Targeted Release of Probiotics from Enteric Microparticulated Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of Enteric Microparticles Containing Probiotic Bacteria
2.3. Microparticles Characterization
2.4. Microbiological Studies
2.5. Long-Term Stability Studies
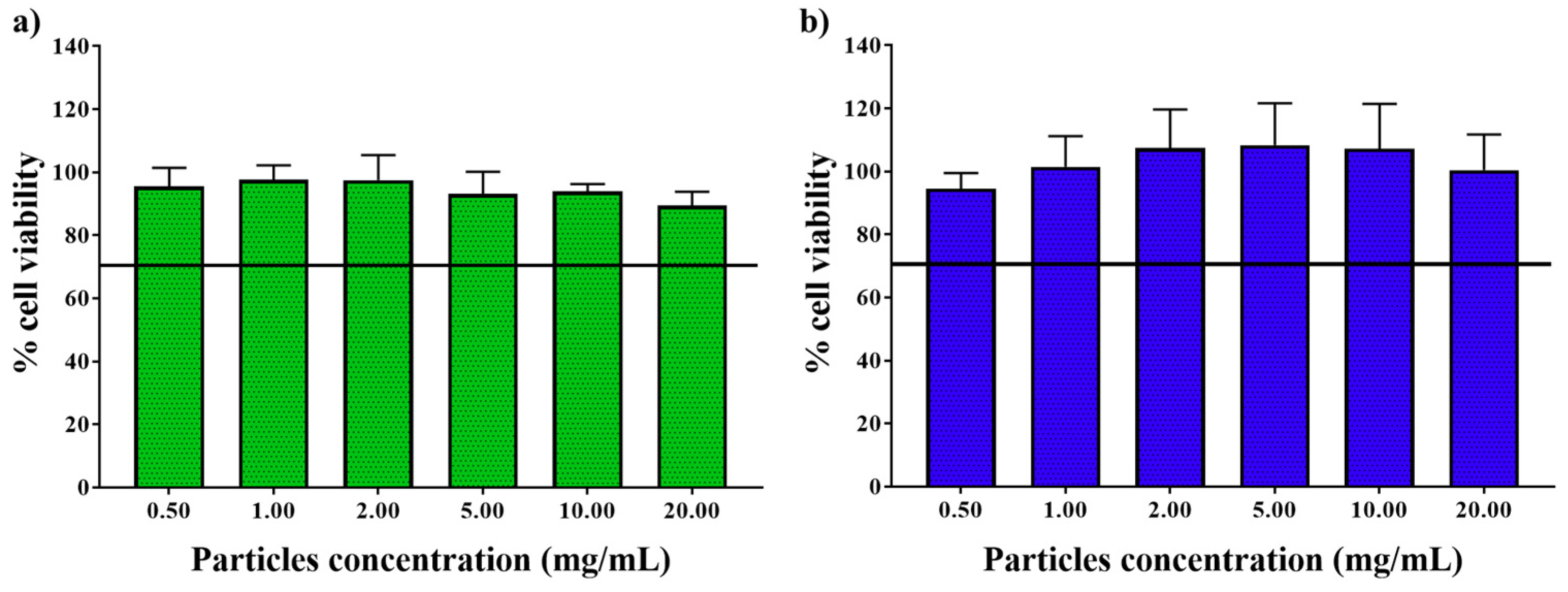
2.6. In Vitro Cell Viability Assay
2.7. Statistical Analyses
3. Results and Discussion
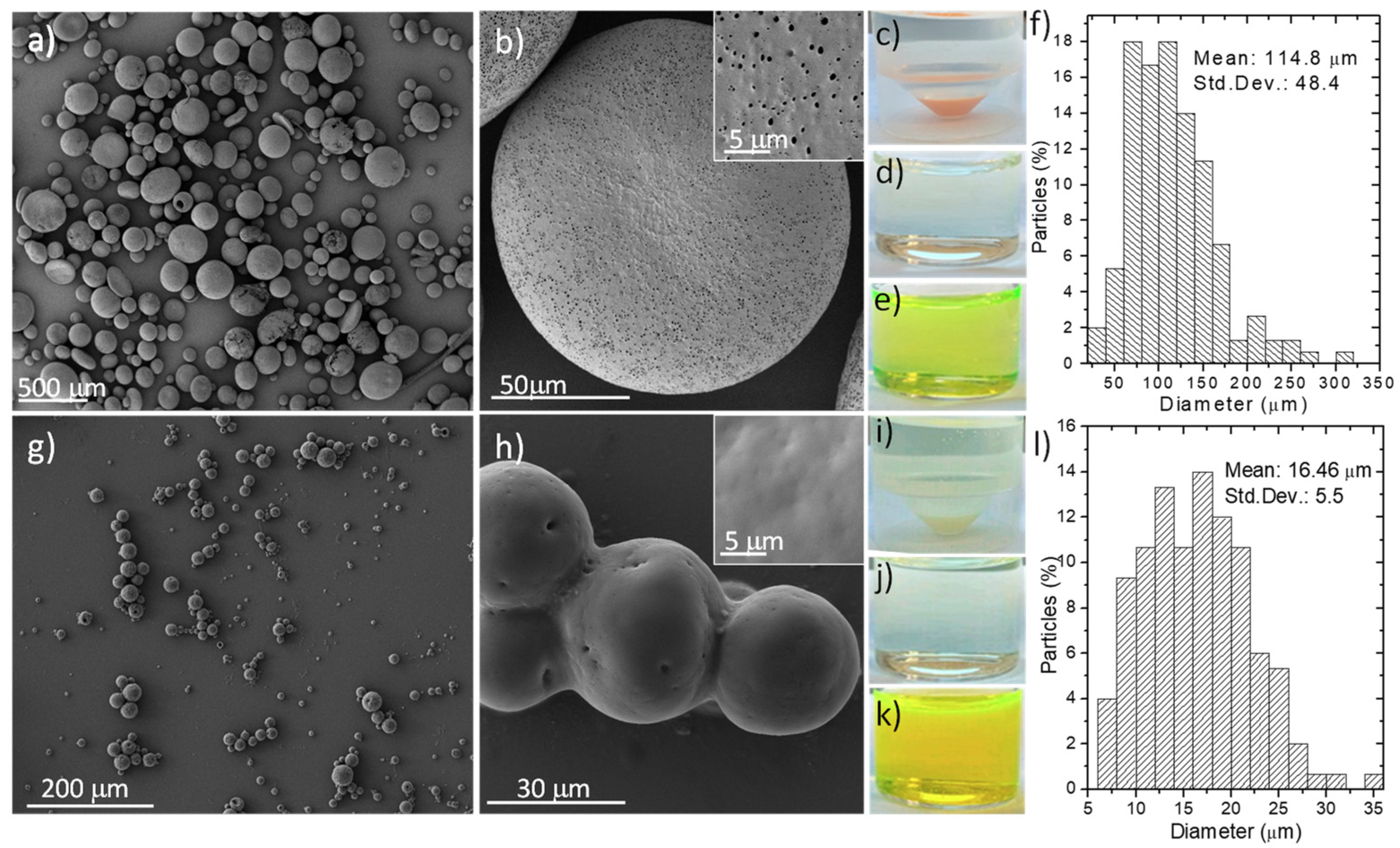
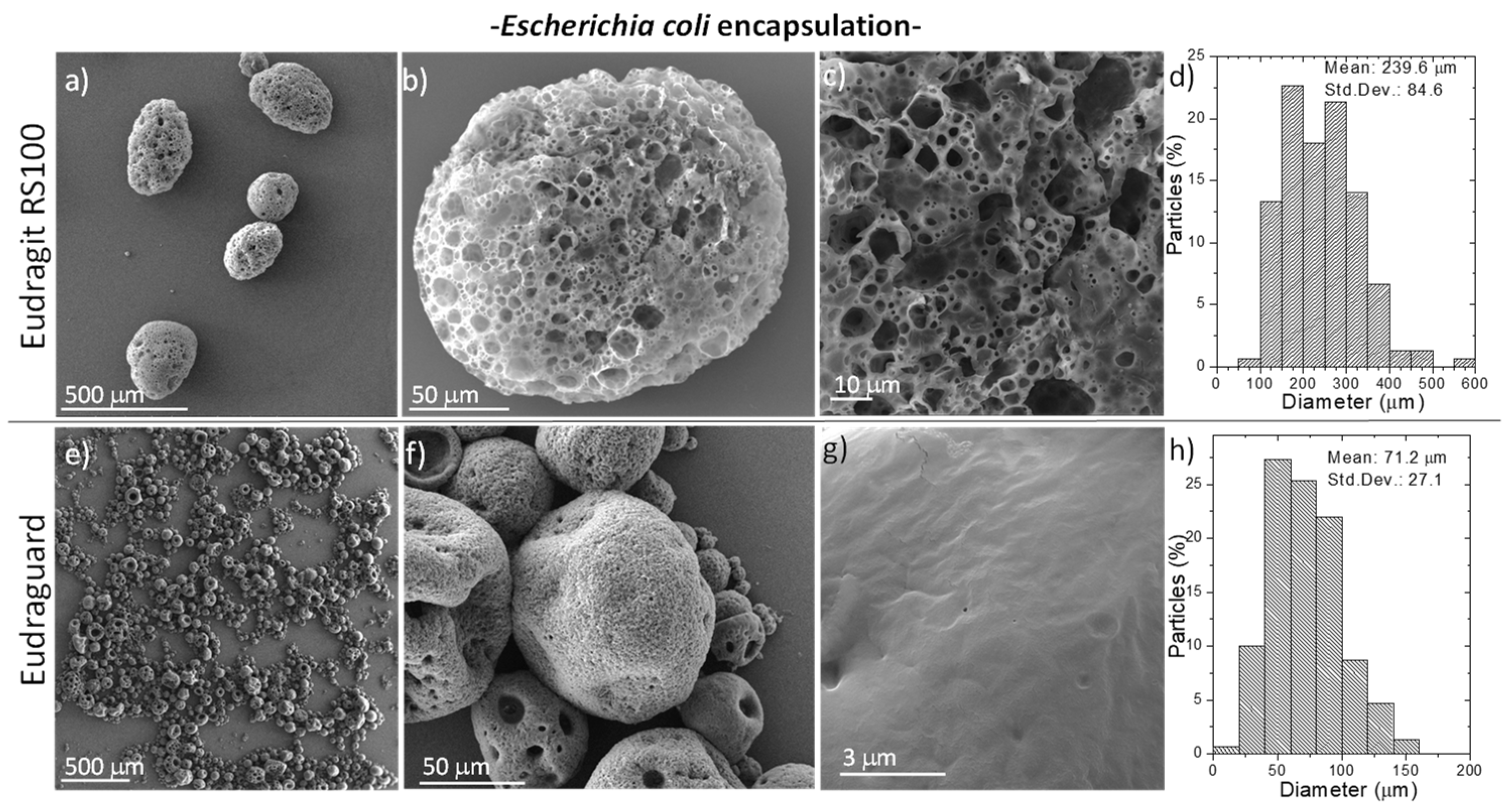
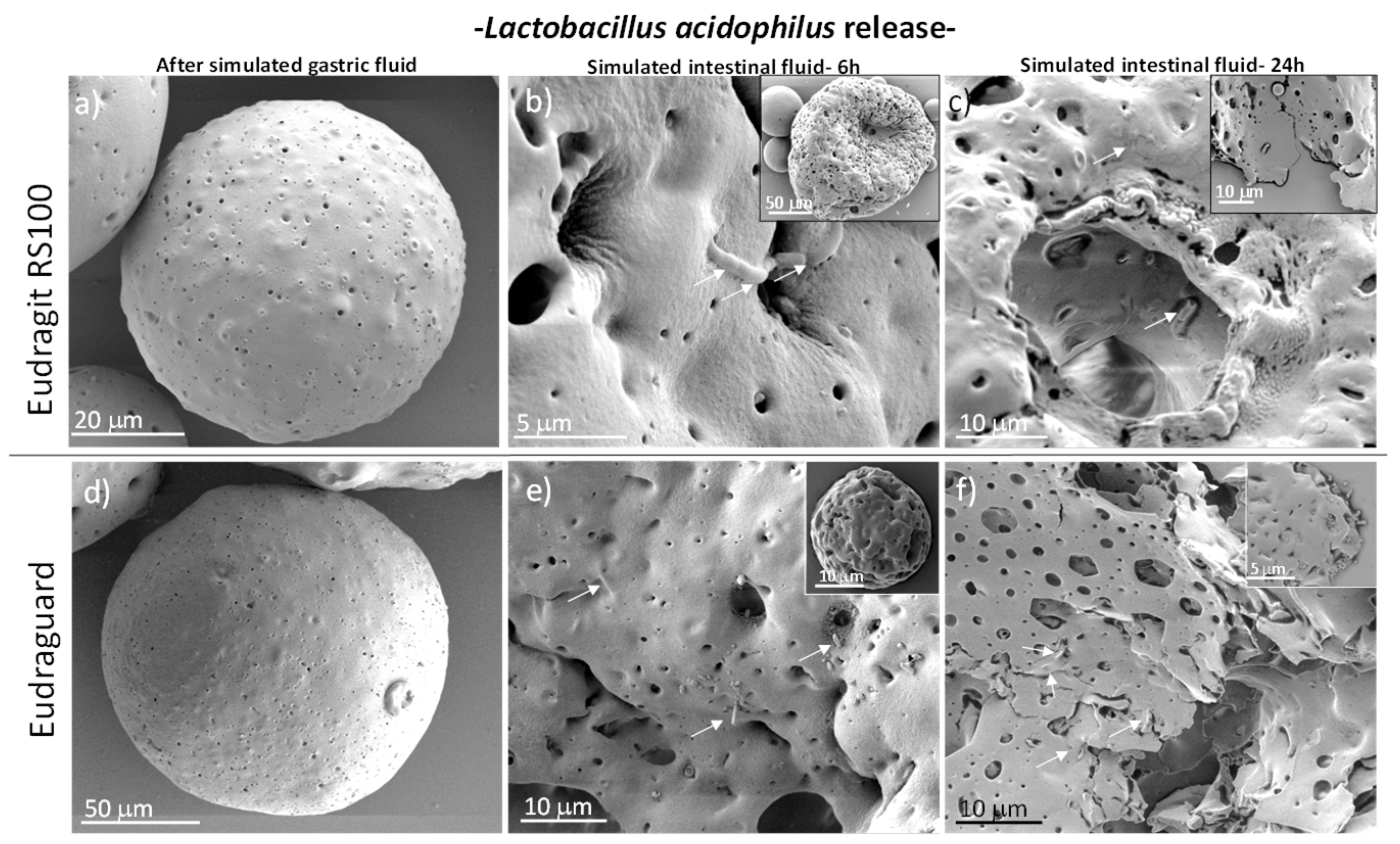
3.1. Synthesis and Characterization of Fabricated Microparticles
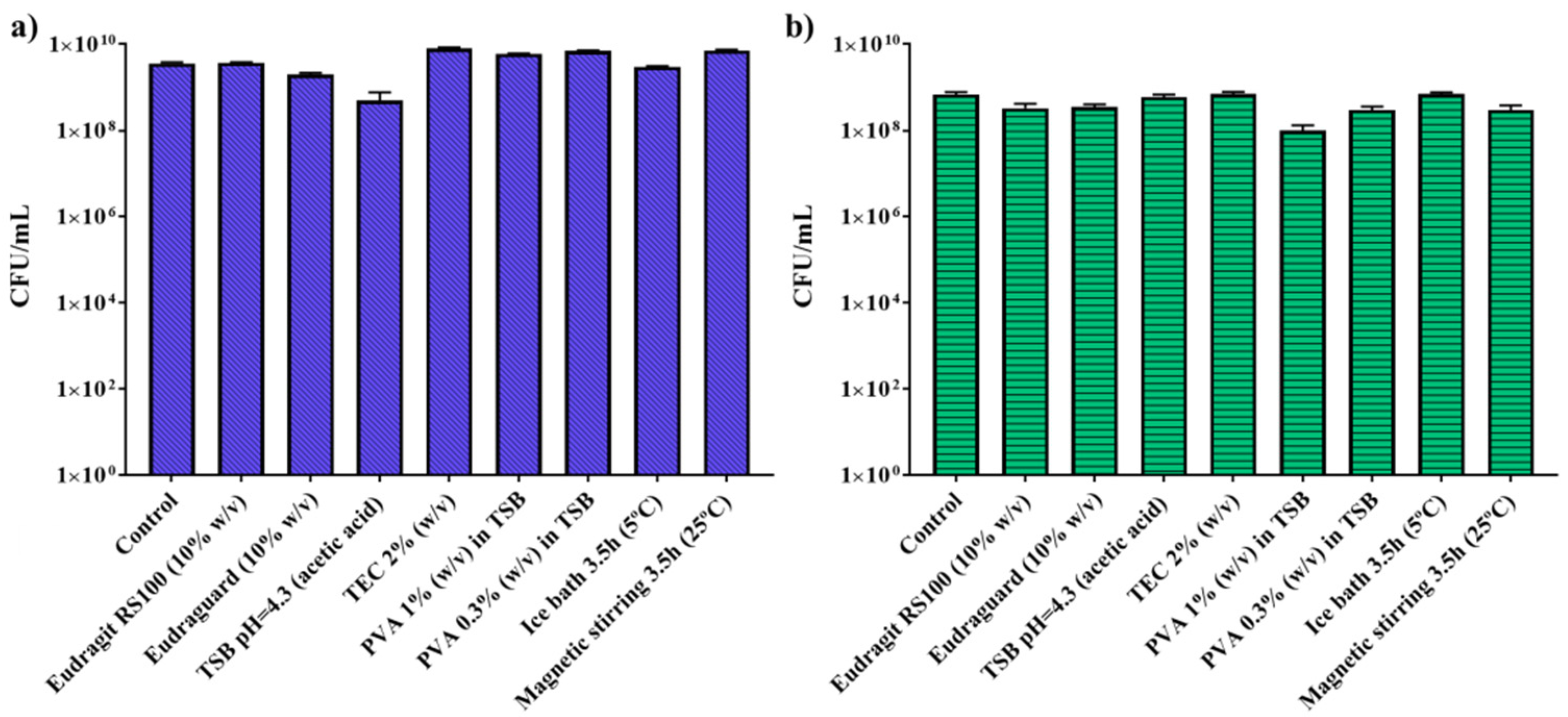
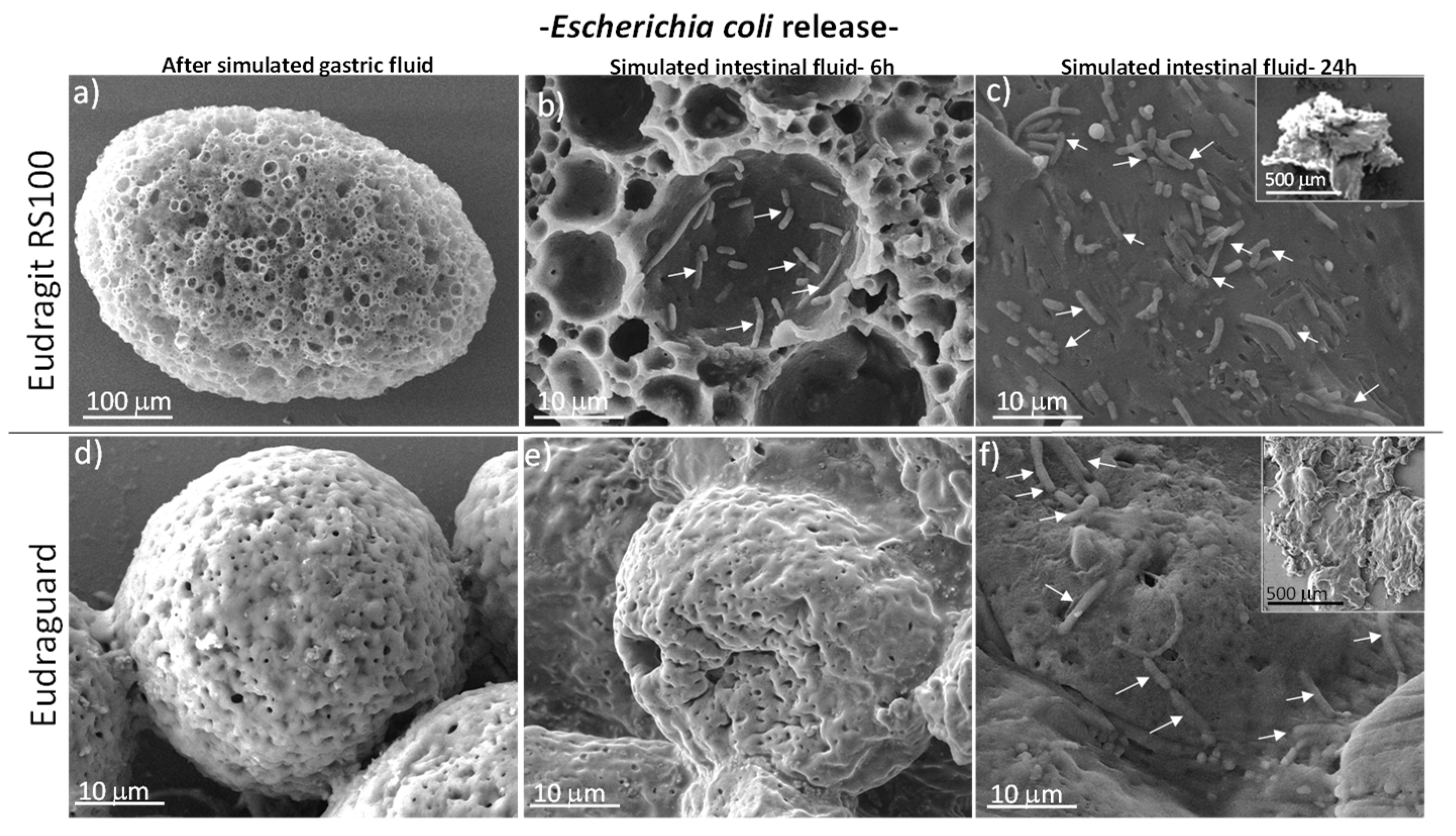
3.2. In Vitro Biological Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nollenberger, K.; Albers, J. Poly(meth)acrylate-based coatings. Int. J. Pharm. 2013, 457, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Eisele, J.; Haynes, G.; Kreuzer, K.; Hall, C. Toxicological assessment of Anionic Methacrylate Copolymer: I. Characterization, bioavailability and genotoxicity. Regul. Toxicol. Pharmacol. 2016, 82, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Beloqui, A.; Coco, R.; Memvanga, P.B.; Ucakar, B.; des Rieux, A.; Préat, V. pH-sensitive nanoparticles for colonic delivery of curcumin in inflammatory bowel disease. Int. J. Pharm. 