Effect of Alkyl Chain Length in POSS Nanocage on Non-Isothermal Crystallization Behavior of PCL/Amino-POSS Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PCL/POSS Nanocomposite Preparation
2.3. Sample Characterization
3. Results
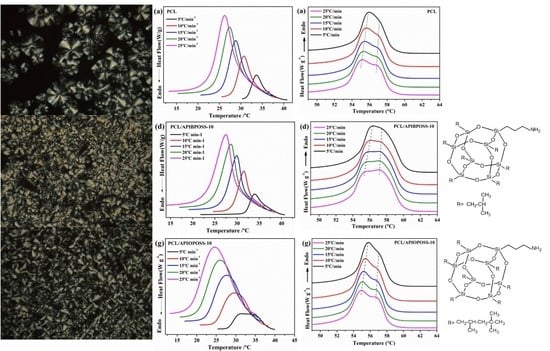
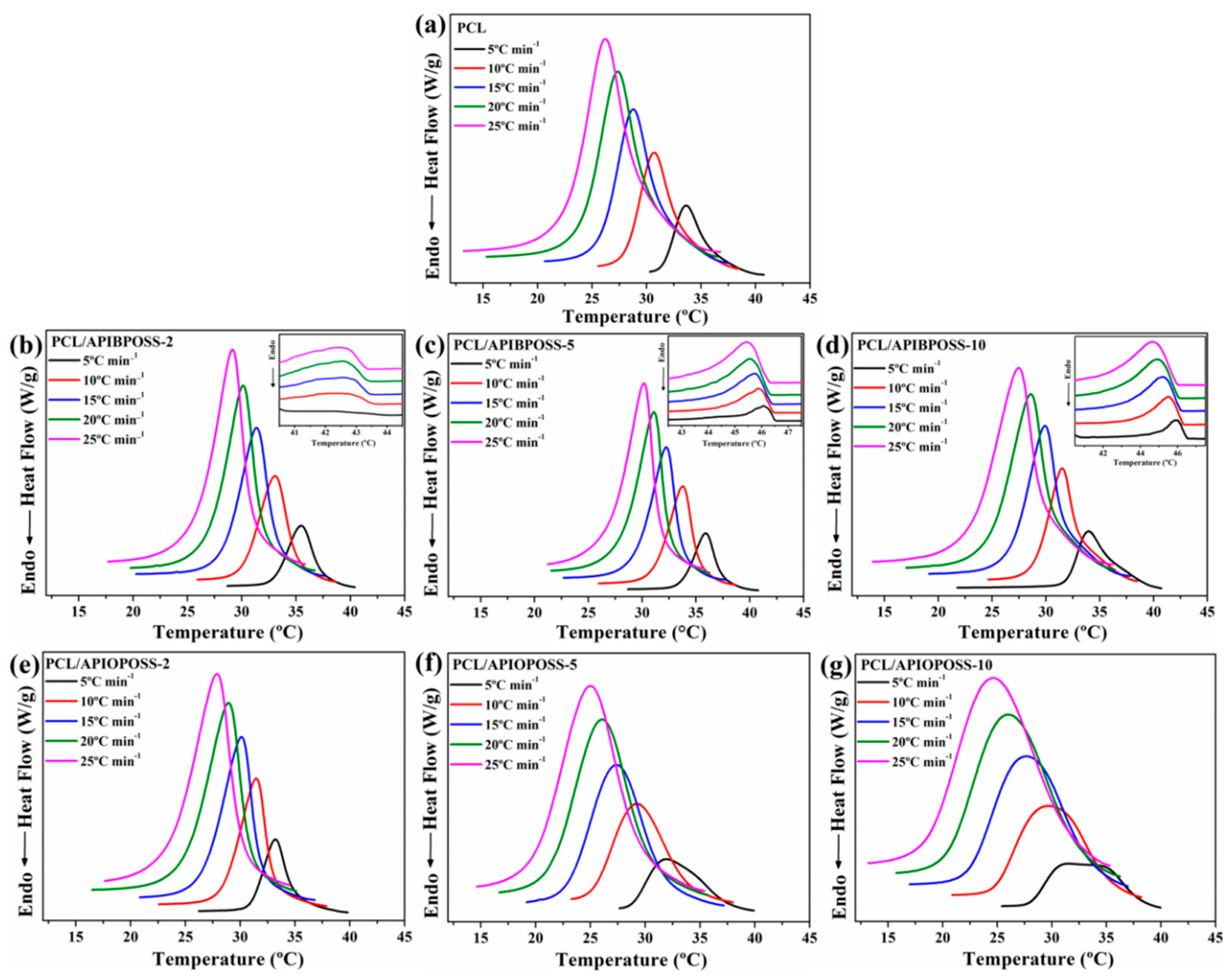
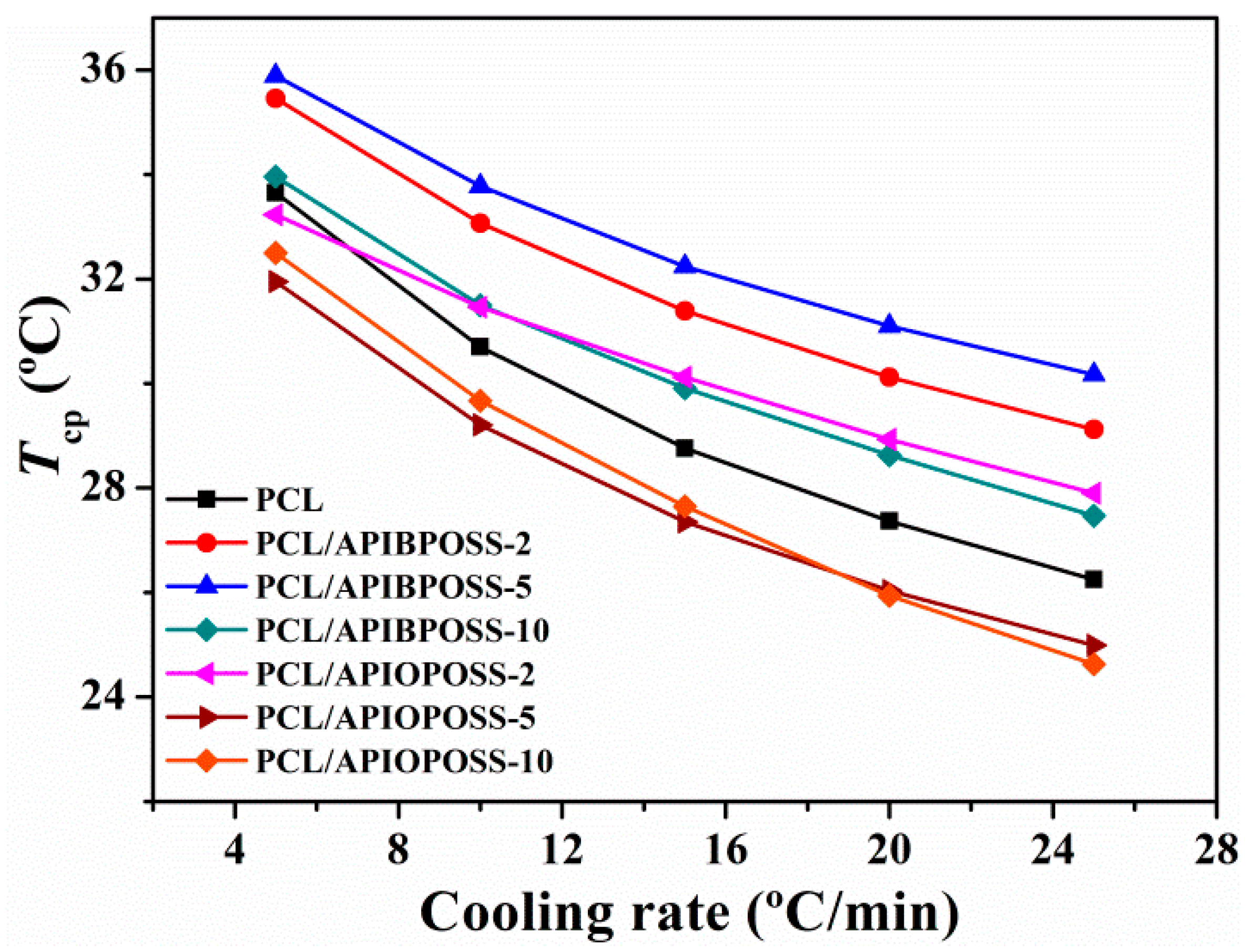
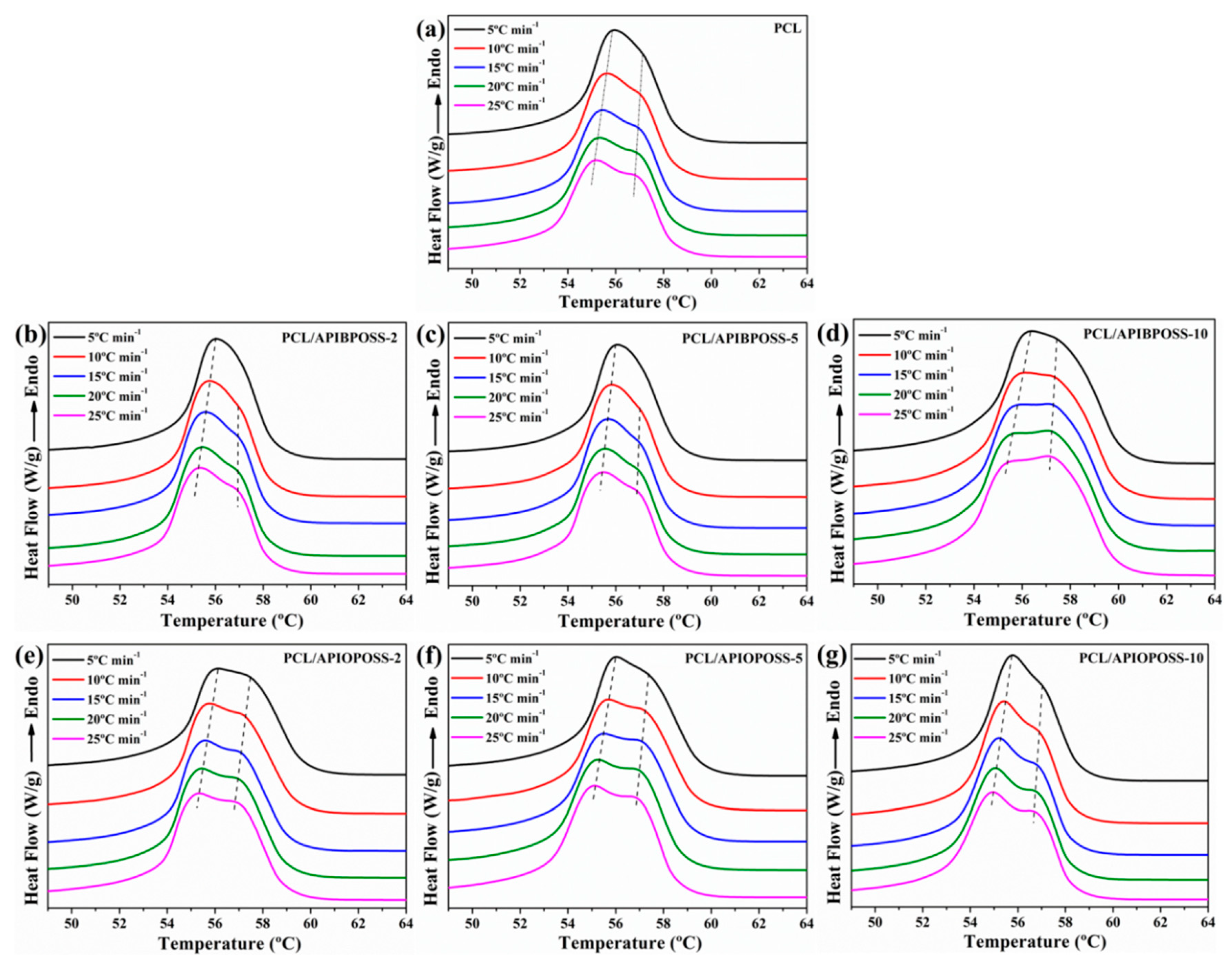
3.1. Non-Isothermal Crystallization and Melting Behavior
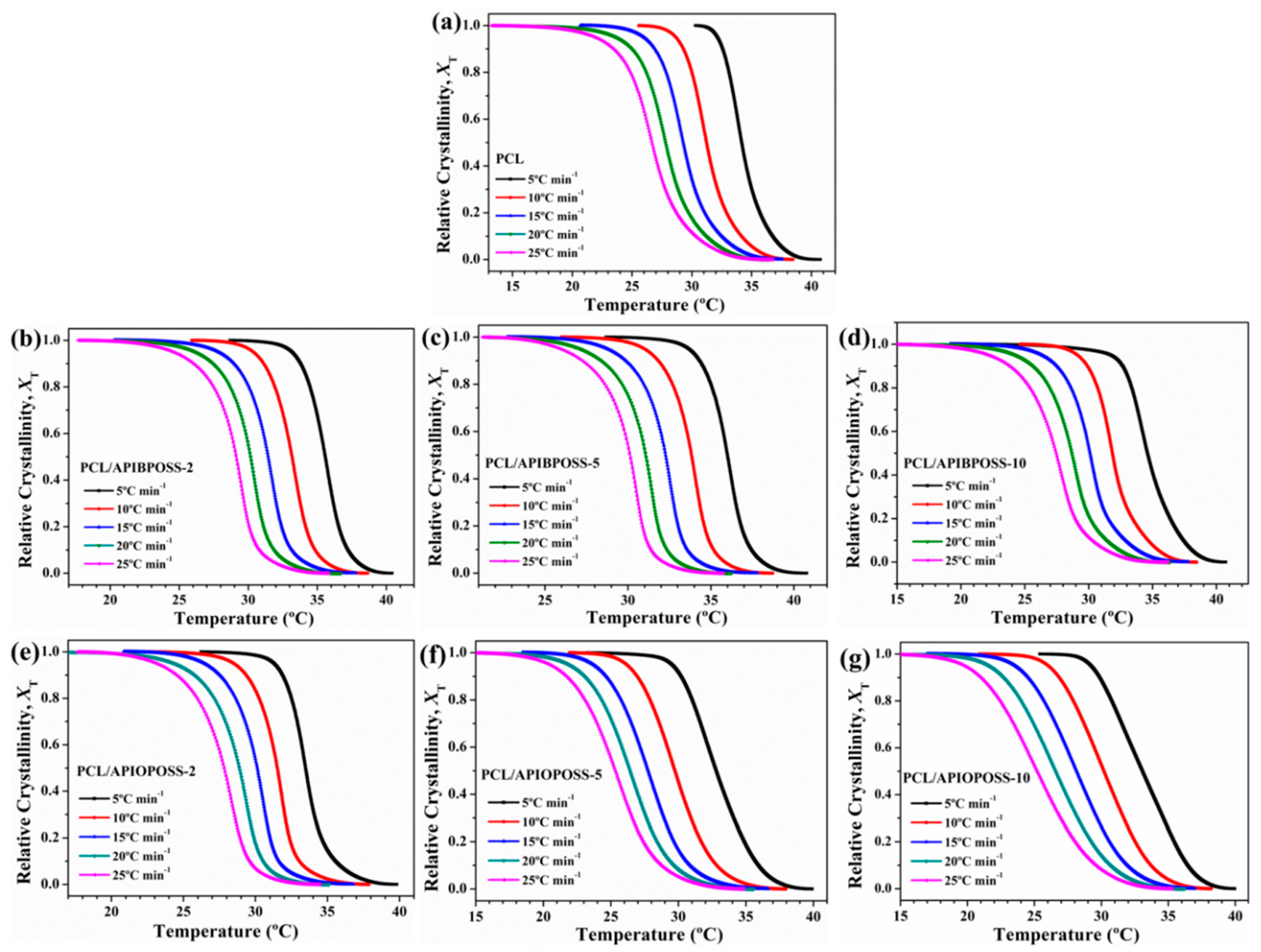
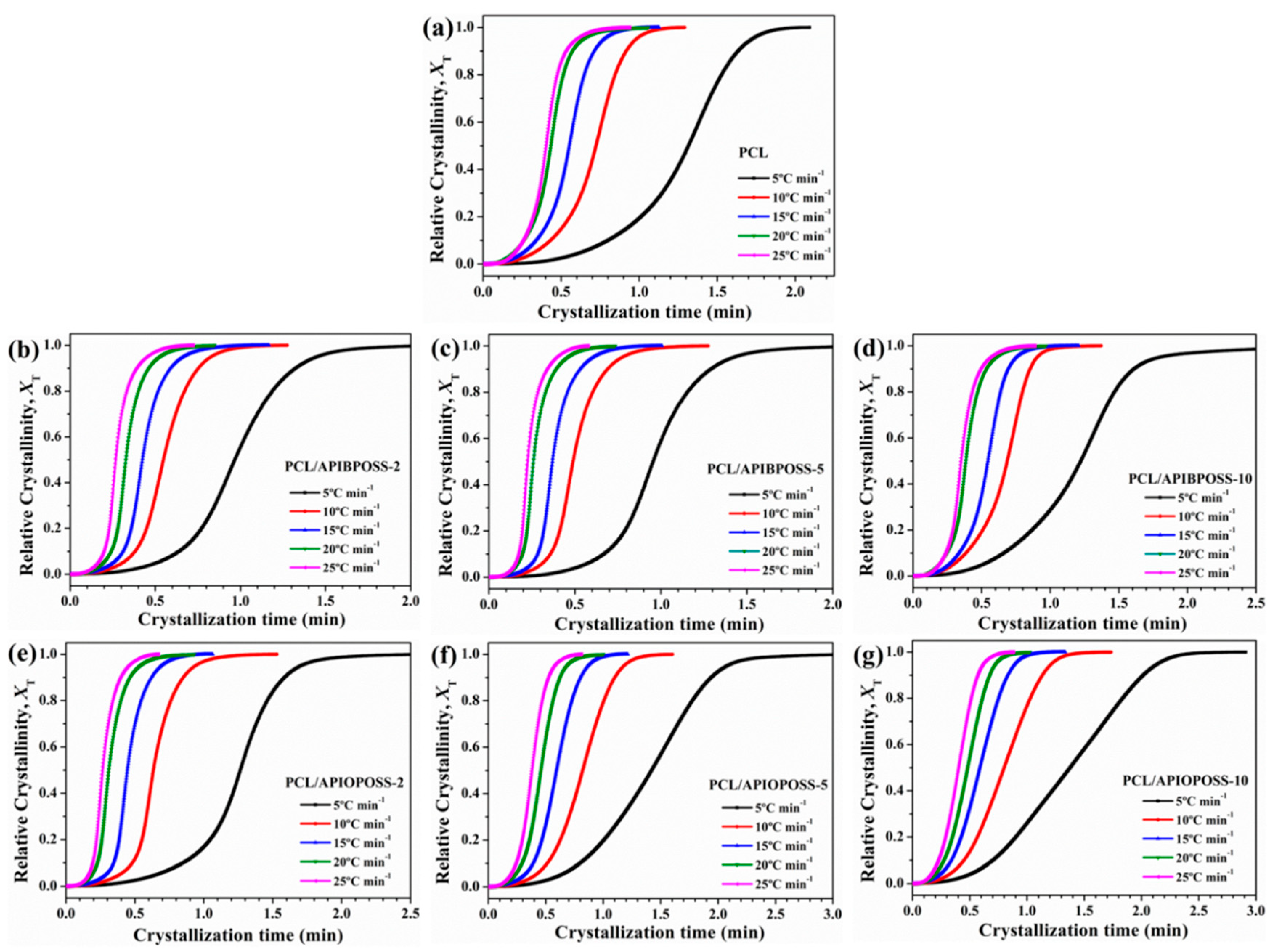
3.2. Non-Isothermal Crystallization Kinetics
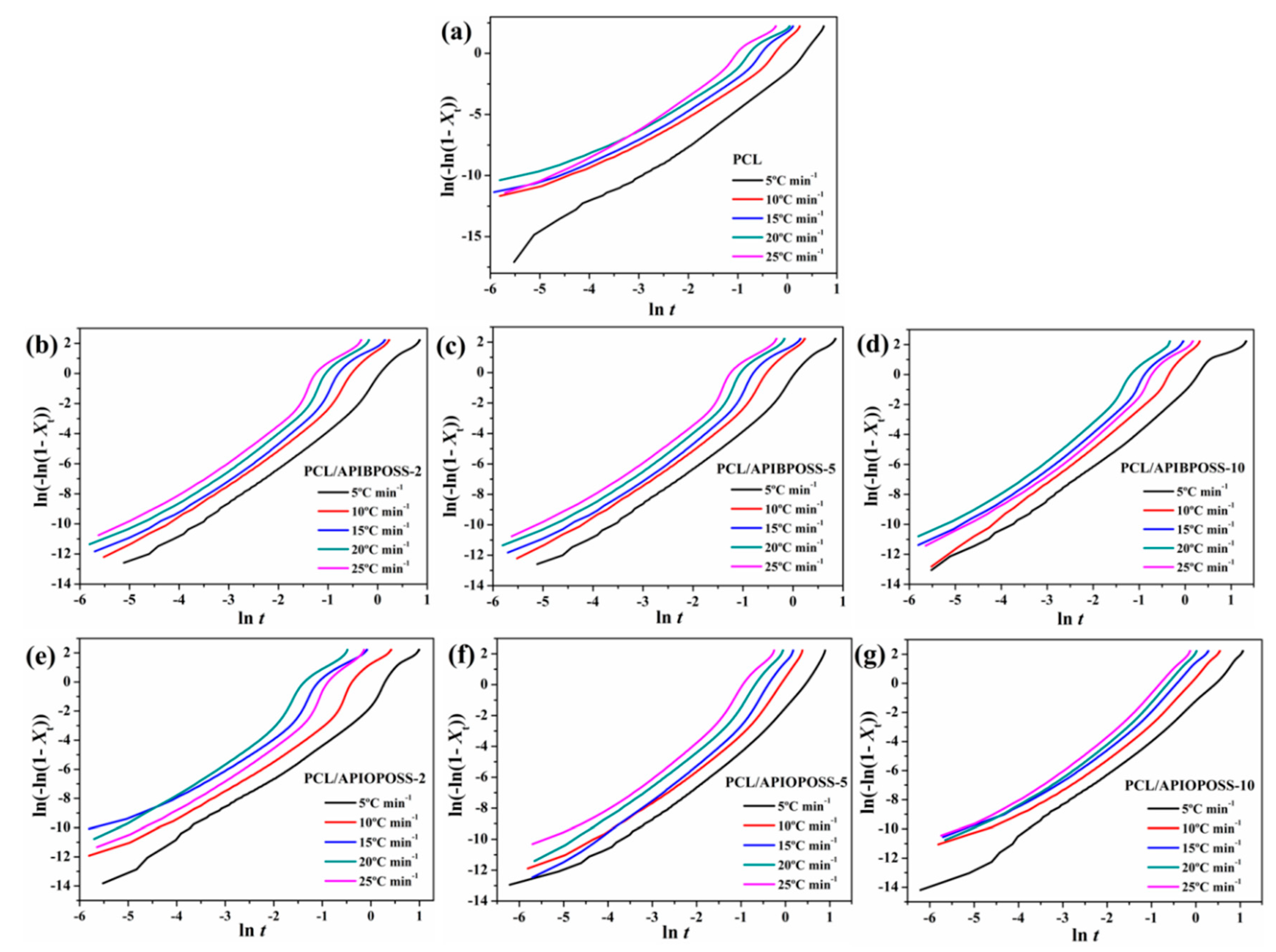
3.2.1. Avrami Analysis
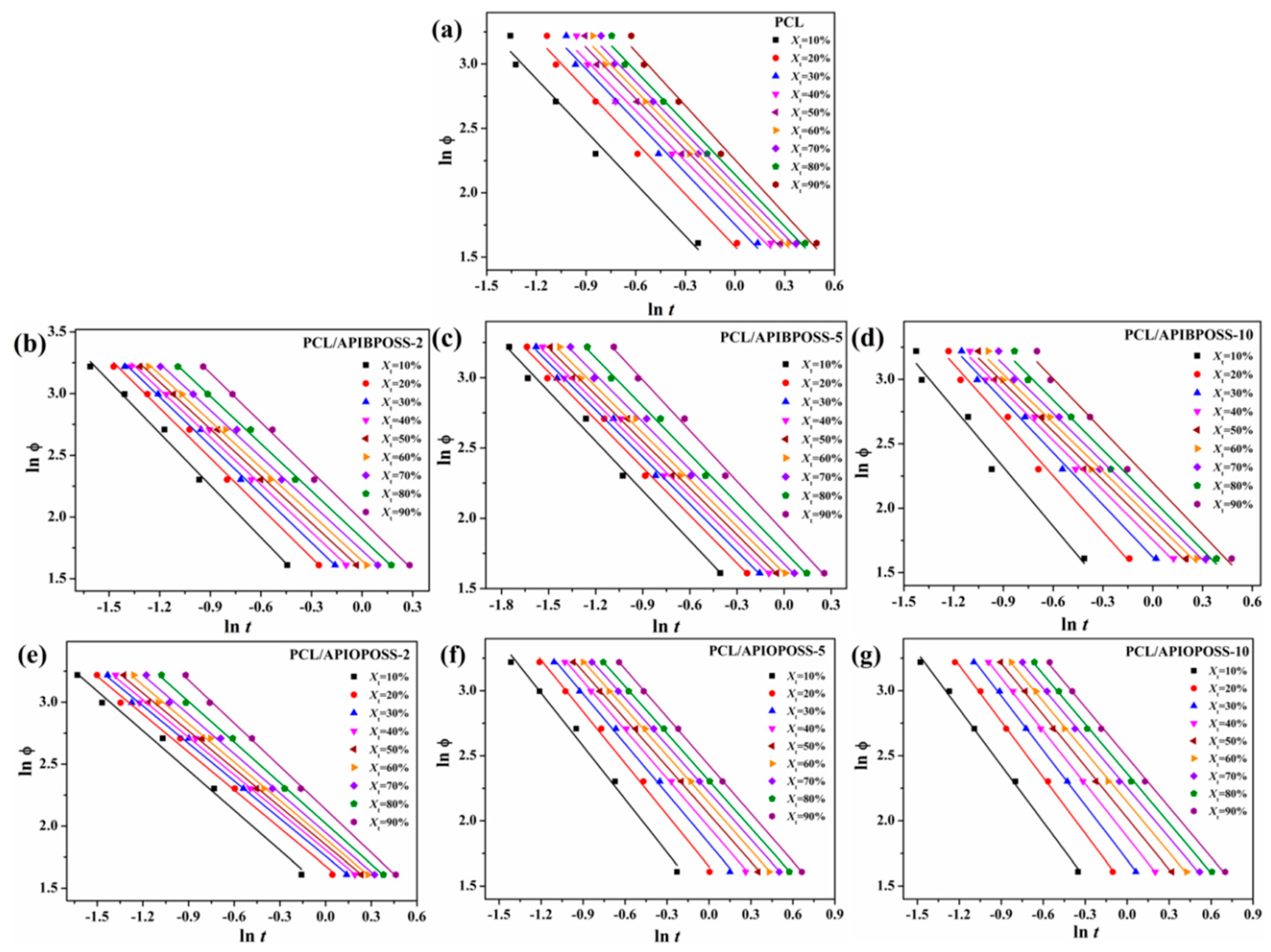
3.2.2. Combined Ozawa–Avrami Method
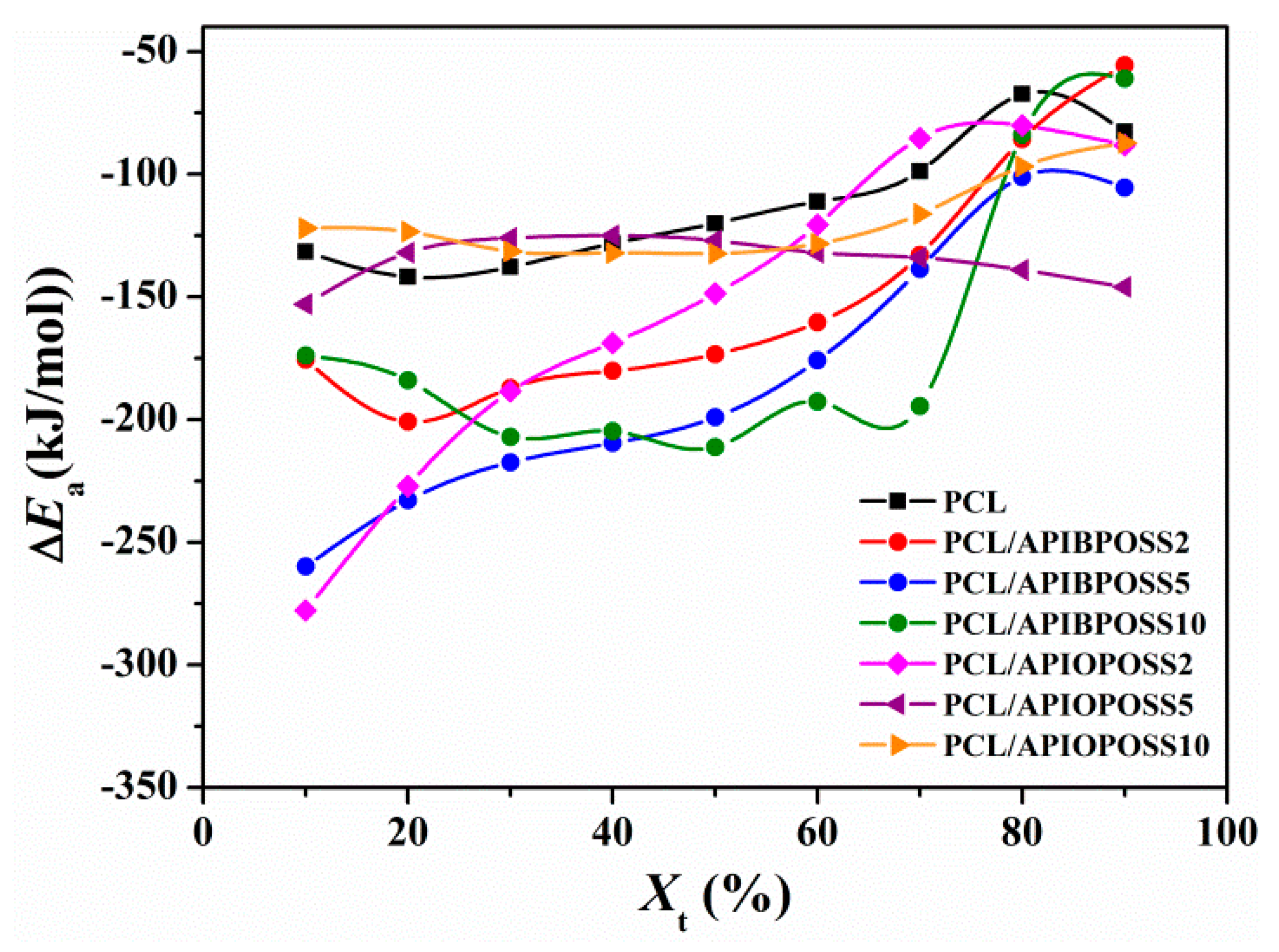
3.2.3. Crystallization Activation Energy
3.3. XRD Characterization
3.4. Spherulitic Morphology
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Kweon, H.Y.; Yoo, M.K.; Park, I.K.; Kim, T.H.; Lee, H.C.; Lee, H.S.; Oh, J.S.; Akaike, T.; Cho, C.S. A novel degradable polycaprolactone networks for tissue engineering. Biomaterials 2003, 24, 801–808. [Google Scholar] [CrossRef]
- Messersmith, P.B.; Giannelis, E.P. Synthesis and barrier properties of poly(ε-caprolactone)-layered silicate nanocomposites. J. Polym. Sci. Polym. Chem. 1995, 33, 1047–1057. [Google Scholar] [CrossRef]
- Fukushima, K.; Tabuani, D.; Camino, G. Nanocomposites of PLA and PCL based on montmorillonite and sepiolite. Mat. Sci. Eng. C-Mater. 2009, 29, 1433–1441. [Google Scholar] [CrossRef]
- Di, Y.; Iannac, S.; Sanguigno, L.; Nicolais, L. Barrier and mechanical properties of poly(caprolactone)/organoclay nanocomposites. Macromol. Symp. 2005, 228, 115–124. [Google Scholar] [CrossRef]
- Chrissafis, K.; Antoniadis, G.; Paraskevopoulos, K.; Vassiliou, A.; Bikiaris, D. Comparative study of the effect of different nanoparticles on the mechanical properties and thermal degradation mechanism of in situ prepared poly(ε-caprolactone) nanocomposites. Compos. Sci. Technol. 2007, 67, 2165–2174. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, Z. Morphology, crystallization behavior and dynamic mechanical properties of biodegradable poly(ε-caprolactone)/thermally reduced graphene nanocomposites. Ind. Eng. Chem. Res. 2011, 50, 13885–13891. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef]
