Synthesis of Bisphenol A Based Phosphazene-Containing Epoxy Resin with Reduced Viscosity
Abstract
:1. Introduction
- (1)
- (2)
2. Materials and Methods
2.1. Starting Materials
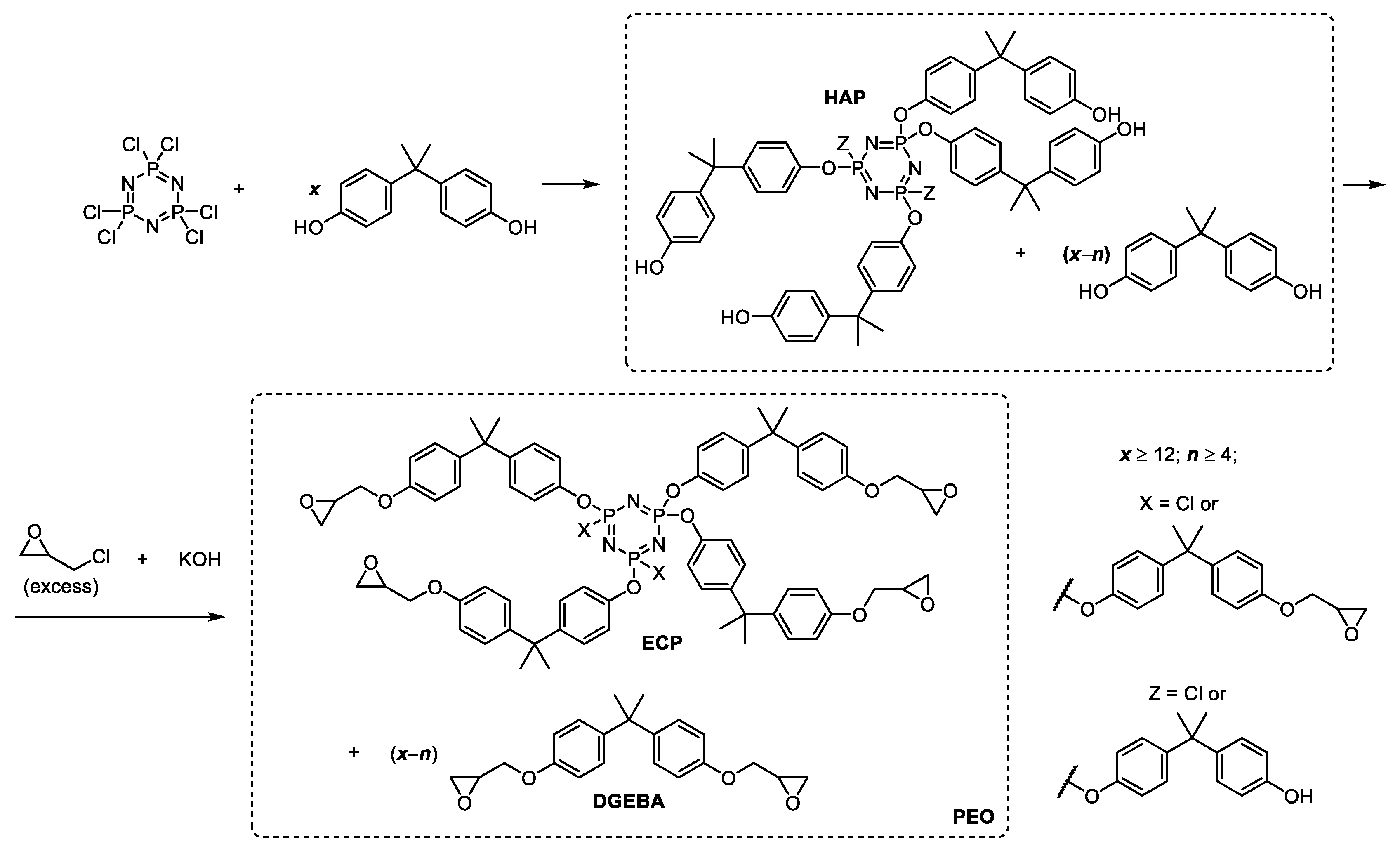
2.2. Synthesis of Epoxyphosphazenes
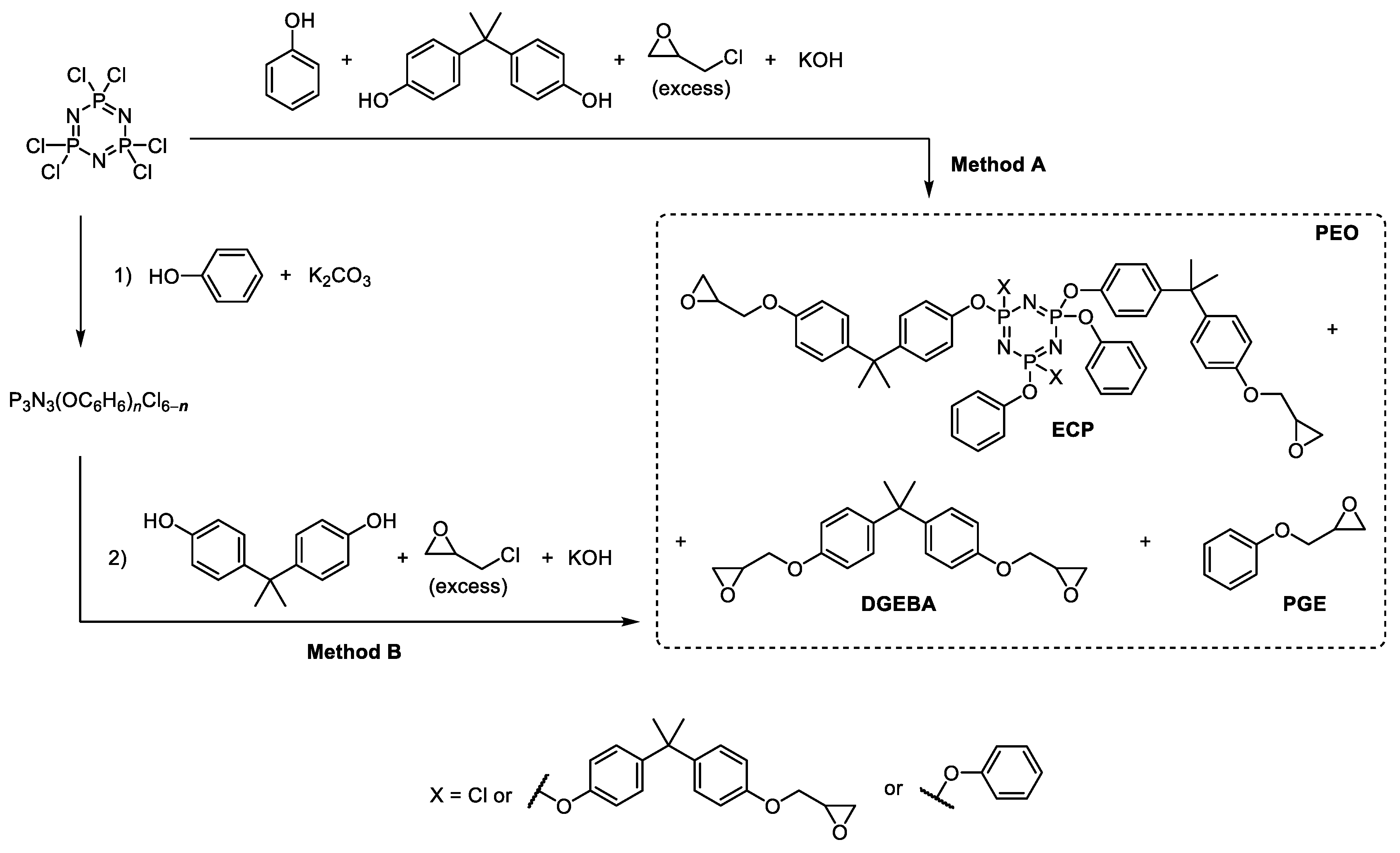
2.2.1. Single-Stage Synthesis of Phosphazene-Containing Epoxy Oligomers (Method A)
2.2.2. Stepwise Synthesis of Phosphazene-Containing Epoxy Oligomers (Method B)
2.3. Methods of Analysis
3. Results and Discussion


4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biron, M. Thermosets and Composites: Material Selection, Applications, Manufacturing and Cost Analysis; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-1-4557-3125-1. [Google Scholar]
- Dodiuk, H.; Goodman, S.H. Handbook of Thermoset Plastics; William Andrew: San Diego, CA, USA, 2013; ISBN 978-1-4557-3109-1. [Google Scholar]
- Petrie, E.M. Epoxy Adhesive Formulations; McGraw Hill Professional: New York, NY, USA, 2005; ISBN 978-0-07-158908-6. [Google Scholar]
- Flick, E.W. Epoxy Resins, Curing Agents, Compounds, and Modifiers: An Industrial Guide; William Andrew: San Diego, CA, USA, 2012; ISBN 978-0-8155-1708-5. [Google Scholar]
