Nitramine-Group-Containing Energetic Prepolymer: Synthesis, and Its Properties as a Binder for Propellant
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials for Monomer Synthesis and Prepolymer Polymerization
2.2. Materials for Binder Preparation and Plasticizer
2.3. Synthesis of Energetic Monomer
2.4. Polymerization of Energetic Prepolymer
2.5. Characterization of Energetic Monomer and Prepolymer
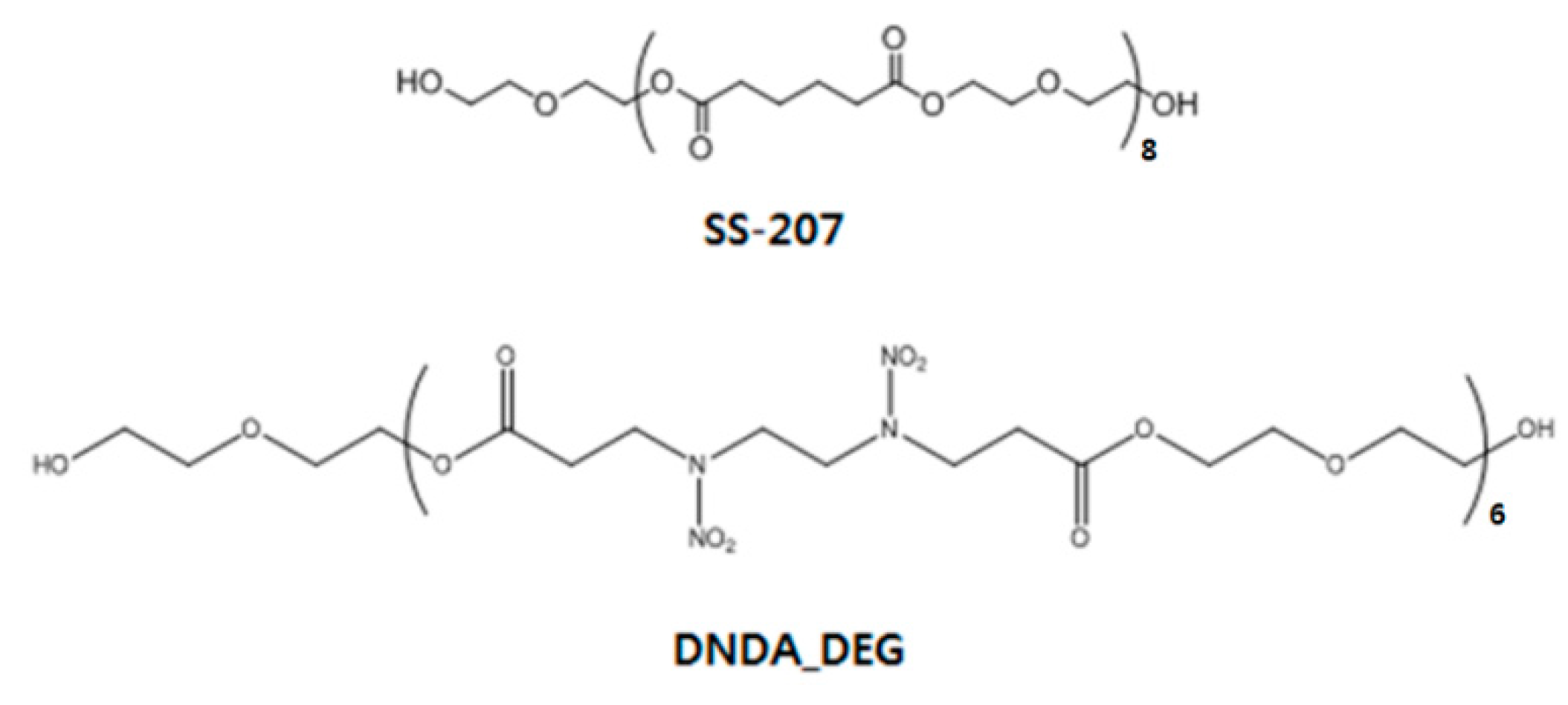
2.6. Formation of Urethane Crosslinked Binder Network
2.7. Thermogram Analysis
2.8. Mechanical Properties
3. Results and Discussion
3.1. Characterization of Energetic Monomer and Prepolymer
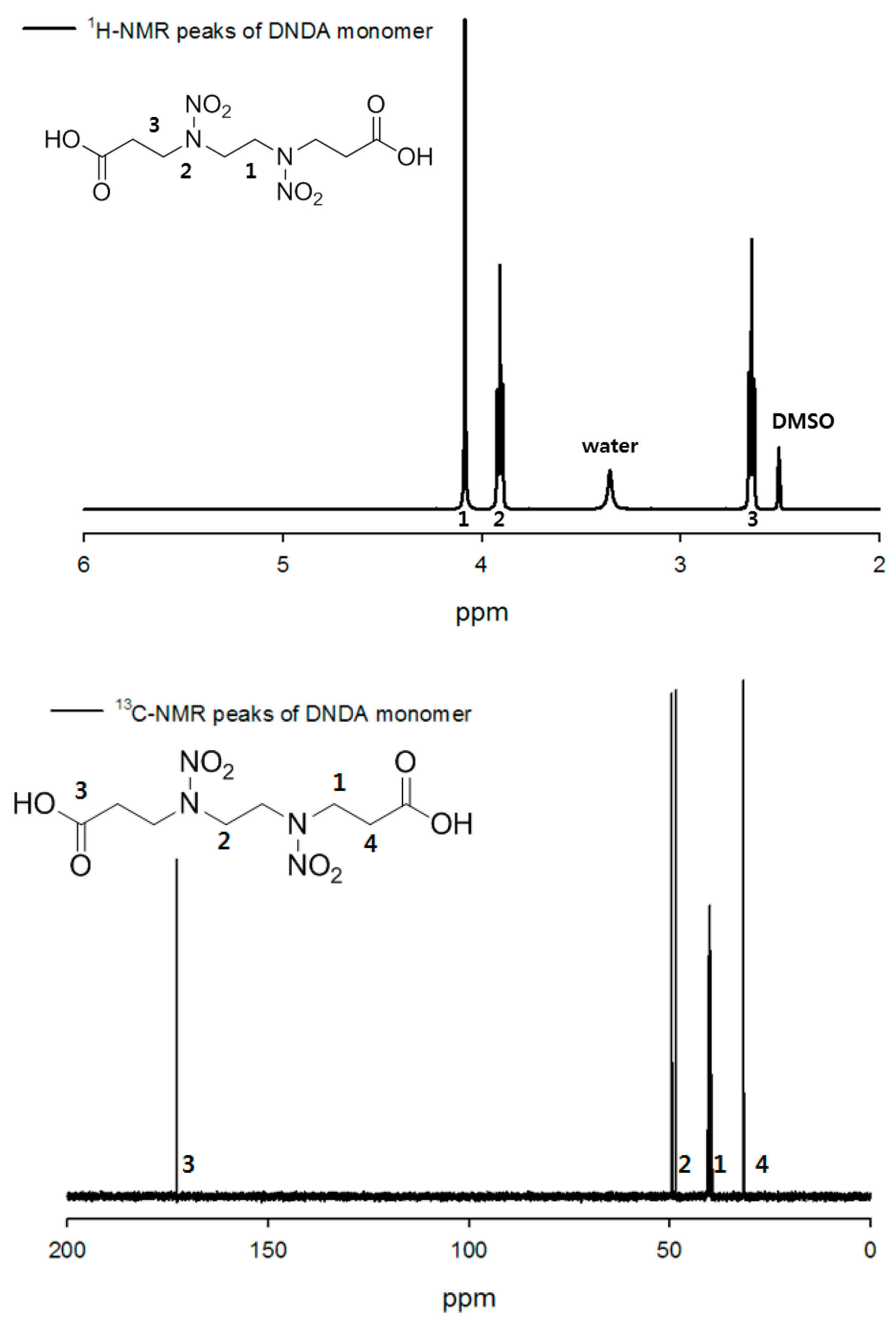
3.1.1. H-NMR and 13C-NMR Spectra of Energetic Monomer
3.1.2. H-NMR Spectrum of Energetic Prepolymer
3.1.3. FT-IR Spectrum of Energetic Prepolymer
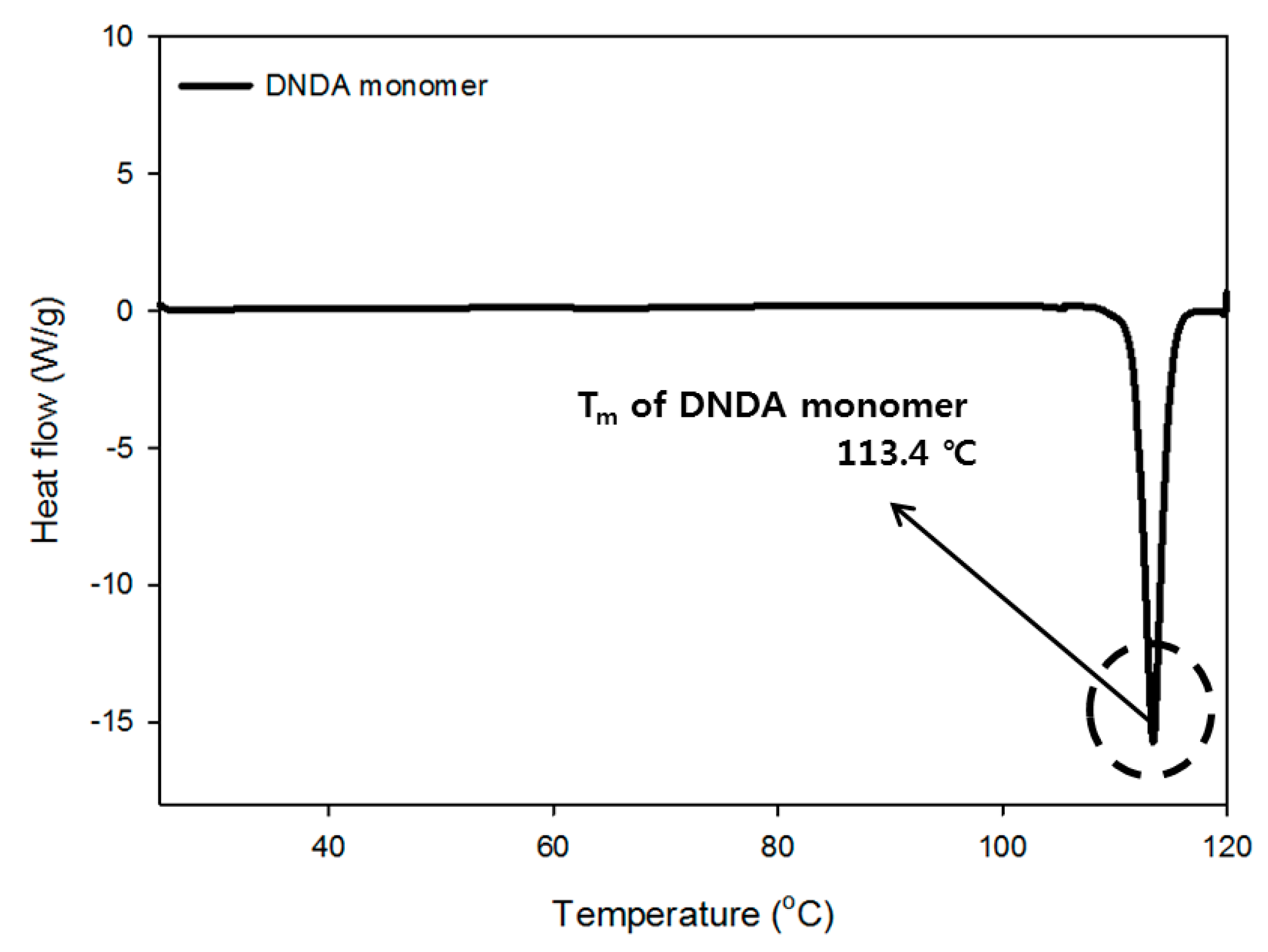
3.1.4. Thermogravimetric Analysis of Monomer and Prepolymer
3.2. Formation of Urethane Crosslinked Binder Network
3.3. Thermogravimetric Analysis of Binders
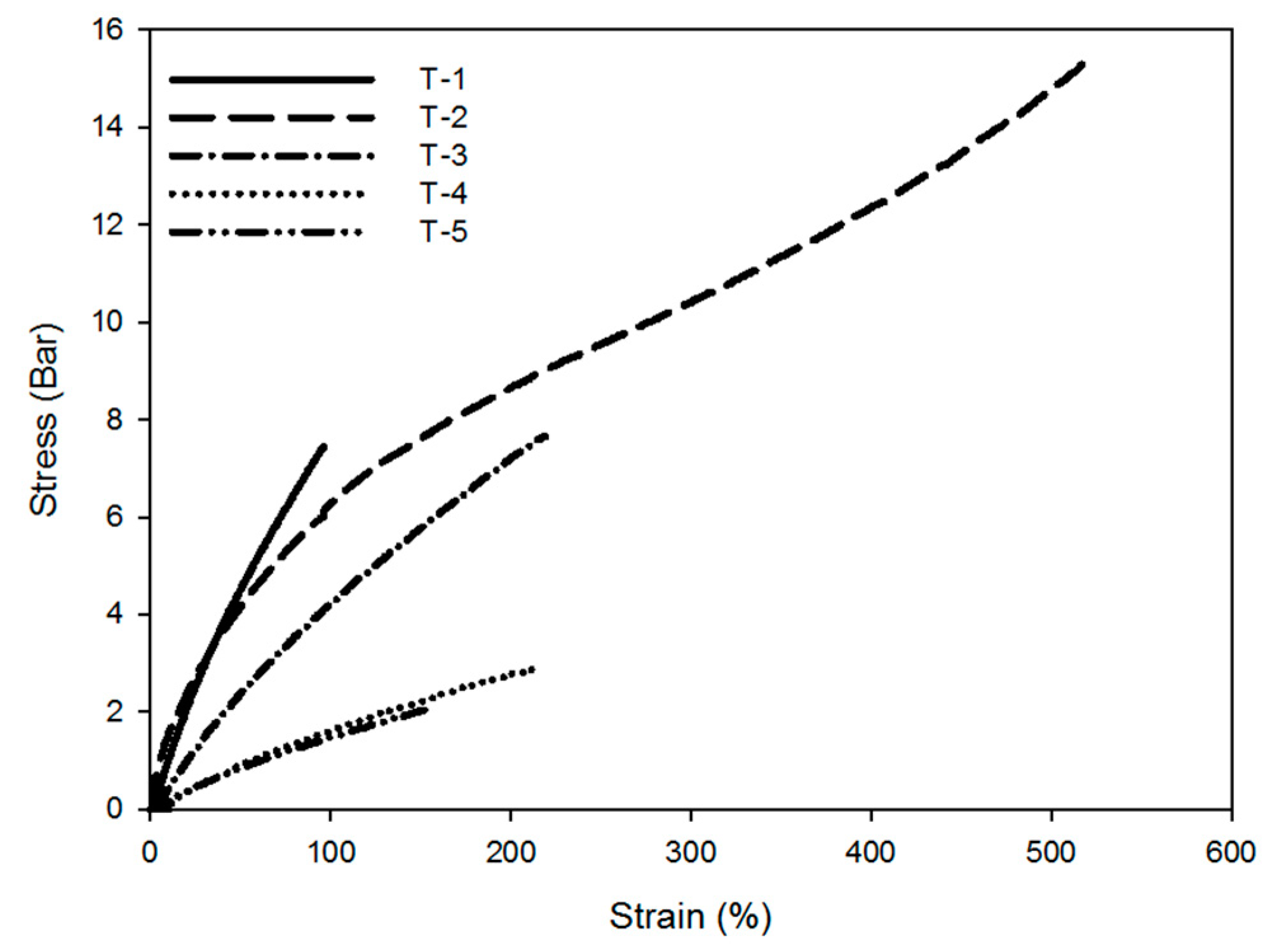
3.4. Mechanical Properties of Binders
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Giovanetti, A.J. Liquid Propellant Inflator for Vehicle Occupant Restraint Apparatus. U.S. Patent No. 5,060,973, 29 October 1991. [Google Scholar]
- Yim, Y.J.; Hwang, K.S. The families, and selection of the solid propellants. Korean Soc. Aeronaut. Space Sci. 1994, 22, 147–154. [Google Scholar]
