Structural Design of Three-Dimensional Graphene/Nano Filler (Al2O3, BN, or TiO2) Resins and Their Application to Electrically Conductive Adhesives
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Few-Layered Graphene (FLG)
2.2. Preparation of Electrically Conductive Adhesives (ECAs)
2.3. Characterizations
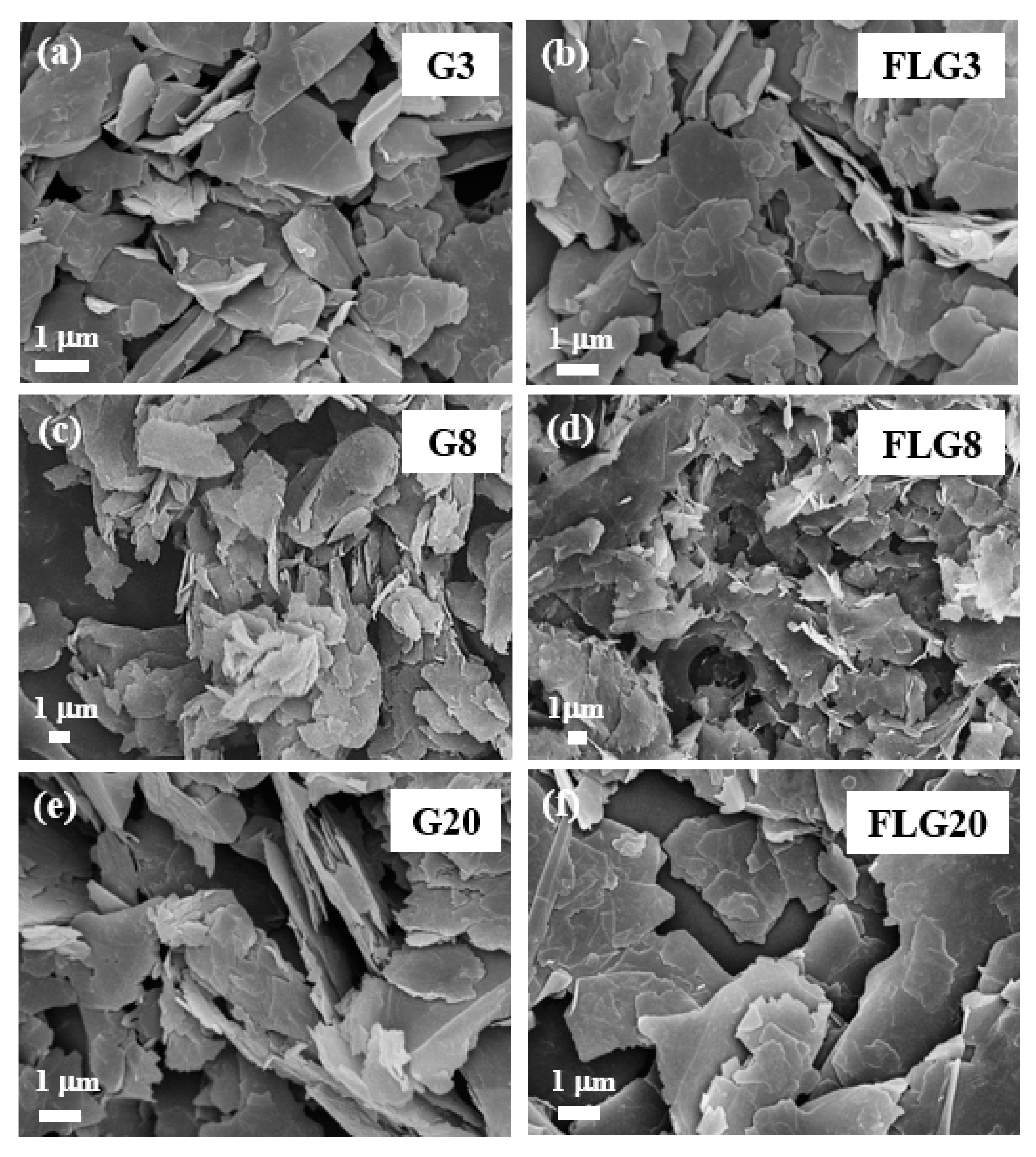
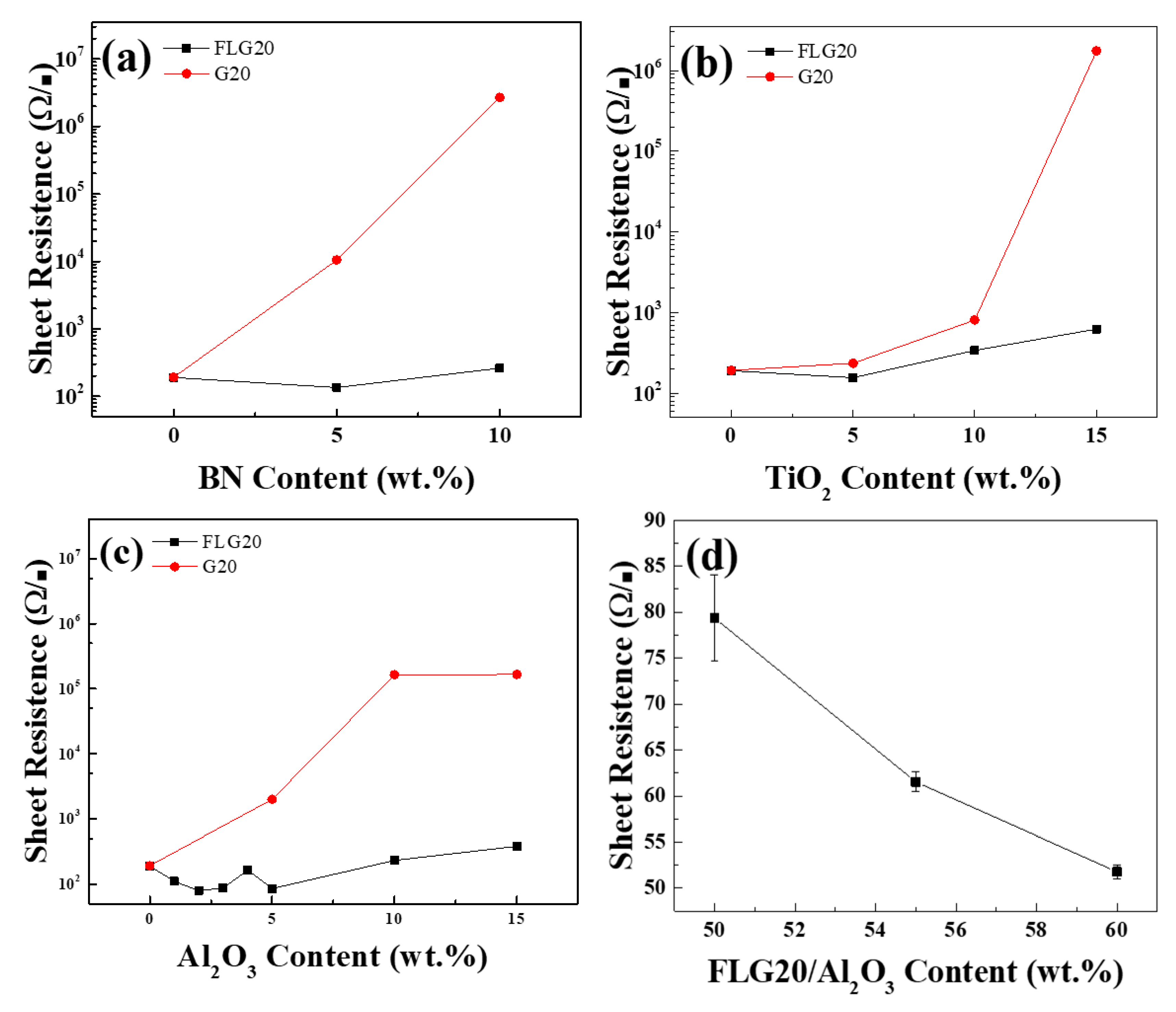
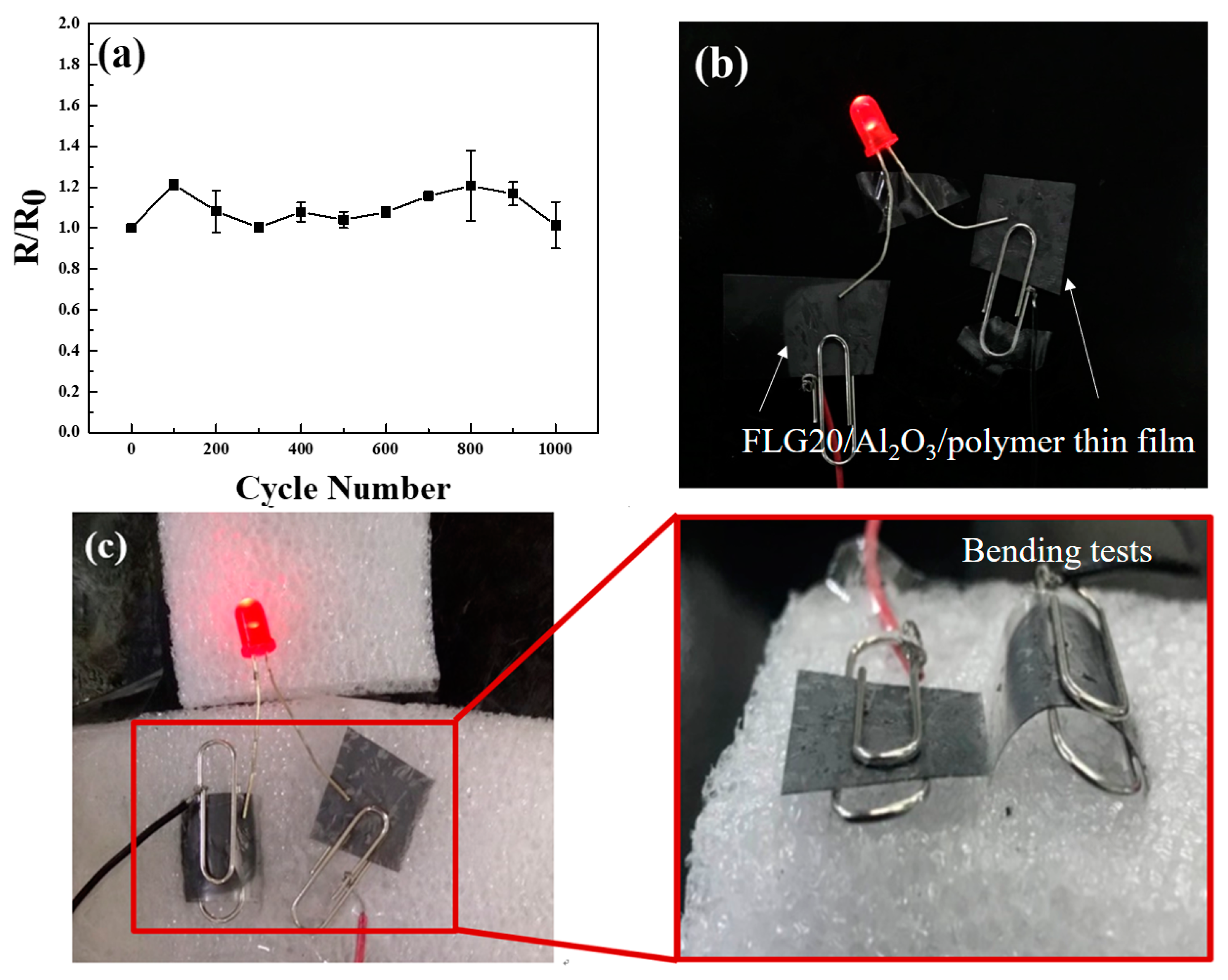
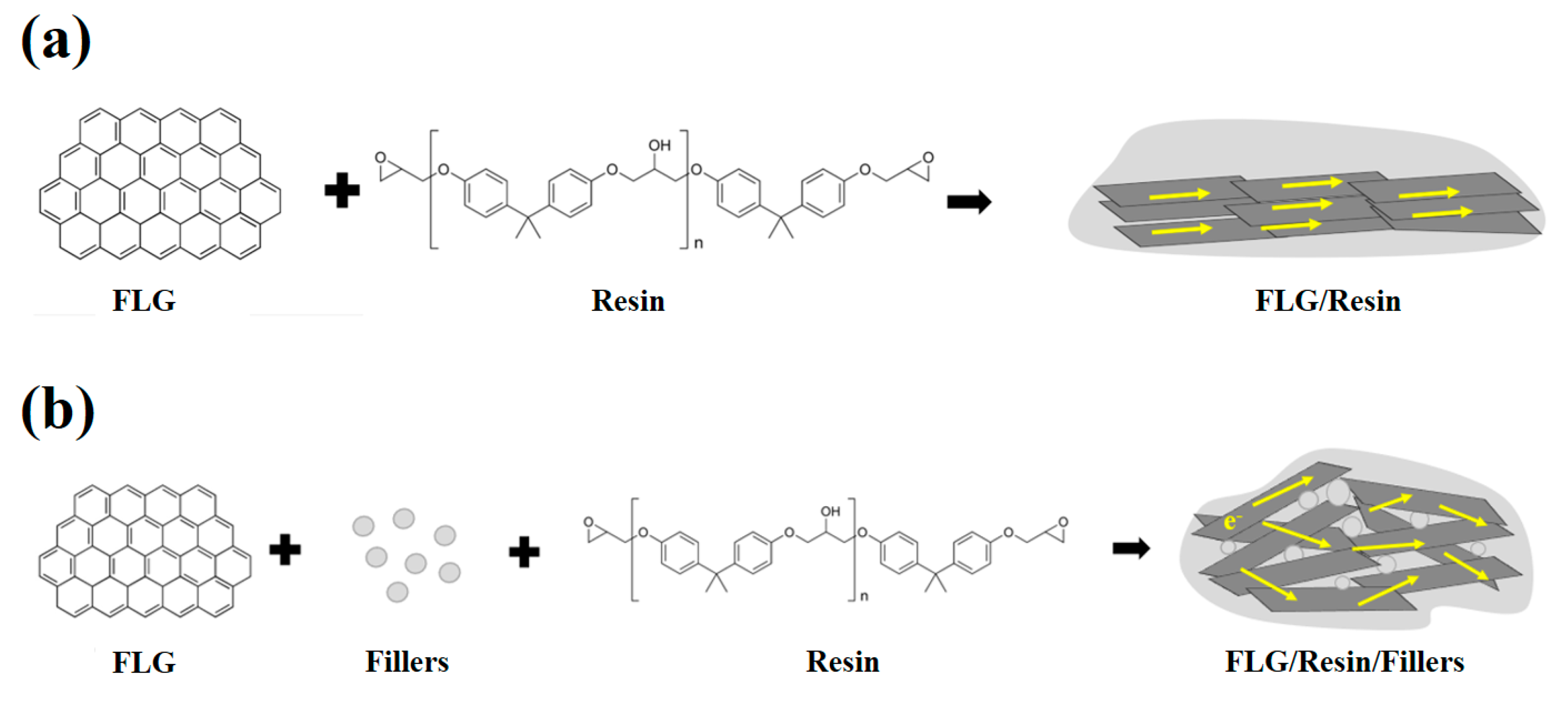
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ling, Q.-D.; Liaw, D.-J.; Zhu, C.; Chan, D.S.-H.; Kang, E.-T.; Neoh, K.-G. Polymer electronic memories: Materials, devices and mechanisms. Prog. Polym. Sci. 2008, 33, 917–978. [Google Scholar] [CrossRef]
- Hsu, C.-W.; Hu, A.H. Green supply chain management in the electronic industry. Int. J. Environ. Sci. Technol. 2008, 5, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wong, C. Recent advances of conductive adhesives as a lead-free alternative in electronic packaging: Materials, processing, reliability and applications. Mater. Sci. Eng. R Rep. 2006, 51, 1–35. [Google Scholar] [CrossRef]
- Yang, X.; He, W.; Wang, S.; Zhou, G.; Tang, Y. Preparation and properties of a novel electrically conductive adhesive using a composite of silver nanorods, silver nanoparticles, and modified epoxy resin. J. Mater. Sci. Mater. Electron. 2012, 23, 108–114. [Google Scholar] [CrossRef]
- Lin, W.; Xi, X.; Yu, C. Research of silver plating nano-graphite filled conductive adhesive. Synth. Met. 2009, 159, 619–624. [Google Scholar] [CrossRef]