- Qian, Q.; Xu, J.; Zhang, M.; He, J.; Ni, P. Versatile Construction of Single-Tailed Giant Surfactants with Hydrophobic Poly(ε-caprolactone) Tail and Hydrophilic POSS Head. Polymers 2019, 11, 311. [Google Scholar] [CrossRef]
- Li, Y.; Dong, X.H.; Zou, Y.; Wang, Z.; Yue, K.; Huang, M.; Liu, H.; Feng, X.; Lin, Z.; Zhang, W.; et al. Polyhedral oligomeric silsesquioxane meets “click” chemistry: Rational design and facile preparation of functional hybrid materials. Polymer 2017, 125, 303–329. [Google Scholar] [CrossRef]
- Fina, A.; Tabuani, D.; Frache, A.; Camino, G. Polypropylene-polyhedral oligomeric silsesquioxanes (POSS) nanocomposites. Polymer 2005, 46, 7855–7866. [Google Scholar] [CrossRef]
- Pan, H.; Yu, J.; Qiu, Z. Crystallization and morphology studies of biodegradable poly(ε-caprolactone)/polyhedral oligomeric silsesquioxanes nanocomposites. Polym. Eng. Sci. 2011, 51, 2159–2165. [Google Scholar] [CrossRef]
- Miltner, H.E.; Watzeels, N.; Gotzen, N.A.; Goffin, A.L.; Duquesne, E.; Benali, S.; Ruelle, B.; Peeterbroeck, S.; Dubois, P.; Goderis, B.; et al. The effect of nano-sized filler particles on the crystalline-amorphous interphase and thermal properties in polyester nanocomposites. Polymer 2012, 53, 1494–1506. [Google Scholar] [CrossRef]
- Goffin, A.L.; Duquesne, E.; Raquez, J.M.; Miltner, H.E.; Ke, X.; Alexandre, M.; Van Tendeloo, G.; Van Mele, B.; Dubois, P. From polyester grafting onto POSS nanocage by ring-opening polymerization to high performance polyester/POSS nanocomposites. J. Mater. Chem. 2010, 209, 9415–9422. [Google Scholar] [CrossRef]
- Miltner, H.E.; Watzeels, N.; Goffin, A.L.; Duquesne, E.; Benali, S.; Dubois, P.; Rahier, H.; Van Mele, B. Quantifying the degree of nanofiller dispersion by advanced thermal analysis: Application to polyester nanocomposites prepared by various elaboration methods. J. Mater. Chem. 2010, 20, 9531–9542. [Google Scholar] [CrossRef]