- Visakh, P.M.; Arao, Y. (Eds.) Flame Retardants: Polymer Blends, Composites and Nanocomposites, 1st ed.; Engineering Materials; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-03466-9. [Google Scholar]
- Lu, S.; Hamerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Rodriguez, F.; Cohen, C.; Ober, C.K.; Archer, L. Principles of Polymer Systems, Sixth Edition; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-1-4822-2378-1. [Google Scholar]
- Amirova, L.M. Fosforsoderzhashhie i Metallkoordinirovannye Jepoksidnye Polimernye Materialy [Фосфорсодержащие и металлкоординированные эпоксидные полимерные материалы]. Ph.D. Thesis, Kazan National Research Technological University, Kazan, Russia, 2004. [Google Scholar]
- Amirova, L.M.; Andrianova, K.A. Gradient polymeric materials based on poorly compatible epoxy oligomers. J. Appl. Polym. Sci. 2006, 102, 96–103. [Google Scholar] [CrossRef]
- Amirova, L.M.; Stroganov, V.F.; Sakhabieva, E.V. Optical Materials the Based on Low Flammable Epoxy Resins. Int. J. Polym. Mater. Polym. Biomater. 2000, 47, 43–60. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Price, D. Advances in Fire Retardant Materials; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 978-1-84569-507-1. [Google Scholar]