- Wallace, I.I.A.; Oyler, J. Nitrate Ester Plasticized Energetic Compositions, Method of Making and Rocket Motor Assemblies Containing the Same. U.S. Patent No. 6,632,378, 14 October 2003. [Google Scholar]
- Chavez, D.E.; Hiskey, M.A.; Naud, D.L.; Parrish, D. Synthesis of an energetic nitrate ester. Angew. Chem. Int. Ed. 2008, 47, 8307–8309. [Google Scholar] [CrossRef] [PubMed]
- Gaur, B.; Lochab, B.; Choudhary, V.; Varma, I.K. Azido Polymers-Energetic Binders for Solid Rocket Propellants. J. Macromol. Sci. C Polym. Rev. 2003, 43, 505–545. [Google Scholar] [CrossRef]
- Muthiah, R.M.; Krishnamurthy, V.N.; Gupta, B.R. Rheology of HTPB propellant. I. Effect of solid loading, oxidizer particle size, and aluminum content. J. Appl. Polym. Sci. 1992, 44, 2043–2052. [Google Scholar] [CrossRef]
- Jeong, H.M.; Lee, J.B.; Lee, S.Y.; Kim, B.K. Shpae memory polyurethane containg mesogenic moiety. J. Mater. Sci. 2000, 35, 279–293. [Google Scholar] [CrossRef]
- Stacer, R.G.; Husband, D.M. Molecular structure of the ideal solid propellant binder. Propellants Explos. Pyrotech. 1991, 16, 167–176. [Google Scholar] [CrossRef]
- Hagen, T. Energetic Binders for Solid Rocket Propellants. Master’s Thesis, Norwegian University of Life Sciences, Ås, Normay, 5 August 2014. [Google Scholar]
- Frankel, M.B.; Grant, L.R.; Flanagan, J.E. Historical development of glycidyl azide polymer. J. Propul. Power 1992, 8, 560–563. [Google Scholar] [CrossRef]
- Mama, H.P. Solid rocket propellants: Latest developments promise significant advances. Space Flight 1994, 38, 409. [Google Scholar]