2014, 473, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, K.; Guhathakarta, S.; Rajaram, R.; Korrapati, P.S. Electrospun zein/eudragit nanofibers based dual drug delivery system for the simultaneous delivery of aceclofenac and pantoprazole. Int. J. Pharm. 2012, 438, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, X.; Meng, L.; Zhang, Y.; Ai, R.; Qi, N.; He, H.; Xu, H.; Tang, X. Thiolated Eudragit nanoparticles for oral insulin delivery: Preparation, characterization and in vivo evaluation. Int. J. Pharm. 2012, 436, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Lin, P.; Xiang, Y.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. A novel nanomatrix system consisted of colloidal silica and pH-sensitive polymethylacrylate improves the oral bioavailability of fenofibrate. Eur. J. Pharm. Biopharm. 2011, 79, 126–134. [Google Scholar] [CrossRef]
- Gao, C.; Huang, J.; Jiao, Y.; Shan, L.; Liu, Y.; Li, Y.; Mei, X. In vitro release and in vivo absorption in beagle dogs of meloxicam from Eudragit® FS 30 D-coated pellets. Int. J. Pharm. 2006, 322, 104–112. [Google Scholar] [CrossRef]
- Zidan, A.; Ahmed, O.; Aljaeid, B. Nicotinamide polymeric nanoemulsified systems: a quality-by-design case study for a sustained antimicrobial activity. Int. J. Nanomedicine 2016, 11, 1501. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C. Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 2003, 67, 429–453. [Google Scholar] [CrossRef]