- Guan, W.; Qiu, Z. Isothermal crystallization kinetics, morphology, and dynamic mechanical properties of biodegradable poly(ε-caprolactone) and octavinyl−polyhedral oligomeric silsesquioxanes nanocomposite. Ind. Eng. Chem. Res. 2012, 51, 3203–3208. [Google Scholar] [CrossRef]
- Lee, K.S.; Chang, Y.W. Thermal and mechanical properties of poly(ε-caprolactone)/polyhedral oligomeric silsesquioxane nanocomposites. Polym. Int. 2013, 62, 64–70. [Google Scholar] [CrossRef]
- Pracella, M.; Chionna, D.; Fina, A.; Tabuani, D.; Frache, A.; Camino, G. Polypropylene–POSS nanocomposites: Morphology and crystallization behaviour. Macromol. Symp. 2006, 234, 59–67. [Google Scholar] [CrossRef]
- Baldi, F.; Bignotti, F.; Fina, A.; Tabuani, D.; Riccò, T. Mechanical characterization of polyhedral oligomeric silsesquioxane/polypropylene blends. J. Appl. Polym. Sci. 2007, 105, 935–943. [Google Scholar] [CrossRef]
- Heeley, E.L.; Hughes, D.J.; Aziz, Y.E.; Taylor, P.G.; Bassindale, A.R. Morphology and crystallization kinetics of polyethylene/long alkyl-chain substituted polyhedral oligomeric silsesquioxanes (POSS) nanocomposite blends: A SAXS/WAXS study. Eur. Polym. J. 2014, 51, 45–56. [Google Scholar] [CrossRef]
- Niemczyk, A.; Dziubek, K.; Sacher-Majewska, B.; Czaja, K.; Dutkiewicz, M.; Marciniec, B. Study of thermal properties of polyethylene and polypropylene nanocomposites with long alkyl chain-substituted POSS fillers. J. Therm. Anal. Calorim. 2016, 125, 1287–1299. [Google Scholar] [CrossRef] [Green Version]
- Cobos, M.; Ramos, J.R.; Guzmán, D.J.; Fernández, M.D.; Fernández, M.J. PCL/POSS nanocomposites: Effect of POSS derivative and preparation method on morphology and properties. Polymers 2019, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, H.S.; Kim, S.H. Unique nucleation of multi-walled carbon nanotube and poly(ethylene 2,6-naphthalate) nanocomposites during non-isothermal crystallization. Polymer 2006, 47, 1379–1389. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, V.B.; Peters, R.H.; Harland, W.G.; Berry, J.P. The effect of addition of high-density polyethylene on the crystallization and mechanical properties of polypropylene and glass-fiber-reinforced polypropylene. J. Appl. Polym. Sci. 1982, 27, 4669–4686. [Google Scholar] [CrossRef]