- Min, B.S. Characterization of the plasticized GAP/PEG and GAP/PCL block copolyurethane binder matrices and its propellants. Propellants Explos. Pyrotech. 2008, 33, 131–138. [Google Scholar] [CrossRef]
- Min, B.S.; Park, Y.C.; Yoo, J.C. A study on the triazole crosslinked polymeric binder based on glycidyl azide polymer and dipolarophile curing agents. Propellants Explos. Pyrotech. 2012, 37, 59–68. [Google Scholar] [CrossRef]
- Ahad, E. Branched Energetic Azido Polymers. U.S. Patent No. 5,605,975, 25 February 1997. [Google Scholar]
- Huisgen, R. 1,3-Dipolar Cycloadditions. Past and Future. Angew. Chem. Int. Ed. Engl. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Kofman, T.P. 5-Amino-3-nitro-1, 2, 4-triazole and Its Derivatives. Russ. J. Org. Chem. 2002, 38, 1231–1243. [Google Scholar] [CrossRef]
- Drake, G.; Hawkins, T.; Brand, A.; Hall, L.; Mckay, M.; Vij, A.; Ismail, I. Energetic, low-melting salts of simple heterocycles. Propellants Explos. Pyrotech. 2003, 28, 174–180. [Google Scholar] [CrossRef]
- Singh, R.P.; Verma, R.D.; Meshri, D.T.; Shreeve, J.N.M. Energetic nitrogen-rich salts and ionic liquids. Angew. Chem. Int. Ed. 2006, 45, 3584–3601. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.A.; Lim, Y.G.; Lee, K.H. Synthesis of polymers including both triazole and tetrazole by click reaction. Bull. Korean Chem. Soc. 2011, 32, 547–552. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, K.T.; Jung, H.; Kim, S.H.; Park, S.; Jeon, H.B.; Kim, W. Characterization of 1, 2, 3-triazole crosslinked polymers based on azide chain-ends prepolymers and a dipolarophile curing agent as propellant binders: The effect of a plasticizer. J. Taiwan Inst. Chem. Eng. 2014, 45, 3110–3116. