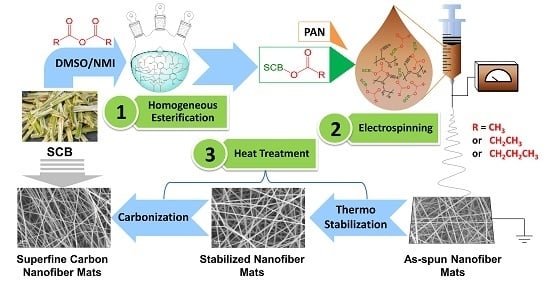
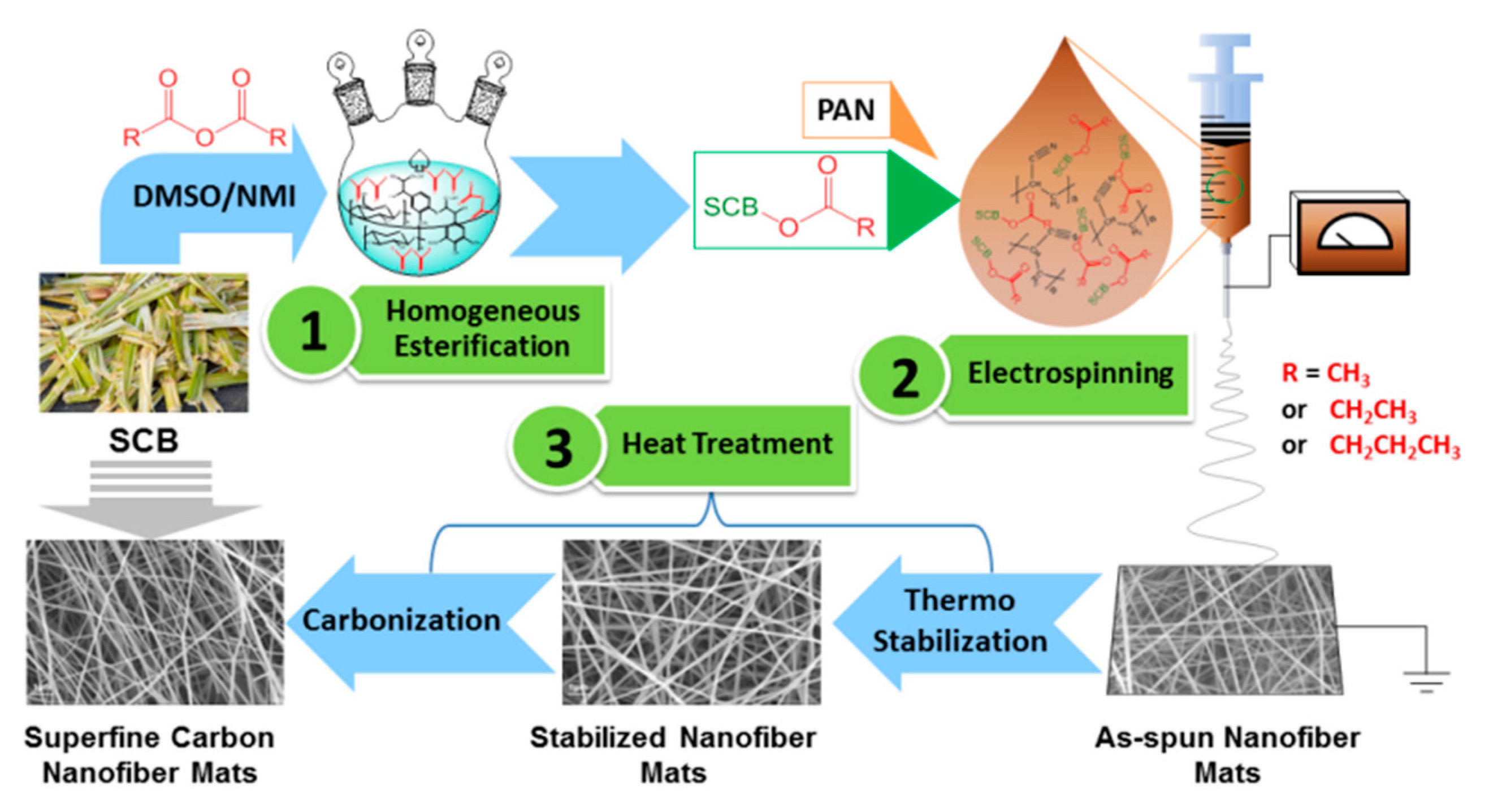
A Feasible Way to Produce Carbon Nanofiber by Electrospinning from Sugarcane Bagasse
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Homogeneous Esterification of SCB
2.3. Preparation of SCB-A/PAN Blends Electrospun Nanofiber Mats (SCB-A/PAN-SNFs)
2.4. Heat Treatment of the Electrospun Nanofiber Mats
2.5. Characterization
3. Results and Discussion
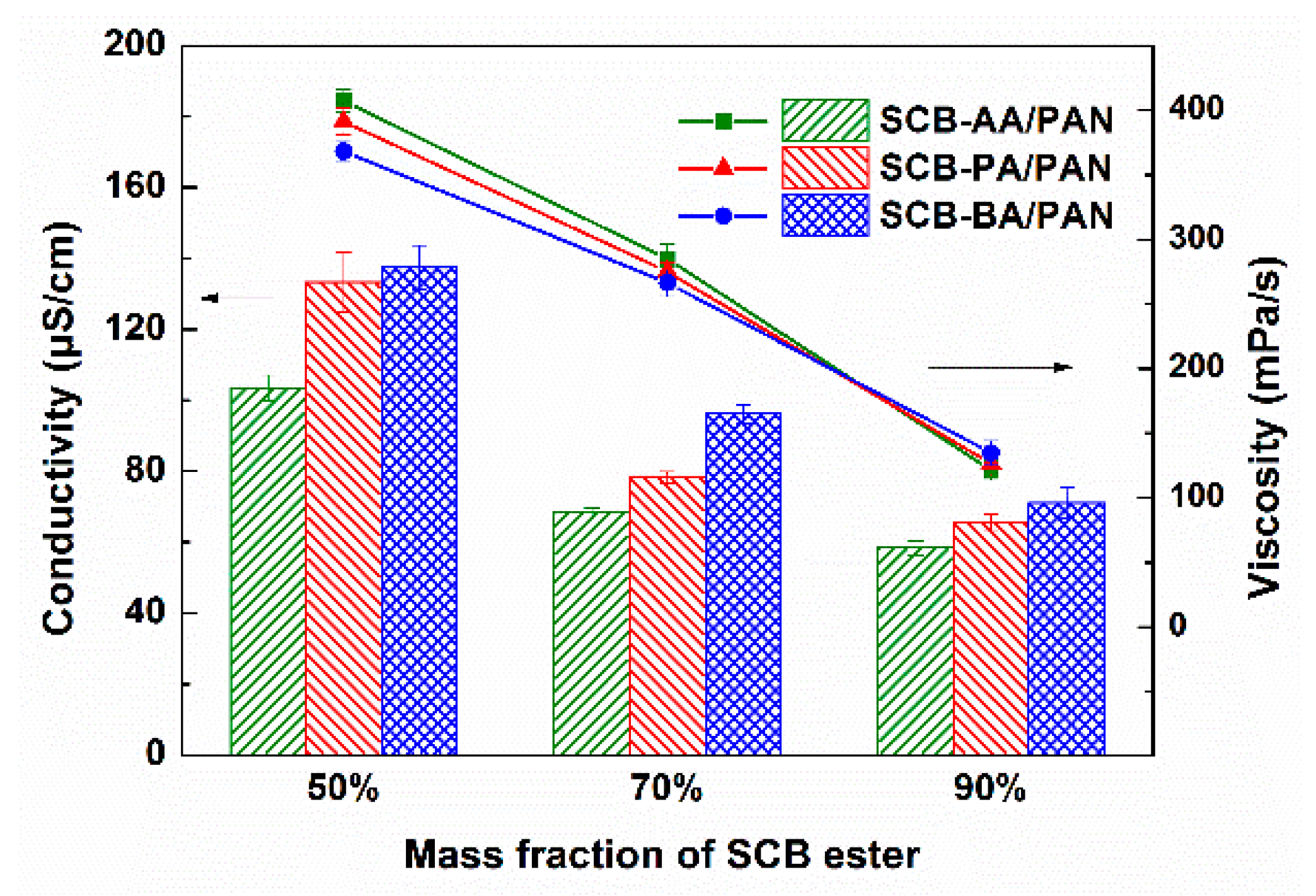
3.1. Spinnability and Rheology of SCB-A/PAN Blends Solutions
3.2. Pyrolysis Behavior of SCB-A and the Corresponding Nanofiber Mats
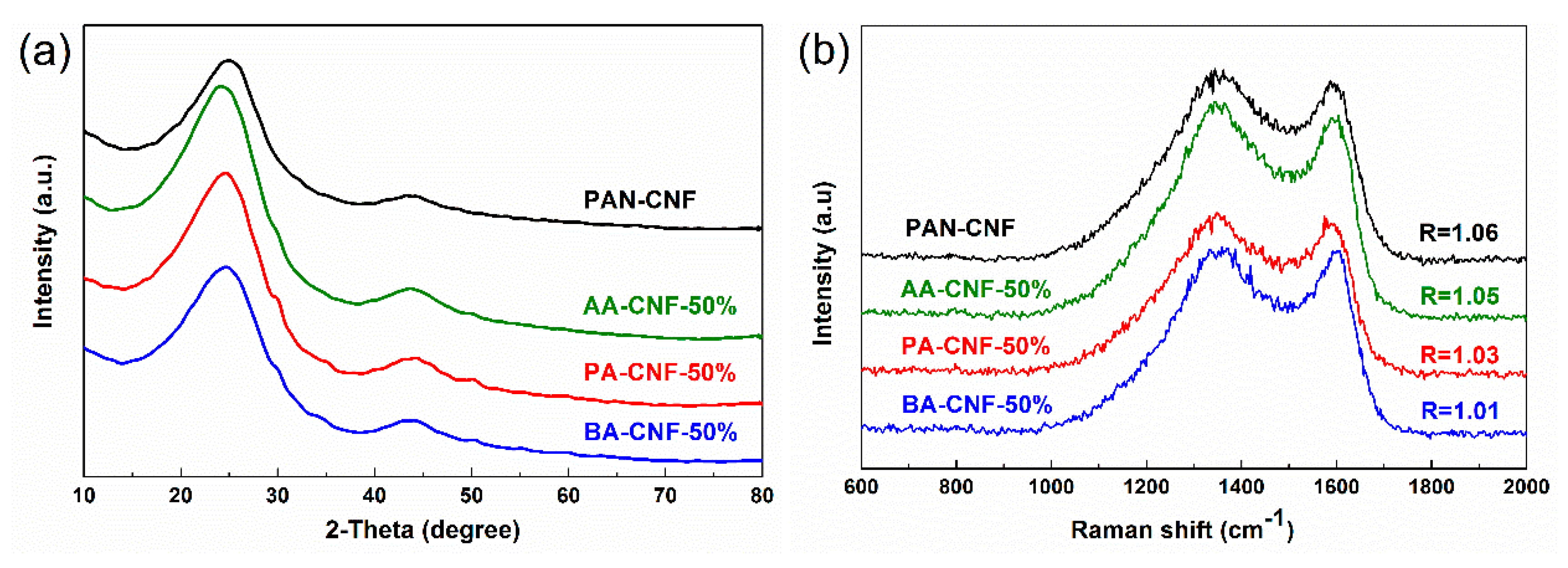
3.3. Thermo Stabilization and Carbonization of SCB-A/PAN-SNFs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SCB | Sugarcane bagasse |
| SCB-A | Esterified products derived from homogeneous esterification of SCB with acid anhydride |
| PAN | Polyacrylonitrile |
| AA | Acetic anhydride |
| PA | Propionic anhydride |
| BA | Butyric anhydride |
| SCB-AA | Esterified products derived from homogeneous esterification of SCB with acetic anhydride |
| SCB-PA | Esterified products derived from homogeneous esterification of SCB with propionic anhydride |
| SCB-BA | Esterified products derived from homogeneous esterification of SCB with butyric anhydride |
| SNF | Electrospun nanofiber mats |
| TNF | Stabilized electrospun nanofiber mats |
| CNF | Carbonized electrospun nanofiber mats |
| DMF | N,N-dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| NMI | N-methylimidazole |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| FT-IR | Fourier transform infrared |
| NMR | Nuclear magnetic resonance |
| SEM | Scanning electron microscope |