- Gupta, A.K.; Purwar, S.N. Crystallization of PP in PP/SEBS blends and its correlation with tensile properties. J. Appl. Polym. Sci. 1984, 29, 1595–1609. [Google Scholar] [CrossRef]
- Crescenzi, V.; Manzini, G.; Calzolari, G.; Borri, C. Thermodynamics of fusion of poly-β-propiolactone and poly-ϵ-caprolactone. Comparative analysis of the melting of aliphatic polylactone and polyester chains. Eur. Polym. J. 1972, 8, 449–463. [Google Scholar] [CrossRef]
- Khanna, Y.P. A barometer of crystallization rates of polymeric materials. Polym. Engng. Sci. 1990, 30, 1615–1619. [Google Scholar] [CrossRef]
- Zhang, U.; Zheng, H.; Lou, X.; Ma, D. Crystallization characteristics of polypropylene and low ethylene content polypropylene copolymer with and without nucleating agents. J. Appl. Polym. Sci. 1994, 51, 51–56. [Google Scholar] [CrossRef]
- Jeziorny, A. Parameters characterizing the kinetics of the non-isothermal crystallization of poly(ethylene terephthalate) determined by d.s.c. Polymer 1978, 19, 1142–1144. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Liu, T.; Mo, Z.; Wang, S.; Zhang, H. Nonisothermal melt and cold crystallization kinetics of poly(aryl ether ether ketone ketone). Polym. Eng. Sci. 1997, 37, 568–575. [Google Scholar] [CrossRef]
- Friedman, H. Kinetics of thermal degradation of char-forming plastics from thermogravimetry application to a phenolic plastic. J. Polym. Sci. C 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Limwanich, W.; Phetsuk, S.; Meepowpan, P.; Kungwan, N.; Punyodom, W. Kinetics studies of non-isothermal melt crystallization of poly(ε-caprolactone) and poly(L-lactide). Chiang Mai J. Sci. 2016, 43, 329–338. [Google Scholar]