- Khoder, G.; Al-Menhali, A.A.; Al-Yassir, F.; Karam, S.M. Potential role of probiotics in the management of gastric ulcer. Exp. Ther. Med. 2016, 12, 3–17. [Google Scholar] [CrossRef]
- Prakash, S.; Tomaro-Duchesneau, C.; Saha, S.; Cantor, A. The Gut Microbiota and Human Health with an Emphasis on the Use of Microencapsulated Bacterial Cells. J. Biomed. Biotechnol. 2011, 2011, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poelvoorde, N.; Huyghebaert, N.; Vervaet, C.; Remon, J.-P. Optimisation of an enteric coated, layered multi-particulate formulation for ileal delivery of viable recombinant Lactococcus lactis. Eur. J. Pharm. Biopharm. 2008, 69, 969–976. [Google Scholar] [CrossRef]
- Huyghebaert, N.; Vermeire, A.; Rottiers, P.; Remaut, E.; Remon, J. Development of an enteric-coated, layered multi-particulate formulation for ileal delivery of viable recombinant Lactococcus lactis. Eur. J. Pharm. Biopharm. 2005, 61, 134–141. [Google Scholar] [CrossRef] [PubMed]
- de Barros, J.M.S.; Scherer, T.; Charalampopoulos, D.; Khutoryanskiy, V.V.; Edwards, A.D. A Laminated Polymer Film Formulation for Enteric Delivery of Live Vaccine and Probiotic Bacteria. J. Pharm. Sci. 2014, 103, 2022–2032. [Google Scholar] [CrossRef] [PubMed]