| TGA/DTG | Thermogravimetric and derivative thermogravimetry analyses |
References
- Fang, W.; Yang, S.; Yuan, T.Q.; Charlton, A.; Sun, R.C. Effects of Various Surfactants on Alkali Lignin Electrospinning Ability and Spun Fibers. Ind. Eng. Chem. Res. 2017, 56, 9551–9559. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. Engl. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Yang, C.M.; Kim, B.H. Incorporation of MnO2 into boron-enriched electrospun carbon nanofiber for electrochemical supercapacitors. J. Alloy. Compd. 2019, 780, 428–434. [Google Scholar] [CrossRef]
- Celebioglu, A.; Topuz, F.; Yildiz, Z.I.; Uyar, T. One-step green synthesis of antibacterial silver nanoparticles embedded in electrospun cyclodextrin nanofibers. Carbohydr. Polym. 2019, 207, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Wang, X.Q.; Dou, L.Y.; Yu, J.Y.; Ding, B. Ultralight and fire-resistant ceramic nanofibrous aerogels with temperature-invariant superelasticity. Sci. Adv. 2018, 4, 8925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ogale, A.A. Effect of temperature and concentration of acetylated-lignin solutions on dry-spinning of carbon fiber precursors. J. Appl. Polym. Sci. 2016, 133, 43663. [Google Scholar] [CrossRef]
- Liu, W.; Chen, W.; Hou, Q.X.; Zhang, J.P.; Wang, B. Surface lignin change pertaining to the integrated process of dilute acid pre-extraction and mechanical refining of poplar wood chips and its impact on enzymatic hydrolysis. Bioresour. Technol. 2017, 228, 125–132. [Google Scholar] [CrossRef]
- Fang, W.; Yang, S.; Wang, X.L.; Yuan, T.Q.; Sun, R.C. Manufacture and application of lignin-based carbon fibers (LCFs) and lignin-based carbon nanofibers (LCNFs). Green Chem. 2017, 19, 1794–1827. [Google Scholar] [CrossRef]