- Salmeia, K.A.; Gaan, S. An overview of some recent advances in DOPO-derivatives: Chemistry and flame retardant applications. Polym. Degrad. Stab. 2015, 113, 119–134. [Google Scholar] [CrossRef]
- Allcock, H. Phosphorus-Nitrogen Compounds: Cyclic, Linear, and High Polymeric Systems; Elsevier: Amsterdam, The Netherlands, 1972; ISBN 978-0-12-050560-9. [Google Scholar]
- Levchik, S.V.; Weil, E.D. A Review of Recent Progress in Phosphorus-based Flame Retardants. J. Fire Sci. 2006, 24, 345–364. [Google Scholar] [CrossRef]
- Allen, C.W. The Use of Phosphazenes as Fire Resistant Materials. J. Fire Sci. 1993, 11, 320–328. [Google Scholar] [CrossRef]
- Leu, T.-S.; Wang, C.-S. Synergistic effect of a phosphorus–nitrogen flame retardant on engineering plastics. J. Appl. Polym. Sci. 2004, 92, 410–417. [Google Scholar] [CrossRef]
- Phosphazene|Chemical Products|Otsuka Chemical Co., Ltd. Available online: https://www.otsukac.co.jp/en/products/flame-retardant/phosphazene/ (accessed on 23 December 2018).