- Villena, M.J.M.; Lara-Villoslada, F.; Martínez, M.A.R.; Hernández, M.E.M. Development of gastro-resistant tablets for the protection and intestinal delivery of Lactobacillus fermentum CECT 5716. Int. J. Pharm. 2015, 487, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Didari, T.; Mozaffari, S.; Nikfar, S.; Abdollahi, M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J. Gastroenterol. 2015, 21, 3072. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Ford, A.C.; Talley, N.J.; Cremonini, F.; Foxx-Orenstein, A.E.; Brandt, L.J.; Quigley, E.M.M. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut 2010, 59, 325–332. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Guo, C.; Mu, D.; Feng, B.; Zuo, X.; Li, Y. Effects of probiotic type, dose and treatment duration on irritable bowel syndrome diagnosed by Rome III criteria: a meta-analysis. BMC Gastroenterol. 2016, 16, 62. [Google Scholar] [CrossRef]
- Miller, L.E.; Ouwehand, A.C. Probiotic supplementation decreases intestinal transit time: Meta-analysis of randomized controlled trials. World J. Gastroenterol. 2013, 19, 4718. [Google Scholar] [CrossRef]
- Miller, L.E.; Zimmermann, A.K.; Ouwehand, A.C. Contemporary meta-analysis of short-term probiotic consumption on gastrointestinal transit. World J. Gastroenterol. 2016, 22, 5122–5131. [Google Scholar] [CrossRef]