- Li, S.; Masoud, T.A.; Questell-Santiago, Y.M.; Florent, H.; Li, Y.; Kim, H.; Meilan, R.; Chapple, C.; Ralph, J.; Luterbacher, J.S. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 2016, 354, 329–333. [Google Scholar]
- Tao, J.; Xiong, J.Q.; Jiao, C.L.; Zhang, D.; Lin, H.; Chen, Y.Y. Cellulose/polymer/silica composite cotton fiber based on a hyperbranch-mesostructure system as versatile adsorbent for water treatment. Carbohydr. Polym. 2017, 166, 271–280. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.J.; Li, D.; Cheng, Y.L.; Adhikari, B. Preparation and characterization of cellulose nanofibers from de-pectinated sugar beet pulp. Carbohydr. Polym. 2014, 102, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.D.; Peng, X.W.; Zhong, L.X.; Wu, L.; Cao, X.F.; Sun, R.C. Electrospun cellulose acetate supported Ag@AgCl composites with facet-dependent photocatalytic properties on degradation of organic dyes under visible-light irradiation. Carbohydr. Polym. 2016, 136, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Qin, Z.Y.; Hu, S.; Dong, S.; Ren, Z.C.; Yu, H.Y. Achieving Long-Term Sustained Drug Delivery for Electrospun Biopolyester Nanofibrous Membranes by Introducing Cellulose Nanocrystals. ACS Biomater. Sci. Eng. 2017, 3, 1666–1676. [Google Scholar] [CrossRef]
- Naeem, M.A.; Alfred, M.; Lv, P.; Zhou, H.; Wei, Q. Three-dimensional bacterial cellulose-electrospun membrane hybrid structures fabricated through in-situ self-assembly. Cellulose 2018, 25, 6823–6830. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Sen, S.; Patil, S.V.; Argyropoulos, D.S. Toward Carbon Fibers from Single Component Kraft Lignin Systems: Optimization of Chain Extension Chemistry. ACS Sustain. Chem. Eng. 2016, 4, 5230–5237. [Google Scholar] [CrossRef]