- Fantin, G.; Medici, A.; Fogagnolo, M.; Pedrini, P.; Gleria, M.; Bertani, R.; Facchin, G. Functionalization of poly(organophosphazenes)—III. Synthesis of phosphazene materials containing carbon-carbon double bonds and epoxide groups. Eur. Polym. J. 2012, 29, 1571–1579. [Google Scholar] [CrossRef]
- Allcock, H.R.; Nelson, C.J.; Coggio, W.D. Photoinitiated graft poly(organophosphazenes): Functionalized immobilization substrates for the binding of amines, proteins, and metals. Chem. Mater. 1994, 6, 516–524. [Google Scholar] [CrossRef]
- Chen-Yang, Y.W.; Lee, H.F.; Yuan, C.Y. A flame-retardant phosphate and cyclotriphosphazene-containing epoxy resin: Synthesis and properties. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 972–981. [Google Scholar] [CrossRef]
- Bertani, R.; Boscolo-Boscoletto, A.; Dintcheva, N.; Ghedini, E.; Gleria, M.; La Mantia, F.; Pace, G.; Pannocchia, P.; Sassi, A.; Scaffaro, R.; et al. New phosphazene-based chain extenders containing allyl and epoxide groups. Des. Monomers Polym. 2003, 6, 245–266. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; La Mantia, F.P.; Magagnini, P.; Acierno, D.; Gleria, M.; Bertani, R. Effect of adding new phosphazene compounds to poly(butylene terephthalate)/polyamide blends. I: Preliminary study in a batch mixer. Polym. Degrad. Stab. 2005, 90, 234–243. [Google Scholar] [CrossRef]
- El Gouri, M.; El Bachiri, A.; Hegazi, S.E.; Rafik, M.; El Harfi, A. Thermal degradation of a reactive flame retardant based on cyclotriphosphazene and its blend with DGEBA epoxy resin. Polym. Degrad. Stab. 2009, 94, 2101–2106. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X. Synthesis, characterization, thermal properties and flame retardancy of a novel nonflammable phosphazene-based epoxy resin. Polym. Degrad. Stab. 2009, 94, 617–624. [Google Scholar] [CrossRef]
- El Gouri, M.; Cherkaoui, O.; Ziraoui, R.; El Harfi, A. Physico-chemical study of DGEBA epoxy resin flame retarded with an ecological flame retardant based on cyclotriphosphazene. J. Mater. Environ. Sci. 2010, 3, 157–162. [Google Scholar]
- El Gouri, M.; El Bachiri, A.; Hegazi, S.E.; Rafik, M.; El Harfi, A. Fireproofing amelioration of epoxy resin material by way a reactive flame retardant based on cyclophosphazene. Phys. Chem. News 2010, 56, 128–137. [Google Scholar]
- El Gouri, M.; Hegazi, S.E.; Rafik, M.; El Harfi, A. Synthesis and thermal degradation of phosphazene containing the epoxy group. Ann. Chim. Sci. Mater. 2010, 35, 27–39. [Google Scholar] [CrossRef]
- Liu, F.; Wei, H.; Huang, X.; Zhang, J.; Zhou, Y.; Tang, X. Preparation and Properties of Novel Inherent Flame-Retardant Cyclotriphosphazene-Containing Epoxy Resins. J. Macromol. Sci. Part B 2010, 49, 1002–1011. [Google Scholar] [CrossRef]
- El Gouri, M.; El Bachiri, A.; Hegazi, S.E.; Ziraoui, R.; Rafik, M.; El Harfi, A. A phosphazene compound multipurpose application-Composite material precursor and reactive flame retardant for epoxy resin materials. J. Mater. Environ. Sci. 2011, 2, 319–334. [Google Scholar]