- Wu, H.; Wu, X.; Liu, J.; Zhang, G.; Wang, Y.; Zeng, Y.; Jing, J. Development of a novel isotropic conductive adhesive filled with silver nanowires. J. Compos. Mater. 2006, 40, 1961–1969. [Google Scholar] [CrossRef]
- Pu, N.-W.; Peng, Y.-Y.; Wang, P.-C.; Chen, C.-Y.; Shi, J.-N.; Liu, Y.-M.; Ger, M.-D.; Chang, C.-L. Application of nitrogen-doped graphene nanosheets in electrically conductive adhesives. Carbon 2014, 67, 449–456. [Google Scholar] [CrossRef]
- Yim, M.J.; Li, Y.; Moon, K.-S.; Paik, K.W.; Wong, C. Review of recent advances in electrically conductive adhesive materials and technologies in electronic packaging. J. Adhes. Sci. Technol. 2008, 22, 1593–1630. [Google Scholar] [CrossRef]
- Yang, C.; Wong, C.P.; Yuen, M.M. Printed electrically conductive composites: Conductive filler designs and surface engineering. J. Mater. Chem. C 2013, 1, 4052–4069. [Google Scholar] [CrossRef]
- Ma, H.; Ma, M.; Zeng, J.; Guo, X.; Ma, Y. Hydrothermal synthesis of graphene nanosheets and its application in electrically conductive adhesives. Mater. Lett. 2016, 178, 181–184. [Google Scholar] [CrossRef]
- Chen, D.; Qiao, X.; Qiu, X.; Tan, F.; Chen, J.; Jiang, R. Effect of silver nanostructures on the resistivity of electrically conductive adhesives composed of silver flakes. J. Mater. Sci. Mater. Electron. 2010, 21, 486–490. [Google Scholar] [CrossRef]
- Amoli, B.M.; Marzbanrad, E.; Hu, A.; Zhou, Y.N.; Zhao, B. Electrical Conductive Adhesives Enhanced with High-A spect-R atio Silver Nanobelts. Macromol. Mater. Eng. 2014, 299, 739–747. [Google Scholar] [CrossRef]