- Li, Q.; Serem, W.K.; Dai, W.; Yue, Y.; Naik, M.T.; Xie, S.; Karki, P.; Liu, L.; Sue, H.J.; Liang, H.; et al. Molecular weight and uniformity define the mechanical performance of lignin-based carbon fiber. J. Mater. Chem. A 2017, 5, 12740–12746. [Google Scholar] [CrossRef]
- Zhang, A.P.; Liu, C.F.; Sun, R.C.; Xie, J.; Chen, X.Y. Homogeneous acylation of eucalyptus wood at room temperature in dimethyl sulfoxide/N-methylimidazole. Bioresour. Technol. 2012, 125, 328–331. [Google Scholar] [CrossRef]
- Wang, H.H.; Chen, W.; Zhang, X.Q.; Liu, C.F.; Sun, R.C. Esterification Mechanism of Bagasse Modified with Glutaric Anhydride in 1-Allyl-3-methylimidazolium Chloride. Materials 2017, 10, 996. [Google Scholar] [CrossRef]
- Wang, H.H.; Zhang, X.Q.; Wei, Y.; Zhang, A.P.; Liu, C.F.; Sun, R.C. Homogeneous Esterification Mechanism of Bagasse Modified with Phthalic Anhydride in Ionic Liquid, Part 3: Structural Transformation of Lignins. BioResources 2017, 12, 4062–4077. [Google Scholar] [CrossRef]
- Chen, M.J.; Chen, C.Y.; Liu, C.F.; Sun, R.C. Homogeneous modification of sugarcane bagasse with maleic anhydride in 1-butyl-3-methylimidazolium chloride without any catalysts. Ind. Crop. Prod. 2013, 46, 380–385. [Google Scholar] [CrossRef]
- Xie, H.B.; King, A.; Kilpelainen, I.; Granstrom, M.; Argyropoulos, D.S. Thorough Chemical Modification of Wood-Based Lignocellulosic Materials in Ionic Liquids. Biomacromolecules 2007, 8, 3740–3748. [Google Scholar] [CrossRef] [PubMed]