- Gu, X.; Huang, X.; Wei, H.; Tang, X. Synthesis of novel epoxy-group modified phosphazene-containing nanotube and its reinforcing effect in epoxy resin. Eur. Polym. J. 2011, 47, 903–910. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, X.; Wu, D. Novel cyclolinear cyclotriphosphazene-linked epoxy resin for halogen-free fire resistance: Synthesis, characterization, and flammability characteristics. Ind. and Eng. Chem. Res. 2012, 51, 15064–15074. [Google Scholar] [CrossRef]
- El Gouri, M.; El Harfi, A. Chemical modification of hexachlorocyclotriphosphazene—Preparation of flame retardants and ecological flame retardant polymers. J. Mater. Environ. Sci. 2012, 3, 17–33. [Google Scholar]
- Liu, J.; Tang, J.; Wang, X.; Wu, D. Synthesis, characterization and curing properties of a novel cyclolinear phosphazene-based epoxy resin for halogen-free flame retardancy and high performance. RSC Adv. 2012, 2, 5789–5799. [Google Scholar] [CrossRef]
- Sun, J.; Wang, X.; Wu, D. Novel Spirocyclic Phosphazene-Based Epoxy Resin for Halogen-Free Fire Resistance: Synthesis, Curing Behaviors, and Flammability Characteristics. ACS Appl. Mater. Interfaces 2012, 4, 4047–4061. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Wang, X.; Wu, D. Fabrication of spirocyclic phosphazene epoxy-based nanocomposites with graphene via exfoliation of graphite platelets and thermal curing for enhancement of mechanical and conductive properties. Ind. Eng. Chem. Res. 2013, 52, 10160–10171. [Google Scholar] [CrossRef]
- Huang, X.; Wei, W.; Wei, H.; Li, Y.; Gu, X.; Tang, X. Preparation of heat-moisture resistant epoxy resin based on phosphazene. J. Appl. Polym. Sci. 2013, 130, 248–255. [Google Scholar] [CrossRef]
- El Gouri, M.; El Mansouri, A.; El Gouri, R.; Hadik, N.; Cherkaoui, O.; Outzourhit, A.; El Harfi, A. Physical behaviour of epoxy resin material flame retarded with a reactive flame retardant based on cyclophosphazene. J. Mater. Environ. Sci. 2014, 5, 400–4007. [Google Scholar]
- Lu, L.; Chen, Y.; Wang, S.; Yang, S.; Dong, X. Preparation and flame retardancy of MMT pattern synergy intumescent flame-retardant epoxy resin. Gaofenzi Cailiao Kexue Yu Gongcheng Polym. Mater. Sci. Eng. 2014, 30, 139–144. [Google Scholar]
- Xu, G.R.; Xu, M.J.; Li, B. Synthesis and characterization of a novel epoxy resin based on cyclotriphosphazene and its thermal degradation and flammability performance. Polym. Degrad. Stab. 2014, 109, 240–248. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D. Novel cyclotriphosphazene-based epoxy compound and its application in halogen-free epoxy thermosetting systems: Synthesis, curing behaviors, and flame retardancy. Polym. Degrad. Stab. 2014, 103, 96–112. [Google Scholar] [CrossRef]
- Lakshmikandhan, T.; Sethuraman, K.; Chandramohan, A.; Alagar, M. Development of phosphazene imine-modified epoxy composites for low dielectric, antibacterial activity, and UV shielding applications. Polym. Compos. 2017, 38, E24–E33. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D. Synthesis of a novel linear polyphosphazene-based epoxy resin and its application in halogen-free flame-resistant thermosetting systems. Polym. Degrad. Stab. 2005, 118, 45–58. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Jiang, L.; Mao, Y.; Zhao, D. Kinetics of thermal decomposition and flame retardance of phosphazene-containing epoxy resin. In Proceedings of the 10th Asia-Pacific Conference on Combustion, Beijing, China, 19–22 June 2015. [Google Scholar]