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, K.T.; Jang, Y.; Lee, S.; Jeon, H.B.; Paik, H.J.; Kim, W. 1, 2, 3-triazole crosslinked polymers as binders for solid rocket propellants. J. App. Polym. Sci. 2014, 131, 40594–40598. [Google Scholar] [CrossRef]
- Rothgery, E.F.; Hani, R.; Dumas, R.H.; Shen, M. Energetic Polymers and Process for Preparation Thereof. WO Patent 1993013051A1, 8 July 1993. [Google Scholar]
- Day, R.W.; Hani, R. Nitramine-Containing Homopolymers and Co-Polymers and a Process for the Preparation Thereof. U.S. Patent No. 4,916,206, 10 April 1990. [Google Scholar]
- Day, R.W.; Hani, R. Nitramine-Containing Polyether Polymers and a Process for the Preparation Thereof. U.S. Patent No. 5,319,068, 7 June 1994. [Google Scholar]
- Zenin, A.A. Study of Combustion Mechanism of Nitramine-Polymer Mixtures; Russian Academy of Sciences Moscow Inst Of Chemical Physics: Moscow, Russia, 2000. [Google Scholar]
- Klöhn, W.; Eisele, S. Nitramine solid rocket propellants with reduced signature. Propellants Explos. Pyrotech. 1987, 12, 71–77. [Google Scholar] [CrossRef]
- Zenin, A.A.; Finjakov, S.V.; Puchkov, V.M.; Ibragimov, N.G. Temperature and pressure sensitivities of burning wave parameters of nitramine-containing propellants and HMX. J. Propul. Power 1999, 15, 753–758. [Google Scholar] [CrossRef]
- Blomquist, H.R. Gas Generating Material for Vehicle Occupant Protection Device. U.S. Patent No. 6,802,533, 12 October 2004. [Google Scholar]