- Cui, H.-W.; Jiu, J.-T.; Nagao, S.; Sugahara, T.; Suganuma, K.; Uchida, H.; Schroder, K.A. Ultra-fast photonic curing of electrically conductive adhesives fabricated from vinyl ester resin and silver micro-flakes for printed electronics. RSC Adv. 2014, 4, 15914–15922. [Google Scholar] [CrossRef]
- Nishikawa, H.; Mikami, S.; Miyake, K.; Aoki, A.; Takemoto, T. Effects of silver coating covered with copper filler on electrical resistivity of electrically conductive adhesives. Mater. Trans. 2010, 51, 1785–1789. [Google Scholar] [CrossRef]
- Ho, L.-N.; Nishikawa, H. Influence of post-curing and coupling agents on polyurethane based copper filled electrically conductive adhesives. J. Mater. Sci. Mater. Electron. 2013, 24, 2077–2081. [Google Scholar] [CrossRef]
- Santamaria, A.; Muñoz, M.E.; Fernández, M.; Landa, M. Electrically conductive adhesives with a focus on adhesives that contain carbon nanotubes. J. Appl. Polym. Sci. 2013, 129, 1643–1652. [Google Scholar] [CrossRef]
- Czech, Z.; Kowalczyk, A.; Pełech, R.; Wróbel, R.; Shao, L.; Bai, Y.; Świderska, J. Using of carbon nanotubes and nano carbon black for electrical conductivity adjustment of pressure-sensitive adhesives. Int. J. Adhes. Adhes. 2012, 36, 20–24. [Google Scholar] [CrossRef]
- Chou, T.-W.; Gao, L.; Thostenson, E.T.; Zhang, Z.; Byun, J.-H. An assessment of the science and technology of carbon nanotube-based fibers and composites. Compos. Sci. Technol. 2010, 70, 1–19. [Google Scholar] [CrossRef]