- Takahashi, K.; Yamamoto, T.; Itoh, S.; Harakawa, K.; Kajiwara, M. Mechanical properties of epoxy resins cured by various amines. Zairyo J. Soc. Mater. Sci. Jpn. 1988, 37, 454–459. [Google Scholar] [CrossRef]
- Yamamoto, T.; Takahashi, K.; Kon, Y.; Harakawa, K. Curing of epoxy resin with phosphazene derivatives. Kobunshi Ronbunshu 1988, 45, 851–856. [Google Scholar] [CrossRef]
- Yamamoto, T.; Takahashi, K.; Kon, Y.; Kobayashi, K. Tensile Behavior and Heat Resistance of Epoxy Resin Cured with Phosphazene Derivatives. Kobunshi Ronbunshu 1989, 46, 177–181. [Google Scholar] [CrossRef]
- Takahashi, K.; Ishikawa, N.; Komori, T.; Yoon, H.-S. Curing of an epoxy resin with P3N3(NH2)2 (OCH2CF3)4 and its mechanical properties. Kobunshi Ronbunshu 1990, 47, 727–734. [Google Scholar] [CrossRef]
- Takahashi, K.; Ishikawa, N.; Yoon, H.-S. Mechanical properties of epoxy resins cured with P3N3(NH2)2 (OC6H4Cl)4. Kobunshi Ronbunshu 1990, 47, 757–762. [Google Scholar] [CrossRef]
- Takahashi, K.; Ishikawa, N.; Yoon, H.-S. Resistance of epoxy resins cured with trichloro-tridimethylamino-cyclotriphosphazene against chemical substances. Zairyo J. Soc. Mater. Sci. Jpn. 1990, 39, 1001–1006. [Google Scholar] [CrossRef]
- Takahashi, K.; Ishikawa, N.; Kohno, T.; Yoon, H.-S. Water resistance of an epoxy resin cured with P3N3Cl3(N(CH3)2)3 and the effect of glass flake reinforcement. Zairyo J. Soc. Mater. Sci. Jpn. 1991, 40, 458–463. [Google Scholar] [CrossRef]
- Takahashi, K.; Nakashima, J.; Ishiguro, S. Mechanical properties of trifunctional epoxy resin with phosphazene derivatives. Kobunshi Ronbunshu 1994, 51, 717–723. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.M.; Liao, Y.L.; Lin, J.J. Synergistic effect of silicate clay and phosphazene-oxyalkyleneamines on thermal stability of cured epoxies. J. Colloid Interface Sci. 2010, 343, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Terekhov, I.V.; Filatov, S.N.; Chistyakov, E.M.; Borisov, R.S.; Kireev, V.V. Synthesis of oligomeric epoxycyclotriphosphazenes and their properties as reactive flame-retardants for epoxy resins. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 544–554. [Google Scholar] [CrossRef]
- Kireev, V.V.; Bilichenko, Y.V.; Borisov, R.S.; Sirotin, I.S.; Filatov, S.N. Laser Mass Spectrometry Analysis of the Formation of Phosphazene-Containing Epoxy Oligomers. Polym. Sci. Ser. B 2018, 60, 243–262. [Google Scholar] [CrossRef]
- Sirotin, I.S.; Bilichenko, Y.V.; Brigadnov, K.A.; Kireev, V.V.; Prudskov, B.M.; Borisov, R.S. Single-stage synthesis of phosphazene-containing epoxy oligomers. Polym. Sci. Ser. B 2014, 56, 471–476. [Google Scholar] [CrossRef]
- Brigadnov, K.A.; Bilichenko, Y.V.; Polyakov, V.A.; Borisov, R.S.; Gusev, K.I.; Rudakova, T.A.; Filatov, S.N.; Kireev, V.V. Epoxy oligomers modified with epoxyphosphazenes. Polym. Sci. Ser. B 2016, 58, 549–555. [Google Scholar] [CrossRef]