- Oliveira Pires, L.S.; Vaz Fernandes, M.H.F.; Marques de Oliveira, J.M. Crystallization kinetics of PCL and PCL–Glass composites for additive manufacturing. J. Therm. Anal. Calorim. 2018, 134, 2115–2125. [Google Scholar] [CrossRef]
- Ahmed, J.; Luciano, G.; Schizzi, I.; Arfat, Y.A.; Maggiore, S.; Thai, T.L.A. Non-isothermal crystallization behavior, rheological properties and morphology of poly(ε-caprolactone)/graphene oxide nanosheets composite films. Thermochim. Acta 2018, 659, 96–104. [Google Scholar] [CrossRef]


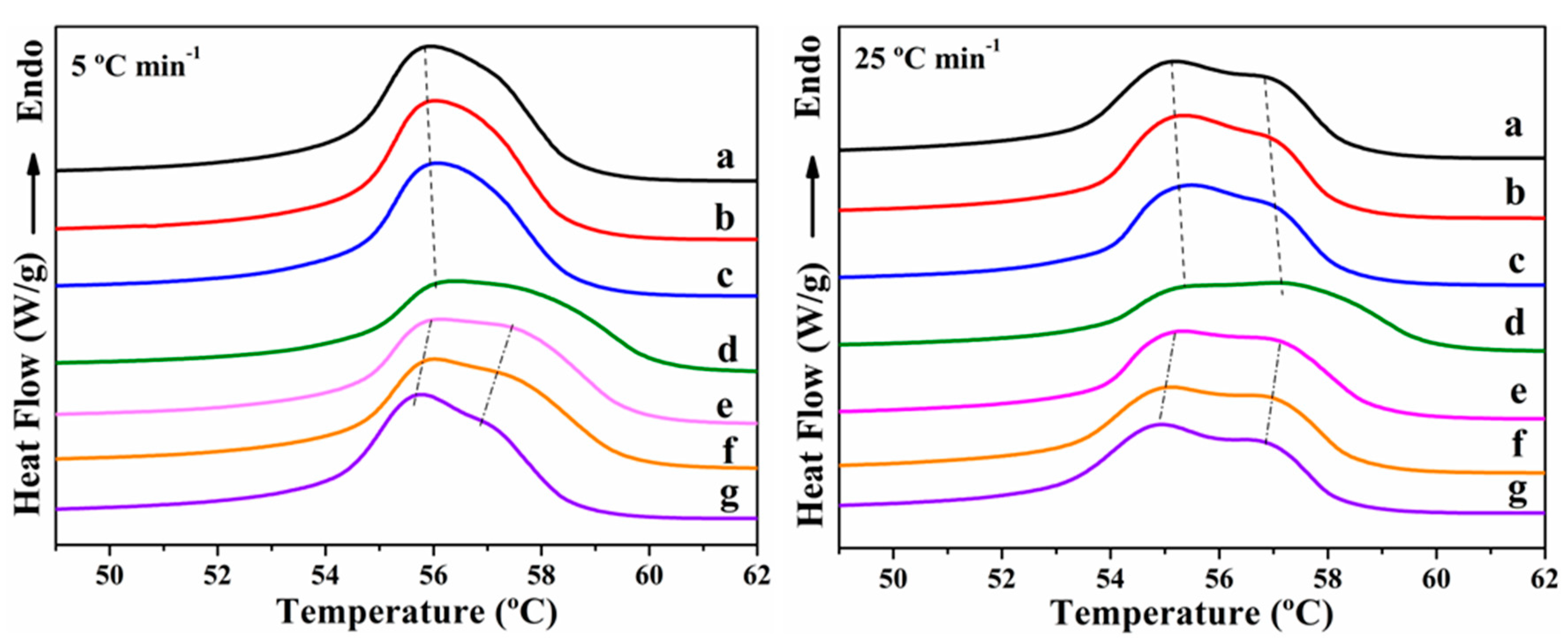
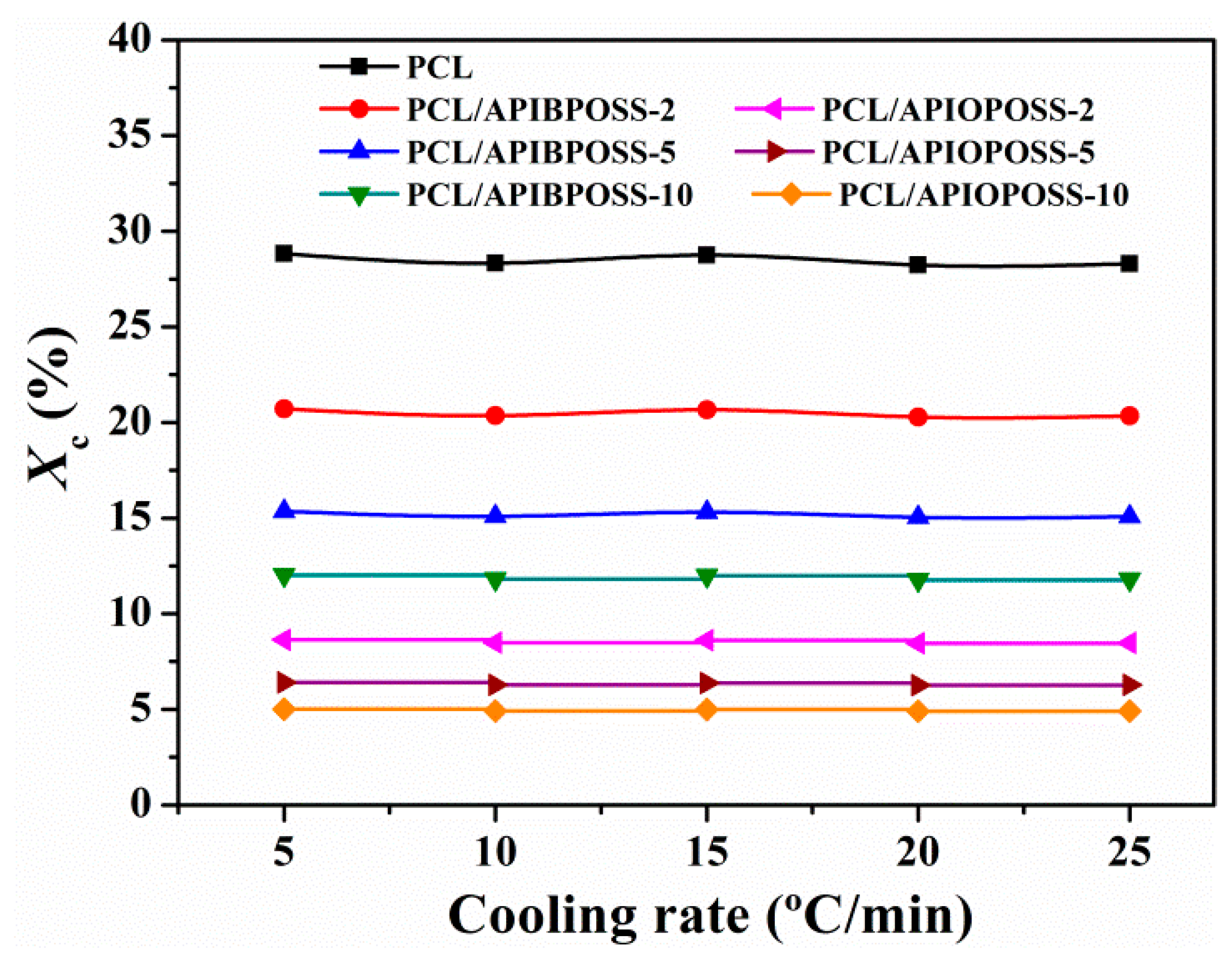
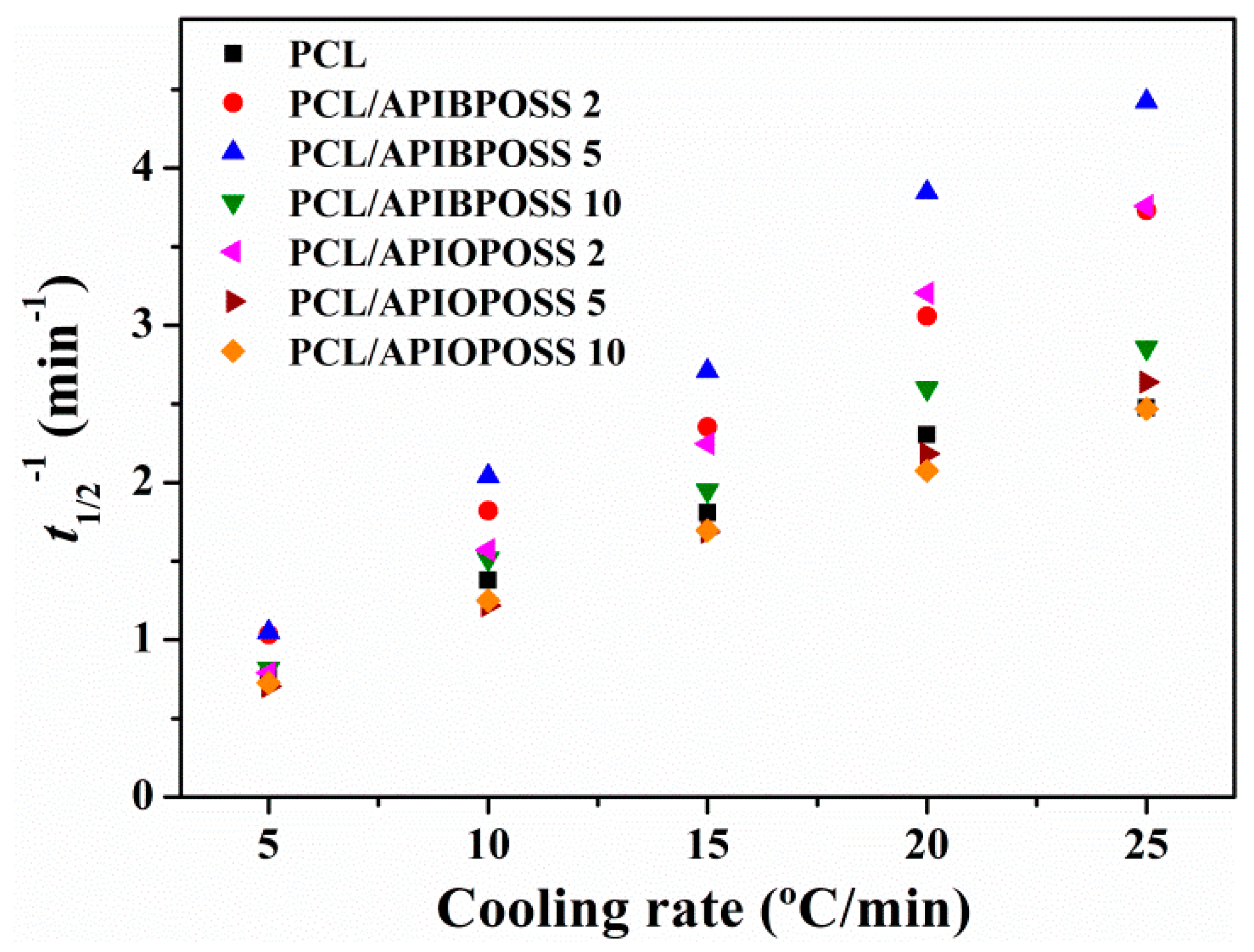


| Sample | ϕ (°C min−1) | Tc0.01 (°C) | Tc0.99 (°C) | Δtc (min) | ΔHc (J g−1) | Tmp (°C) | Tmp − Tcp (°C) | ΔHm (J g−1) | CRC (min−1) | CRP (°C−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| PCL | 5 | 38.92 | 31.38 | 1.508 | 37.34 | 55.96 | 22.31 | 40.94 | 3.270 | 0.0854 |
| 10 | 36.70 | 27.38 | 0.932 | 37.20 | 55.65 | 24.95 | 40.22 | |||
| 15 | 35.41 | 23.86 | 0.770 | 37.45 | 55.45 | 26.69 | 40.82 | |||
| 20 | 34.33 | 20.43 | 0.695 | 37.43 | 55.34 | 27.97 | 40.09 | |||
| 25 | 33.73 | 18.35 | 0.615 | 37.45 | 55.20 | 28.55 | 40.20 | |||
| PCL/APIBPOSS-2 | 5 | 39.03 | 31.48 | 1.510 | 36.85 | 56.05 | 20.59 | 41.08 | 3.806 | 0.1260 |
| 10 | 36.97 | 28.66 | 0.831 | 35.72 | 55.75 | 22.68 | 41.50 | |||
| 15 | 35.68 | 24.89 | 0.719 | 36.25 | 55.57 | 24.18 | 40.80 | |||
| 20 | 34.55 | 23.39 | 0.558 | 36.40 | 55.47 | 25.35 | 41.02 | |||
| 25 | 33.61 | 21.59 | 0.481 | 36.31 | 55.33 | 26.21 | 40.28 | |||
| PCL/APIBPOSS-5 | 5 | 39.21 | 31.83 | 1.476 | 35.04 | 56.10 | 20.21 | 39.29 | 4.171 | 0.1684 |
| 10 | 37.06 | 29.04 | 0.802 | 35.54 | 55.82 | 22.04 | 37.24 | |||
| 15 | 35.74 | 26.28 | 0.631 | 35.95 | 55.68 | 23.44 | 37.09 | |||
| 20 | 34.43 | 24.67 | 0.488 | 35.74 | 55.56 | 24.46 | 37.93 | |||
| 25 | 33.63 | 23.58 | 0.402 | 35.77 | 55.51 | 25.34 | 36.98 | |||
| PCL/APIBPOSS-10 | 5 | 39.37 | 27.23 | 2.428 | 33.29 | 56.39 | 22.43 | 38.64 | 3.334 | 0.1029 |
| 10 | 36.93 | 27.44 | 0.949 | 32.76 | 56.18 | 24.68 | 37.87 | |||
| 15 | 35.59 | 23.51 | 0.805 | 32.86 | 56.13 | 26.22 | 37.52 | |||
| 20 | 34.30 | 21.19 | 0.656 | 32.94 | 55.95 | 27.32 | 37.54 | |||
