevsky, and Pavel Ivchenko. 2019. "DFT Visualization and Experimental Evidence of BHT-Mg-Catalyzed Copolymerization of Lactides, Lactones and Ethylene Phosphates" Polymers 11, no. 10: 1641. https://doi.org/10.3390/polym11101641
APA StyleNifant’ev, I., Shlyakhtin, A., Kosarev, M., Gavrilov, D., Karchevsky, S., & Ivchenko, P. (2019). DFT Visualization and Experimental Evidence of BHT-Mg-Catalyzed Copolymerization of Lactides, Lactones and Ethylene Phosphates. Polymers, 11(10), 1641. https://doi.org/10.3390/polym11101641