- Keplinger, T.; Cabane, E.; Chanana, M.; Hass, P.; Merk, V.; Gierlinger, N.; Burgert, I. A versatile strategy for grafting polymers to wood cell walls. Acta Biomater. 2015, 11, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Jogunola, O.; Eta, V.; Hedenström, M.; Sundman, O.; Salmi, T.; Mikkola, J.-P. Ionic liquid mediated technology for synthesis of cellulose acetates using different co-solvents. Carbohydr. Polym. 2016, 135, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.H.; Zhang, J.M.; Zhan, M.S.; Wu, J.; Zhang, J.; He, J.S. Highly efficient propionylation and butyralation of cellulose in an ionic liquid catalyzed by 4-dimethylminopyridine. Carbohydr. Polym. 2013, 92, 307–311. [Google Scholar] [CrossRef]
- Thenmozhi, S.; Dharmaraj, N.; Kadirvelu, K.; Kim, H.Y. Electrospun nanofibers: New generation materials for advanced applications. Mater. Sci. Eng. B 2017, 217, 36–48. [Google Scholar] [CrossRef]
- Oroumei, A.; Fox, B.; Naebe, M. Thermal and Rheological Characteristics of Biobased Carbon Fiber Precursor Derived from Low Molecular Weight Organosolv Lignin. ACS Sustain. Chem. Eng. 2015, 3, 758–769. [Google Scholar] [CrossRef]
- Li, Z.Y.; Wang, C. Effects of Working Parameters on Electrospinning. In One-Dimensional Nanostructures: Electrospinning Technique and Unique Nanofibers; Springer: Berlin/Heidelberg, Germany, 2013; pp. 15–28. [Google Scholar]
- Xu, Y.J.; Zou, L.M.; Lu, H.W.; Kang, T.J. Effect of different solvent systems on PHBV/PEO electrospun fibers. RSC Adv. 2017, 7, 4000–4010. [Google Scholar] [CrossRef]
- Hohman, M.; Shin, M.; Rutledge, G.P.; Brenner, M. Electrospinning and Electrically Forced Jets. I. Stability Theory. Phys. Fluids 2001, 13, 2201–2220. [Google Scholar] [CrossRef]