| 25 | 33.43 | 18.54 | 0.596 | 32.67 | 55.75 | 28.28 | 37.25 | |||
| PCL/APIOPOSS-2 | 5 | 38.13 | 29.52 | 1.722 | 37.60 | 56.13 | 22.90 | 38.46 | 4.655 | 0.1463 |
| 10 | 35.84 | 26.29 | 0.955 | 36.91 | 55.74 | 24.28 | 38.62 | |||
| 15 | 34.44 | 24.14 | 0.687 | 36.81 | 55.6 | 25.48 | 37.95 | |||
| 20 | 33.03 | 21.26 | 0.588 | 36.63 | 55.43 | 26.50 | 37.96 | |||
| 25 | 32.21 | 20.56 | 0.466 | 36.41 | 55.34 | 27.44 | 38.36 | |||
| PCL/APIOPOSS-5 | 5 | 38.25 | 27.41 | 2.168 | 36.74 | 56.02 | 24.07 | 37.03 | 3.512 | 0.0897 |
| 10 | 35.83 | 24.56 | 1.127 | 36.59 | 55.72 | 26.51 | 37.10 | |||
| 15 | 34.25 | 21.54 | 0.847 | 36.32 | 55.50 | 28.15 | 37.47 | |||
| 20 | 33.16 | 19.28 | 0.694 | 36.57 | 55.28 | 29.24 | 37.34 | |||
| 25 | 32.39 | 17.82 | 0.583 | 36.53 | 55.12 | 30.13 | 37.15 | |||
| PCL/APIOPOSS-10 | 5 | 38.52 | 28.09 | 2.086 | 36.87 | 55.78 | 23.28 | 37.66 | 3.236 | 0.0814 |
| 10 | 36.36 | 24.44 | 1.192 | 36.52 | 55.4 | 25.73 | 37.78 | |||
| 15 | 34.92 | 21.41 | 0.901 | 36.22 | 55.2 | 27.55 | 38.36 | |||
| 20 | 33.93 | 19.33 | 0.730 | 36.24 | 55.05 | 29.11 | 37.54 | |||
| 25 | 32.94 | 17.08 | 0.634 | 35.75 | 54.98 | 30.35 | 37.19 |
| Sample | ϕ (°C min−1) | n1 | Zc1 | r2 | n2 | Zc2 | r2 |
|---|---|---|---|---|---|---|---|
| PCL | 5 | 3.06 | 0.735 | 0.999 | 5.38 | 0.694 | 0.999 |
| 10 | 2.56 | 0.999 | 0.997 | 5.38 | 1.148 | 0.999 | |
| 15 | 2.81 | 1.058 | 0.998 | 5.47 | 1.212 | 0.999 | |
| 20 | 2.45 | 1.066 | 0.998 | 5.20 | 1.220 | 0.998 | |
| 25 | 3.07 | 1.081 | 0.999 | 5.36 | 1.213 | 0.999 | |
| PCL/APIBPOSS-2 | 5 | 2.67 | 0.805 | 0.995 | 4.67 | 0.950 | 0.997 |
| 10 | 2.67 | 1.027 | 0.993 | 5.05 | 1.299 | 0.993 | |
| 15 | 2.78 | 1.066 | 0.993 | 6.03 | 1.372 | 0.991 | |
| 20 | 2.71 | 1.079 | 0.994 | 6.55 | 1.411 | 0.989 | |
| 25 | 2.57 | 1.073 | 0.993 | 7.02 | 1.425 | 0.993 | |
| PCL/APIBPOSS-5 | 5 | 2.94 | 0.803 | 0.995 | 4.54 | 0.966 | 0.993 |
| 10 | 2.74 | 1.046 | 0.989 | 5.92 | 1.462 | 0.991 | |
| 15 | 2.84 | 1.082 | 0.990 | 7.01 | 1.549 | 0.991 | |
| 20 | 2.80 | 1.117 | 0.987 | 6.90 | 1.563 | 0.989 | |