- Sarychev, I.A.; Sirotin, I.S.; Borisov, R.S.; Mu, J.; Sokolskaya, I.B.; Bilichenko, J.V.; Filatov, S.N.; Kireev, V.V. Synthesis of Resorcinol-Based Phosphazene-Containing Epoxy Oligomers. Polymers 2019, 11, 614. [Google Scholar] [CrossRef] [Green Version]
- Sirotin, I.S.; Bilichenko, Y.V.; Suraeva, O.V.; Solodukhin, A.N.; Kireev, V.V. Synthesis of oligomeric chlorophosphazenes in the presence of ZnCl2. Polym. Sci. Ser. B 2013, 55, 63–68. [Google Scholar] [CrossRef]
- Riddick, J.A.; Bunger, W.B.; Sakano, T.K. Organic Solvents: Physical Properties and Methods of Purification; Wiley: Hoboken, NJ, USA, 1986; ISBN 978-0-471-08467-9. [Google Scholar]
- Sirotin, I.S.; Bilichenko, Y.V.; Brigadnov, K.A.; Kireev, V.V.; Suraeva, O.V.; Borisov, R.S. Oligomeric hydroxy-aryloxy phosphazene based on cyclic chlorophosphazenes. Russ. J. Appl. Chem. 2013, 86, 1903–1912. [Google Scholar] [CrossRef]
- Simonov-Emel’yanov, I.D.; Apeksimov, N.V.; Kochergina, L.M.; Bilichenko, Y.V.; Kireev, V.V.; Brigadnov, K.A.; Sirotin, I.S.; Filatov, S.N. Rheological and rheokinetic properties of phosphazene-containing epoxy oligomers. Polym. Sci. Ser. B 2016, 58, 168–172. [Google Scholar] [CrossRef]
- Li, S.; Guan, W.; Wu, Z.; Lu, J.; Guo, J. An Improved Method to Determine Epoxy Index of Epoxy Resins. Polym.-Plast. Technol. Eng. 2007, 46, 901–903. [Google Scholar] [CrossRef]






| m/z | Compound Formula 1 | Relative Content of the Compound (% weight) in the Product, Obtained via | |
|---|---|---|---|
| Method A | Method B | ||
| 636 | P3N3Cl(OPh)5 | 12.3 | 3.6 |
| 654 | P3N3Cl4(OPh)(OArOGly) | - | 3.0 |
| 768 | P3N3Cl2(OPh)3(OArOGly) | 1.6 | 7.2 |
| 826 | P3N3Cl(OPh)4(OArOGly) | 14.0 | 8.6 |
| 958 | P3N3Cl(OPh)3(OArOGly)(OArOGly′) | - | 5.0 |
| 960 | P3N3Cl(OPh)3(OArOH)(OArOGly) | - | 8.5 |
| 1016 | P3N3Cl(OPh)3(OArOGly)2 | 22.2 | 9.2 |
| 1150 | P3N3Cl(OPh)2(OArOH)(OArOGly)2 | - | 3.0 |
| 1206 | P3N3Cl(OPh)2(OArOGly)3 | 25.0 | 19.3 |
| 1397 | P3N3Cl(OPh)(OArOGly)4 | 16.7 | - |
| 1587 | P3N3Cl(OArOGly)5 | 1.0 | - |
 .
.| Chromatography Data (Figure 6) | Mass-Spectrometric Data (Figure 7) | |||||||
|---|---|---|---|---|---|---|---|---|
| Peak No | Elution Time (min) | Probable Formula 1 of the Compound at the Output of the Column | Calculated Molecular Weight | Content 2 of the Compound (wt%) | The Most Likely Formula | Calculated Molecular Weight | Observed m/z Values of the Products Obtained by Method | |
| A | B | |||||||
| 1 | 7.8 | PhOH | 93 | 2.0/7.6 | PhOH | 93 | 93 | 93 |
| 7.9 | PhOCH2CH=CH2 | 134 | 0.5/- | PhOCH2CH=CH2 | 134 | 133 | none | |
| 2 | 9.3 | PhOGly | 150 | 0.9/4.8 | PhOGly | 150 | none | 149 |
| 3 | 14.6 | HOArOH | 228 | 1.0/6.3 | HOAr′OH | 212 | 212 | 212 |
| 4 | 15.8 | HOArOGly | 284 | 0.8/1.9 | HOAr′OCH2CH=CH2 | 252 | 250 | 250 |
| 5 | 16.7 | HOArOGly | 284 | 2.0/8.2 | HOAr′OGly | 268 | 268 + 283 | 268 + 283 |
| 6 | 18.7 | HOAr′OGly′ | 306.5 | - /4.1 | HOAr′OGly′ | 306.5 | none | 305 |
| 7 | 19.8 | GlyOArOGly | 340 | 66.8/45.9 | GlyOArOGly | 325 | 325 + 340 | 325 + 340 |
| 8 | 22.4 | Gly′OAr′OGly | 361 | 21.2/2.,1 | Gly′OAr′OGly | 361 | 361 | 361 |
| 9 | 26.8 | Gly′OAr′OGly′ | 397 | 4.6/ - | Gly′OAr′OGly′ | 397 | 397 | none |
 ; 2 in the numerator for method A and in the denominator for method B.