- Wang, H.H.; Wen, X.X.; Zhang, X.Q.; Liu, C.F. Acetylation of Microcrystalline Cellulose by Transesterification in AmimCl/DMSO Cosolvent System. Molecules 2017, 22, 1419. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, A.P.; Liu, C.F.; Sun, R.C. Modification of sugarcane bagasse with acetic anhydride and butyric anhydride in ionic liquid 1-butyl-3-methylimidazolium chloride. BioResources 2012, 7, 3476–3487. [Google Scholar]
- Zhang, Q.; Zhang, X.Q.; Zhu, Z.Y.; Zhang, A.P.; Zhang, C.H.; Wang, X.Y.; Liu, C.F. Mechanocatalytic Solvent-Free Esterification of Sugarcane Bagasse. Polymers 2018, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S.; Lei, H.W.; Zhu, L.; Zhu, X.L.; Qian, M.; Yadavalli, G.; Wu, J.; Chen, S.L. Thermal behavior and kinetic study for catalytic co-pyrolysis of biomass with plastics. Bioresour. Technol. 2016, 220, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.P.; Liang, J.H.; Morgan, H.M.; Liu, Y.Y.; Mao, H.P.; Bu, Q. Thermal behavior and kinetic study for co-pyrolysis of lignocellulosic biomass with polyethylene over Cobalt modified ZSM-5 catalyst by thermogravimetric analysis. Bioresour. Technol. 2018, 247, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.I.; Lee, J.N.; Song, J.C.; Kang, P.H.; Park, J.K.; Lee, Y.M. Fabrication of polyacrylonitrile/lignin-based carbon nanofibers for high-power lithium ion battery anodes. J. Solid State Electrochem. 2013, 17, 2471–2475. [Google Scholar] [CrossRef]
- Jin, J.; Yu, B.J.; Shi, Z.Q.; Wang, C.Y.; Chong, C.B. Lignin-based electrospun carbon nanofibrous webs as free-standing and binder-free electrodes for sodium ion batteries. J. Power Sources 2014, 272, 800–807. [Google Scholar] [CrossRef]