| 25 | 2.88 | 1.110 | 0.988 | 7.60 | 1.551 | 0.989 | |
| PCL/APIBPOSS-10 | 5 | 2.48 | 0.624 | 0.997 | 3.95 | 0.804 | 0.995 |
| 10 | 2.55 | 1.023 | 0.999 | 5.51 | 1.212 | 0.998 | |
| 15 | 2.77 | 1.057 | 0.998 | 6.67 | 1.252 | 0.993 | |
| 20 | 2.59 | 1.069 | 0.998 | 5.22 | 1.256 | 0.991 | |
| 25 | 2.68 | 1.061 | 0.993 | 5.67 | 1.247 | 0.993 | |
| PCL/APIOPOSS-2 | 5 | 2.45 | 0.688 | 0.996 | 6.27 | 0.690 | 0.998 |
| 10 | 2.44 | 0.942 | 0.992 | 7.74 | 1.367 | 0.997 | |
| 15 | 2.74 | 1.036 | 0.993 | 7.62 | 1.472 | 0.995 | |
| 20 | 2.30 | 1.040 | 0.990 | 6.26 | 1.414 | 0.995 | |
| 25 | 2.43 | 1.055 | 0.992 | 6.15 | 1.364 | 0.997 | |
| PCL/APIOPOSS-5 | 5 | 2.55 | 0.711 | 0.995 | 3.40 | 0.738 | 0.999 |
| 10 | 2.70 | 0.951 | 0.995 | 4.23 | 1.047 | 0.999 | |
| 15 | 2.66 | 0.998 | 0.997 | 4.60 | 1.125 | 0.999 | |
| 20 | 2.51 | 1.032 | 0.997 | 4.50 | 1.165 | 0.999 | |
| 25 | 2.62 | 1.047 | 0.998 | 4.59 | 1.163 | 0.999 | |
| PCL/APIOPOSS-10 | 5 | 2.67 | 0.770 | 0.998 | 2.69 | 0.785 | 0.999 |
| 10 | 2.75 | 0.996 | 0.997 | 3.29 | 1.038 | 0.999 | |
| 15 | 2.74 | 1.052 | 0.997 | 3.46 | 1.103 | 1.000 | |
| 20 | 2.81 | 1.070 | 0.998 | 3.55 | 1.117 | 1.000 | |
| 25 | 2.75 | 1.076 | 0.998 | 3.47 | 1.118 | 1.000 |
| Sample | Xt (%) | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
|---|---|---|---|---|---|---|---|---|---|---|
| PCL | α | 1.39 | 1.38 | 1.36 | 1.34 | 1.34 | 1.34 | 1.34 | 1.37 | 1.43 |
| F(T) | 3.39 | 4.77 | 5.68 | 6.30 | 6.85 | 7.32 | 7.81 | 8.42 | 9.42 | |
| r2 | 0.9849 | 0.9899 | 0.9911 | 0.9892 | 0.9931 | 0.9932 | 0.9938 | 0.9936 | 0.9925 | |
| PCL/APIBPOSS-2 | α | 1.44 | 1.38 | 1.34 | 1.31 | 1.29 | 1.29 | 1.29 | 1.31 | 1.37 |
| F(T) | 2.58 | 3.43 | 3.93 | 4.34 | 4.69 | 5.07 | 5.52 | 6.10 | 7.05 | |
| r2 | 0.9958 | 0.9966 | 0.9971 | 0.9975 | 0.9978 | 0.9981 | 0.9982 | 0.9982 | 0.9967 | |
| PCL/APIBPOSS-5 | α | 1.18 | 1.13 | 1.12 | 1.11 | 1.11 | 1.12 | 1.13 | 1.16 | 1.23 |
| F(T) | 3.09 | 3.79 | 4.15 | 4.43 | 4.68 | 4.95 | 5.29 | 5.77 | 6.61 | |
| r2 | 0.9907 | 0.9928 | 0.9935 | 0.9938 | 0.9951 | 0.9948 | 0.9956 | 0.9962 | 0.9953 | |