- Ford, A.C.; Quigley, E.M.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.R.; Moayyedi, P. Efficacy of Prebiotics, Probiotics and Synbiotics in Irritable Bowel Syndrome and Chronic Idiopathic Constipation: Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2014, 109, 1547–1561. [Google Scholar] [CrossRef] [PubMed]
- Demers, M.; Dagnault, A.; Desjardins, J. A randomized double-blind controlled trial: Impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin. Nutr. 2014, 33, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Sawh, S.C.; Deshpande, S.; Jansen, S.; Reynaert, C.J.; Jones, P.M. Prevention of necrotizing enterocolitis with probiotics: A systematic review and meta-analysis. PeerJ 2016, 4, e2429. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Jiang, X.-Y.; Zhou, L.-S.; Song, J.-H.; Zhang, X. Effects of Probiotics on Intestinal Mucosa Barrier in Patients With Colorectal Cancer after Operation. Medicine (Baltimore). 2016, 95, e3342. [Google Scholar] [CrossRef] [PubMed]
- Wassenaar, T.M. Insights from 100 Years of Research with Probiotic E. Coli. Eur. J. Microbiol. Immunol. (Bp). 2016, 6, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, S.; Tran, V.H.; Duke, C.C. Stability of [6]-gingerol and [6]-shogaol in simulated gastric and intestinal fluids. J. Pharm. Biomed. Anal. 2007, 45, 648–653. [Google Scholar] [CrossRef]
- Lee, B.-J.; Min, G.-H. Oral controlled release of melatonin using polymer-reinforced and coated alginate beads. Int. J. Pharm. 1996, 144, 37–46. [Google Scholar] [CrossRef]