- Tao, L.; Huang, Y.B.; Yang, X.Q.; Zheng, Y.W.; Liu, C.; Di, M.W.; Zheng, Z.F. Flexible anode materials for lithium-ion batteries derived from waste biomass-based carbon nanofibers: I. Effect of carbonization temperature. RSC Adv. 2018, 8, 7102–7109. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.Y.; Guo, Y.; Yu, B.J.; Zhang, J.; Wang, C.Y. Biotechnology humic acids-based electrospun carbon nanofibers as cost-efficient electrodes for lithium-ion batteries. Electrochim. Acta 2016, 203, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.C.; Pan, F.S.; Li, W.H.; Jiang, Y.; Zhong, X.W.; Yu, Y. Free-standing porous carbon nanofibers-sulfur composite for flexible Li-S battery cathode. Nanoscale 2014, 6, 9579–9587. [Google Scholar] [CrossRef]
- Chen, H.; Liu, D.; Shen, Z.H.; Bao, B.F.; Zhao, S.Y.; Wu, L.M. Functional Biomass Carbons with Hierarchical Porous Structure for Supercapacitor Electrode Materials. Electrochim. Acta 2015, 180, 241–251. [Google Scholar] [CrossRef]
- Hassan, F.M.; Chabot, V.; Li, J.; Kim, B.K.; Ricardez-Sandoval, L.; Yu, A. Pyrrolic-structure enriched nitrogen doped graphene for highly efficient next generation supercapacitors. J. Mater. Chem. A 2013, 1, 2904–2912. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Srivastava, A.; Gowda, S.R.; Gullapalli, H.; Dubey, M.; Ajayan, P.M. Synthesis Of Nitrogen-Doped Graphene Films For Lithium Battery Application. ACS Nano 2010, 4, 6337–6342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, X.H.; Wang, T.; Liu, Z.L.; Zheng, X.Y.; Zheng, J.; Li, X.G. Ultrafine Sn nanocrystals in a hierarchically porous N-doped carbon for lithium ion batteries. Nano Res. 2017, 10, 1950–1958. [Google Scholar] [CrossRef]
- Wu, Z.S.; Ren, W.C.; Xu, L.; Li, F.; Cheng, H.M. Doped Graphene Sheets As Anode Materials with Superhigh Rate and Large Capacity for Lithium Ion Batteries. ACS Nano 2011, 5, 5463–5471. [Google Scholar] [CrossRef] [PubMed]