- Highsmith, T.K.; Sanderson, A.J.; Cannizzo, L.F.; Hajik, R.M. Polymerization Of Poly(glycidyl Nitrate) From High Purity Glycidyl Nitrate Synthesized From Glycerol. U.S. Patent No. 6,362,311, 26 March 2002. [Google Scholar]
- Dee, G.T.; Sauer, B.B. The cohesive energy density of polymers and its relationship to surface tension, bulk thermodynamic properties, and chain structure. J. Appl. Polym. Sci. 2017, 134, 44431–44435. [Google Scholar] [CrossRef]
- Colclough, M.E.; Desai, H.; Millar, R.W.; Paul, N.C.; Stewart, M.J.; Golding, P. Energetic polymers as binders in composite propellants and explosives. Polym. Adv. Tech. 1994, 5, 554–560. [Google Scholar] [CrossRef]
- Philippides, A.; Budd, P.M.; Price, C.; Cuncliffe, A.V. The nitration of polystyrene. Polymer 1993, 34, 3509–3513. [Google Scholar] [CrossRef]
- Mohan, Y.M.; Mani, Y.; Raju, K.M. Synthesis of azido polymers as potential energetic propellant binders. Des. Monomers Polym. 2006, 9, 201–236. [Google Scholar] [CrossRef]
- Shen, M.C.; Tobolsky, A.V. Rubber elasticity of preswollen polymer networks: Highly crosslinked vinyl–divinyl systems. J. Polym. Sci. A Gen. Pap. 1965, 3, 629640. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: New York, NY, USA, 1971; Volume 464. [Google Scholar]








| Step | Unit | T-1 | T-2 | T-3 | T-4 | T-5 |
|---|---|---|---|---|---|---|
| Prepolymer –OH value | - | SS-207 | DNDA_DEG | |||
| mgKOH/g | 56 | 56 | 56 | 56 | 56 | |
| mole/kg | 1 | 1 | 1 | 1 | 1 | |