- Samak, Y.O.; El Massik, M.; Coombes, A.G.A. A Comparison of Aerosolization and Homogenization Techniques for Production of Alginate Microparticles for Delivery of Corticosteroids to the Colon. J. Pharm. Sci. 2017, 106, 208–216. [Google Scholar] [CrossRef]
- Cameron, N.R.; Sherrington, D.C. High Internal Phase Emulsions (HIPEs)—Structure, Properties and Use in Polymer Preparation; Springer: Berlin/Heidelberg, Germany, 1996; pp. 163–214. [Google Scholar]
- Lei, L.; Zhang, Q.; Shi, S.; Zhu, S. High internal phase emulsion with double emulsion morphology and their templated porous polymer systems. J. Colloid Interface Sci. 2016, 483, 232–240. [Google Scholar] [CrossRef]
- Roucher, A.; Morvan, M.; Pekin, D.; Depardieu, M.; Blin, J.-L.; Schmitt, V.; Konrad, M.; Baret, J.-C.; Backov, R. From Compartmentalization of Bacteria within Inorganic Macrocellular Beads to the Assembly of Microbial Consortia. Adv. Biosyst. 2018, 2, 1700233. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of sustained-action medication. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Haznedar, S.; Dortunç, B. Preparation and in vitro evaluation of Eudragit microspheres containing acetazolamide. Int. J. Pharm. 2004, 269, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Bujacz, G.; Wrzesniewska, B.; Bujacz, A. Cryoprotection properties of salts of organic acids: a case study for a tetragonal crystal of HEW lysozyme. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Portela, R.; Almeida, P.L.; Patrício, P.; Cidade, T.; Sobral, R.G.; Leal, C.R. Real-time rheology of actively growing bacteria. Phys. Rev. E 2013, 87, 030701. [Google Scholar] [CrossRef] [Green Version]
- Florence, A.T.; Hillery, A.M.; Hussain, N.; Jani, P.U. Nanoparticles as carriers for oral peptide absorption: Studies on particle uptake and fate. J. Control. Release 1995, 36, 39–46. [Google Scholar] [CrossRef]
- Boylston, T.D.; Vinderola, C.G.; Ghoddusi, H.B.; Reinheimer, J.A. Incorporation of bifidobacteria into cheeses: challenges and rewards. Int. Dairy J. 2004, 14, 375–387. [Google Scholar] [CrossRef]
- Knorr, D. Technology aspects related to microorganisms in functional foods. Trends Food Sci. Technol. 1998, 9, 295–306. [Google Scholar] [CrossRef]
- Froder, J.G.; Dupeyrón, D.; Carvalho, J.C.T.; Maistro, E.L. In vitro study of the cytotoxic and genotoxic effects of indomethacin-loaded Eudragit® L 100 nanocapsules. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Naha, P.C.; Byrne, H.J.; Panda, A.K. Role of Polymeric Excipients on Controlled Release Profile of Glipizide from PLGA and Eudragit RS 100 Nanoparticles. J. Nanopharmaceutics Drug Deliv. 