; 2 in the numerator for method A and in the denominator for method B.| Raw Reagents Ratio | Mixture Average Functionality 6 | Content (wt%) | Average Molecular Weight | Viscosity (Pa⋅s) at the Temperature of (°C) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Epoxy Group | P | Cl | Phosphazene Fraction 2 | Entire Mixture 3 Mw/Mn | Organic Fraction | Phosphazene Fraction 4 | 20 | 40 | 70 | ||
| DGEBA | |||||||||||
| - | 2.0 | 24.6–25.1 | - | - | - | 346/- | - | - | 5.83 | 0.86 | 0.06 |
| RDGE | |||||||||||
| - | 2.0 | 34.4–36.4 | - | - | - | 243/- | - | - | 1.10 | 0.11 | 0.03 |
| HCP:BPA | PEOs obtained by interaction of HCP with BPA and ECH [55,56,61] | ||||||||||
| 1:8 | 2.5 | 17.1 | 3.1 | 2.7 | 49/49 | 1487/717 | 3405 | 1473 | - | 220 | 3 |
| 1:12 | 2.3 | 20.0 | 1.8 | 1.5 | 36/30 | 1212/681 | 1486 | - | 130 | 2 | |
| 1:16 | 2.2 | 21.4 | 1.5 | 1.3 | 30/25 | 931/627 | 1492 | 440 | 78 | 2 | |
| HCP:Resorcinol | PEOs obtained by interaction of HCP with resorcinol and ECH [57] | ||||||||||
| 1:12 | 2.4 | 21.0 | 4.0 | 4.4 | 45/43 | 2350/380 | 220 3 | 1054 | 8.33 | 6.15 | 0.36 |
| 1:16 | 2.3 | 28.6 | 3.0 | 2.4 | 30/32 | 1260/260 | 999 | 2.43 | 1.94 | 0.15 | |
| 1:24 | 2.2 | 29.6 | 2.0 | 1.9 | 23/21 | 1130/260 | 957 | 1.71 | 0.45 | 0.05 | |
| HCP:PhOH:BPA | PEOs obtained by interaction of HCP with BPA, phenol and ECH 7 (this work) | ||||||||||
| 1:2:6 | 2.2 | 16.1 | 4.6 | 2.2 | 61/54 | 4246/288 | 340 5 | 1211 | 64.6 | 13.7 | 0.8 |
| 1:3:5 | 2.0 | 15.5 | 5.0 | 2.3 | 61/51 | 3459/413 | 1058 | 58.6 | 10.6 | 0.8 | |
| 1:4:4 | 1.9 | 14.7 | 5.4 | 2.7 | 57/47 | 3248/317 | 930 | 9.4 | 6.0 | 0.8 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kireev, V.V.; Bilichenko, Y.V.; Borisov, R.S.; Mu, J.; Kuznetsov, D.A.; Eroshenko, A.V.; Filatov, S.N.; Sirotin, I.S. Synthesis of Bisphenol A Based Phosphazene-Containing Epoxy Resin with Reduced Viscosity. Polymers 2019, 11, 1914. https://doi.org/10.3390/polym11121914
Kireev VV, Bilichenko YV, Borisov RS, Mu J, Kuznetsov DA, Eroshenko AV, Filatov SN, Sirotin IS. Synthesis of Bisphenol A Based Phosphazene-Containing Epoxy Resin with Reduced Viscosity. Polymers. 2019; 11(12):1914. https://doi.org/10.3390/polym11121914
Chicago/Turabian StyleKireev, Vyacheslav V., Yulya V. Bilichenko, Roman S. Borisov, Jianxin Mu, Dmitry A. Kuznetsov, Anastasiya V. Eroshenko, Sergey N. Filatov, and Igor S. Sirotin. 2019. "Synthesis of Bisphenol A Based Phosphazene-Containing Epoxy Resin with Reduced Viscosity" Polymers 11, no. 12: 1914. https://doi.org/10.3390/polym11121914
APA StyleKireev, V. V., Bilichenko, Y. V., Borisov, R. S., Mu, J., Kuznetsov, D. A., Eroshenko, A. V., Filatov, S. N., & Sirotin, I. S. (2019). Synthesis of Bisphenol A Based Phosphazene-Containing Epoxy Resin with Reduced Viscosity. Polymers, 11(12), 1914. https://doi.org/10.3390/polym11121914