- Janczak, D.; Słoma, M.; Wróblewski, G.; Młożniak, A.; Jakubowska, M. Thick Film Polymer Composites with Graphene Nanoplatelets for Use in Printed Electronics. In Mechatronics 2013; Březina, T., Jabloński, R., Eds.; Springer: Cham, Switzerland, 2014. [Google Scholar]
- Jang, B.Z.; Zhamu, A. Nano Graphene Platelet-Based Conductive Inks. US Patent US20120007913A1, 7 January 2010. [Google Scholar]
- Crain, J.M.; Lettow, J.S.; Aksay, I.A.; Korkut, S.A.; Chiang, K.S.; Chen, C.-H.; Prud’homme, R.K. Printed Electronics. US Patent 8,278,757, 2 October 2012. [Google Scholar]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2010; pp. 11–19. [Google Scholar]
- Compton, O.C.; Nguyen, S.T. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Ruoff, R.S.; Bielawski, C.W. From conception to realization: An historial account of graphene and some perspectives for its future. Angew. Chem. Int. Ed. 2010, 49, 9336–9344. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Gadipelli, S.; Guo, Z.X. Graphene-based materials: Synthesis and gas sorption, storage and separation. Prog. Mater. Sci. 2015, 69, 1–60. [Google Scholar] [CrossRef] [Green Version]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282. [Google Scholar] [CrossRef]
- Gao, J.; Liu, F.; Liu, Y.; Ma, N.; Wang, Z.; Zhang, X. Environment-friendly method to produce graphene that employs vitamin C and amino acid. Chem. Mater. 2010, 22, 2213–2218. [Google Scholar] [CrossRef]
- Webb, M.J.; Palmgren, P.; Pal, P.; Karis, O.; Grennberg, H. A simple method to produce almost perfect graphene on highly oriented pyrolytic graphite. Carbon 2011, 49, 3242–3249. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Shen, Z.; Li, J.; Yi, M.; Zhang, X.; Ma, S. Preparation of graphene by jet cavitation. Nanotechnology 2011, 22, 365306. [Google Scholar] [CrossRef]