| Curatives –NCO value | mole/kg | 5.45 | 5.45 | 5.45 | 5.45 | 5.45 |
| –NCO: –OH | - | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 |
| Prepolymer | g | 2.909 | 2.909 | 1.588 | 1.092 | 0.832 |
| Curatives | g | 0.587 | 0.587 | 0.321 | 0.220 | 0.168 |
| Catalyst | g | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 |
| Plasticizer | g | - | - | 1.588 | 2.184 | 2.496 |
| Title | T-1 | T-2 | T-3 | T-4 | T-5 |
|---|---|---|---|---|---|
| Tg (°C) | −40 | 5.5 | −26 | −44 | −49 |
| Title | Unit | T-1 | T-2 | T-3 | T-4 | T-5 |
|---|---|---|---|---|---|---|
| Elongation at break | % | 96 | 517 | 219 | 211 | 157 |
| Tensile strength | bar | 7.4 | 15.3 | 7.6 | 2.9 | 2.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, K.; Mun, H.; Jung, J.Y.; Cho, H.L.; Kim, S.J.; Min, B.S.; Jeon, H.B.; Kim, W. Nitramine-Group-Containing Energetic Prepolymer: Synthesis, and Its Properties as a Binder for Propellant. Polymers 2019, 11, 1966. https://doi.org/10.3390/polym11121966
Hwang K, Mun H, Jung JY, Cho HL, Kim SJ, Min BS, Jeon HB, Kim W. Nitramine-Group-Containing Energetic Prepolymer: Synthesis, and Its Properties as a Binder for Propellant. Polymers. 2019; 11(12):1966. https://doi.org/10.3390/polym11121966
Chicago/Turabian StyleHwang, Kiwon, Hyunsung Mun, Jin Young Jung, Hye Lim Cho, Sung June Kim, Byoung Sun Min, Heung Bae Jeon, and Wonho Kim. 2019. "Nitramine-Group-Containing Energetic Prepolymer: Synthesis, and Its Properties as a Binder for Propellant" Polymers 11, no. 12: 1966. https://doi.org/10.3390/polym11121966