| PCL/APIBPOSS-10 | α | 1.53 | 1.43 | 1.33 | 1.28 | 1.26 | 1.26 | 1.28 | 1.32 | 1.38 |
| F(T) | 2.53 | 3.82 | 5.09 | 5.78 | 6.27 | 6.70 | 7.15 | 7.77 | 8.88 | |
| r2 | 0.9814 | 0.9889 | 0.9933 | 0.9947 | 0.9946 | 0.9933 | 0.9927 | 0.9917 | 0.9896 | |
| PCL/APIOPOSS-2 | α | 1.05 | 1.01 | 1.01 | 1.01 | 1.03 | 1.04 | 1.07 | 1.11 | 1.17 |
| F(T) | 4.54 | 5.42 | 5.82 | 6.09 | 6.34 | 6.62 | 6.98 | 7.49 | 8.39 | |
| r2 | 0.9942 | 0.9961 | 0.9969 | 0.9969 | 0.9971 | 0.9969 | 0.9968 | 0.9973 | 0.9971 | |
| PCL/APIOPOSS-5 | α | 1.37 | 1.33 | 1.29 | 1.26 | 1.242 | 1.23 | 1.229 | 1.24 | 1.26 |
| F(T) | 3.93 | 5.27 | 6.27 | 7.09 | 7.82 | 8.53 | 9.25 | 10.12 | 11.39 | |
| r2 | 0.9916 | 0.9955 | 0.9969 | 0.9982 | 0.9982 | 0.9987 | 0.9987 | 0.9990 | 0.9990 | |
| PCL/APIOPOSS-10 | α | 1.38 | 1.39 | 1.37 | 1.34 | 1.32 | 1.29 | 1.28 | 1.28 | 1.29 |
| F(T) | 3.39 | 4.65 | 5.71 | 6.72 | 7.70 | 8.67 | 9.66 | 10.76 | 13.33 | |
| r2 | 0.9897 | 0.9937 | 0.9955 | 0.9967 | 0.9970 | 0.9972 | 0.9970 | 0.9970 | 0.9966 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, M.D.; Guzmán, D.J.; Ramos, J.R.; Fernández, M.J. Effect of Alkyl Chain Length in POSS Nanocage on Non-Isothermal Crystallization Behavior of PCL/Amino-POSS Nanocomposites. Polymers 2019, 11, 1719. https://doi.org/10.3390/polym11101719
Fernández MD, Guzmán DJ, Ramos JR, Fernández MJ. Effect of Alkyl Chain Length in POSS Nanocage on Non-Isothermal Crystallization Behavior of PCL/Amino-POSS Nanocomposites. Polymers. 2019; 11(10):1719. https://doi.org/10.3390/polym11101719
Chicago/Turabian StyleFernández, M. Dolores, Dailyn J. Guzmán, Johnny R. Ramos, and M. Jesús Fernández. 2019. "Effect of Alkyl Chain Length in POSS Nanocage on Non-Isothermal Crystallization Behavior of PCL/Amino-POSS Nanocomposites" Polymers 11, no. 10: 1719. https://doi.org/10.3390/polym11101719
APA StyleFernández, M. D., Guzmán, D. J., Ramos, J. R., & Fernández, M. J. (2019). Effect of Alkyl Chain Length in POSS Nanocage on Non-Isothermal Crystallization Behavior of PCL/Amino-POSS Nanocomposites. Polymers, 11(10), 1719. https://doi.org/10.3390/polym11101719