2013, 1, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Gemeinhart, R.A. Cisplatin delivery from poly(acrylic acid-co-methyl methacrylate) microparticles. J. Control. Release 2005, 106, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Patra, C.N.; Priya, R.; Swain, S.; Kumar Jena, G.; Panigrahi, K.C.; Ghose, D. Pharmaceutical significance of Eudragit: A review. Futur. J. Pharm. Sci. 2017, 3, 33–45. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yus, C.; Gracia, R.; Larrea, A.; Andreu, V.; Irusta, S.; Sebastian, V.; Mendoza, G.; Arruebo, M. Targeted Release of Probiotics from Enteric Microparticulated Formulations. Polymers 2019, 11, 1668. https://doi.org/10.3390/polym11101668
Yus C, Gracia R, Larrea A, Andreu V, Irusta S, Sebastian V, Mendoza G, Arruebo M. Targeted Release of Probiotics from Enteric Microparticulated Formulations. Polymers. 2019; 11(10):1668. https://doi.org/10.3390/polym11101668
Chicago/Turabian StyleYus, Cristina, Ruben Gracia, Ane Larrea, Vanesa Andreu, Silvia Irusta, Victor Sebastian, Gracia Mendoza, and Manuel Arruebo. 2019. "Targeted Release of Probiotics from Enteric Microparticulated Formulations" Polymers 11, no. 10: 1668. https://doi.org/10.3390/polym11101668
APA StyleYus, C., Gracia, R., Larrea, A., Andreu, V., Irusta, S., Sebastian, V., Mendoza, G., & Arruebo, M. (2019). Targeted Release of Probiotics from Enteric Microparticulated Formulations. Polymers, 11(10), 1668. https://doi.org/10.3390/polym11101668