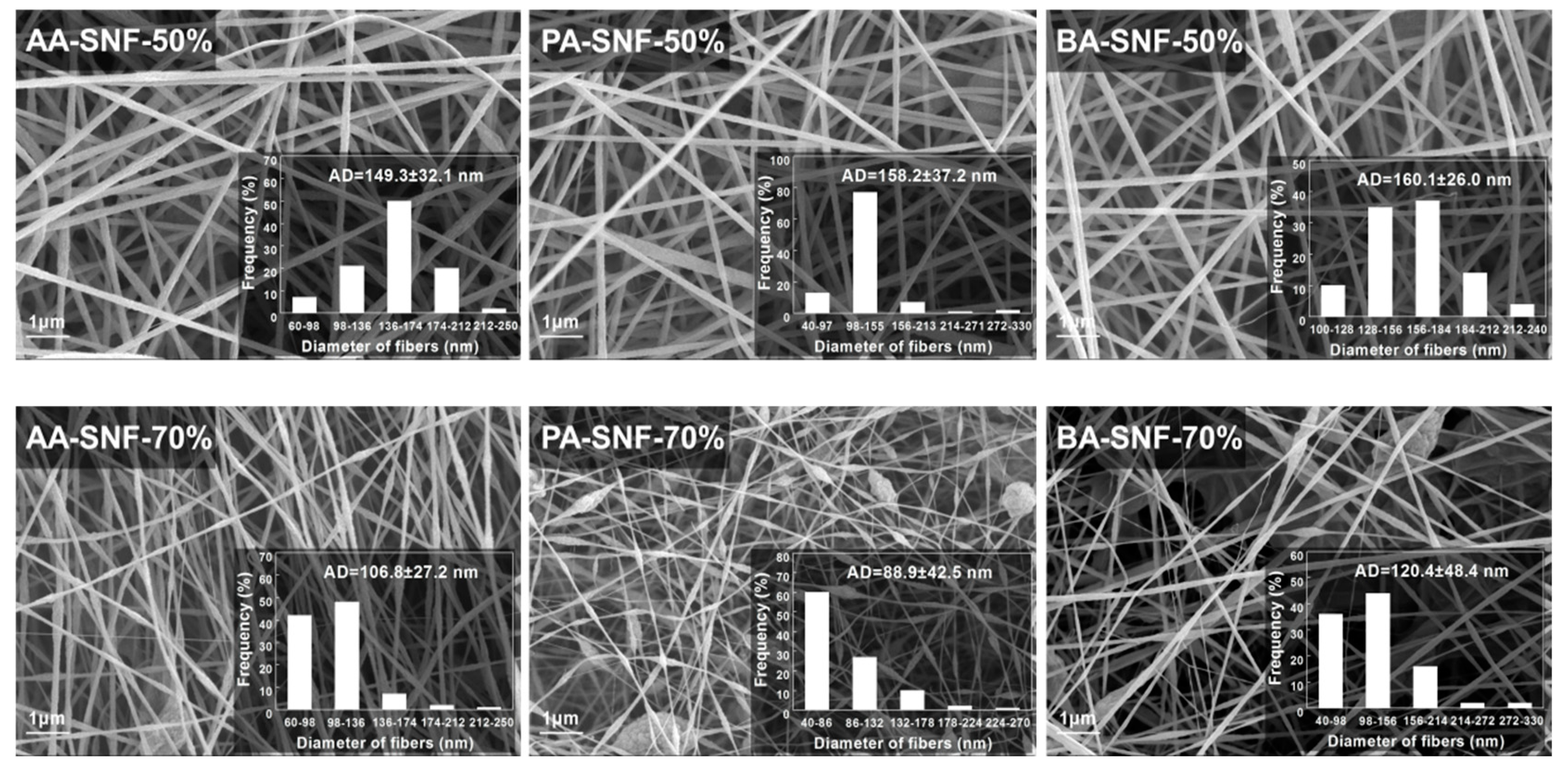


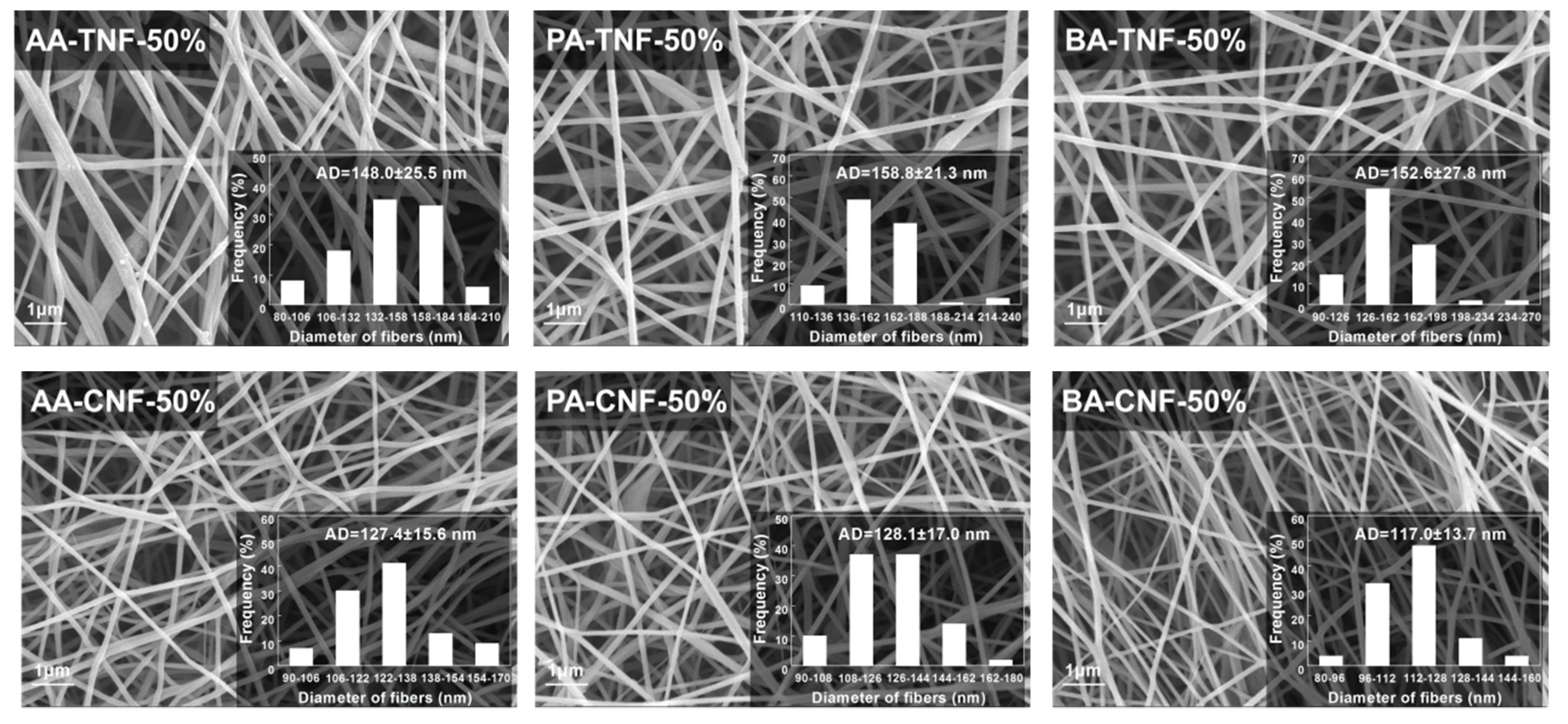

| Sample | Elemental Analysis | XPS Analysis | EC (S/cm) 2 | AD (nm) 3 | SBET (m2/g) 4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| C (%) | N (%) | O (%) 1 | C (%) | N (%) | O (%) | ||||
| PAN-CNF | 74.2 | 15.4 | 8.70 | 87.8 | 3.80 | 8.42 | 8.97 × 10−5 | 254.9 ± 37.7 | 12.9 |
| AA-CNF-50% | 75.7 | 11.1 | 11.7 | 86.9 | 8.22 | 4.86 | 2.18 × 10−4 | 127.4 ± 15.6 | 36.8 |
| PA-CNF-50% | 74.5 | 11.8 | 13.2 | 86.0 | 9.96 | 4.35 | 2.49 × 10−4 | 128.1 ± 17.0 | 35.8 |
| BA-CNF-50% | 75.4 | 11.0 | 12.5 | 86.7 | 8.80 | 4.47 | 3.71 × 10−4 | 117.0 ± 13.7 | 34.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Meng, X.-T.; Wang, H.-H.; Zhang, X.-Q.; Wei, Y.; Li, Z.-Y.; Li, D.; Zhang, A.-P.; Liu, C.-F. A Feasible Way to Produce Carbon Nanofiber by Electrospinning from Sugarcane Bagasse. Polymers 2019, 11, 1968. https://doi.org/10.3390/polym11121968
Chen W, Meng X-T, Wang H-H, Zhang X-Q, Wei Y, Li Z-Y, Li D, Zhang A-P, Liu C-F. A Feasible Way to Produce Carbon Nanofiber by Electrospinning from Sugarcane Bagasse. Polymers. 2019; 11(12):1968. https://doi.org/10.3390/polym11121968
Chicago/Turabian StyleChen, Wei, Xin-Tong Meng, Hui-Hui Wang, Xue-Qin Zhang, Yi Wei, Zeng-Yong Li, Di Li, Ai-Ping Zhang, and Chuan-Fu Liu. 2019. "A Feasible Way to Produce Carbon Nanofiber by Electrospinning from Sugarcane Bagasse" Polymers 11, no. 12: 1968. https://doi.org/10.3390/polym11121968
APA StyleChen, W., Meng, X.-T., Wang, H.-H., Zhang, X.-Q., Wei, Y., Li, Z.-Y., Li, D., Zhang, A.-P., & Liu, C.-F. (2019). A Feasible Way to Produce Carbon Nanofiber by Electrospinning from Sugarcane Bagasse. Polymers, 11(12), 1968. https://doi.org/10.3390/polym11121968