- Yi, M.; Li, J.; Shen, Z.; Zhang, X.; Ma, S. Morphology and structure of mono-and few-layer graphene produced by jet cavitation. Appl. Phys. Lett. 2011, 99, 123112. [Google Scholar] [CrossRef]
- He, Y.; Chen, W.; Li, X.; Zhang, Z.; Fu, J.; Zhao, C.; Xie, E. Freestanding three-dimensional graphene/MnO2 composite networks as ultralight and flexible supercapacitor electrodes. ACS Nano 2012, 7, 174–182. [Google Scholar] [CrossRef]
- Peng, X.; Tan, F.; Wang, W.; Qiu, X.; Sun, F.; Qiao, X.; Chen, J. Conductivity improvement of silver flakes filled electrical conductive adhesives via introducing silver–graphene nanocomposites. J. Mater. Sci. Mater. Electron. 2014, 25, 1149–1155. [Google Scholar] [CrossRef]
- Ghaleb, Z.; Mariatti, M.; Ariff, Z. Properties of graphene nanopowder and multi-walled carbon nanotube-filled epoxy thin-film nanocomposites for electronic applications: The effect of sonication time and filler loading. Compos. Part A Appl. Sci. Manuf. 2014, 58, 77–83. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, L.; Li, J.; Li, J.; Sun, S.; Chen, F.; Chen, L.; Jiang, W. Preparation and electrical properties of graphene nanosheet/Al2O3 composites. Carbon 2010, 48, 1743–1749. [Google Scholar] [CrossRef]
- Breckenridge, R.G.; Hosler, W.R. Electrical properties of titanium dioxide semiconductors. Phys. Rev. 1953, 91, 793. [Google Scholar] [CrossRef]
- Cui, H.-W.; Li, D.-S.; Fan, Q.; Lai, H.-X. Electrical and mechanical properties of electrically conductive adhesives from epoxy, micro-silver flakes, and nano-hexagonal boron nitride particles after humid and thermal aging. Int. J. Adhes. Adhes. 2013, 44, 232–236. [Google Scholar] [CrossRef]
- Cui, H.-W.; Li, D.-S.; Fan, Q. Using nano hexagonal boron nitride particles and nano cubic silicon carbide particles to improve the thermal conductivity of electrically conductive adhesives. Electron. Mater. Lett. 2013, 9, 1–5. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.-H.; Huang, C.-H.; Liu, W.-R. Structural Design of Three-Dimensional Graphene/Nano Filler (Al2O3, BN, or TiO2) Resins and Their Application to Electrically Conductive Adhesives. Polymers 2019, 11, 1713. https://doi.org/10.3390/polym11101713
Zhang C-H, Huang C-H, Liu W-R. Structural Design of Three-Dimensional Graphene/Nano Filler (Al2O3, BN, or TiO2) Resins and Their Application to Electrically Conductive Adhesives. Polymers. 2019; 11(10):1713. https://doi.org/10.3390/polym11101713
Chicago/Turabian StyleZhang, Chia-Hsin, Chia-Hung Huang, and Wei-Ren Liu. 2019. "Structural Design of Three-Dimensional Graphene/Nano Filler (Al2O3, BN, or TiO2) Resins and Their Application to Electrically Conductive Adhesives" Polymers 11, no. 10: 1713. https://doi.org/10.3390/polym11101713
APA StyleZhang, C.-H., Huang, C.-H., & Liu, W.-R. (2019). Structural Design of Three-Dimensional Graphene/Nano Filler (Al2O3, BN, or TiO2) Resins and Their Application to Electrically Conductive Adhesives. Polymers, 11(10), 1713. https://doi.org/10.3390/polym11101713





