Isotactic Polybutene-1/Bamboo Powder Composites with Excellent Properties at Initial Stage of Molding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of KBP
2.3. KBP Characterization
2.3.1. Infiltration Test
2.3.2. FTIR Analysis
2.4. Preparation of iPB/KBP Composites
2.5. Mechanical Properties of iPB/KBP Composites
2.6. Thermal Property of the iPB/KBP Composites
2.7. Crystallization Behavior of the iPB/KBP Composites
2.8. Scanning Electron Microscopy of the iPB/KBP Composites
3. Results and Discussion
3.1. Infiltration Test of BP and KBP
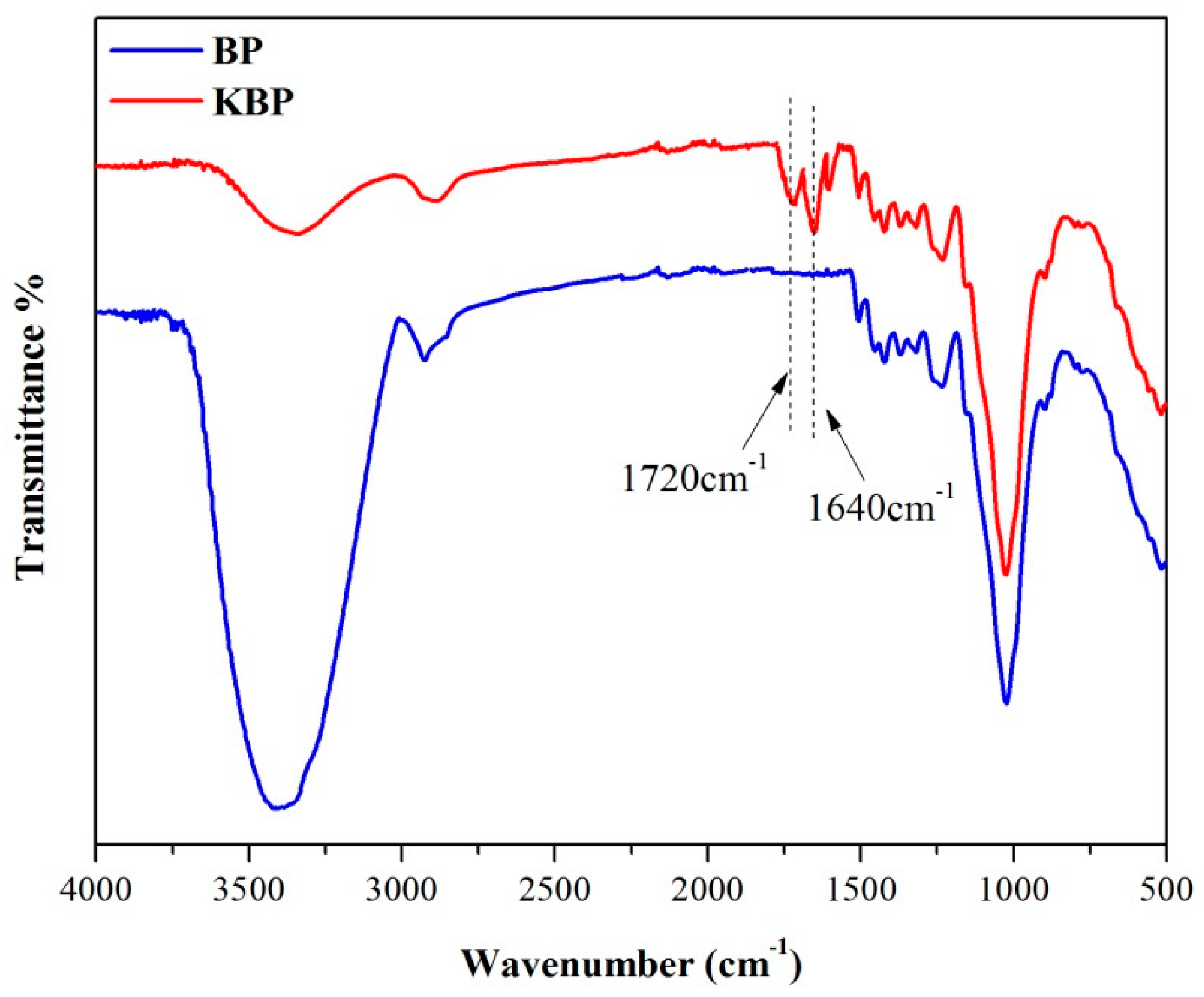
3.2. FTIR Spectra of BP and KBP
3.3. Mechanical Properties of iPB/KBP Composites
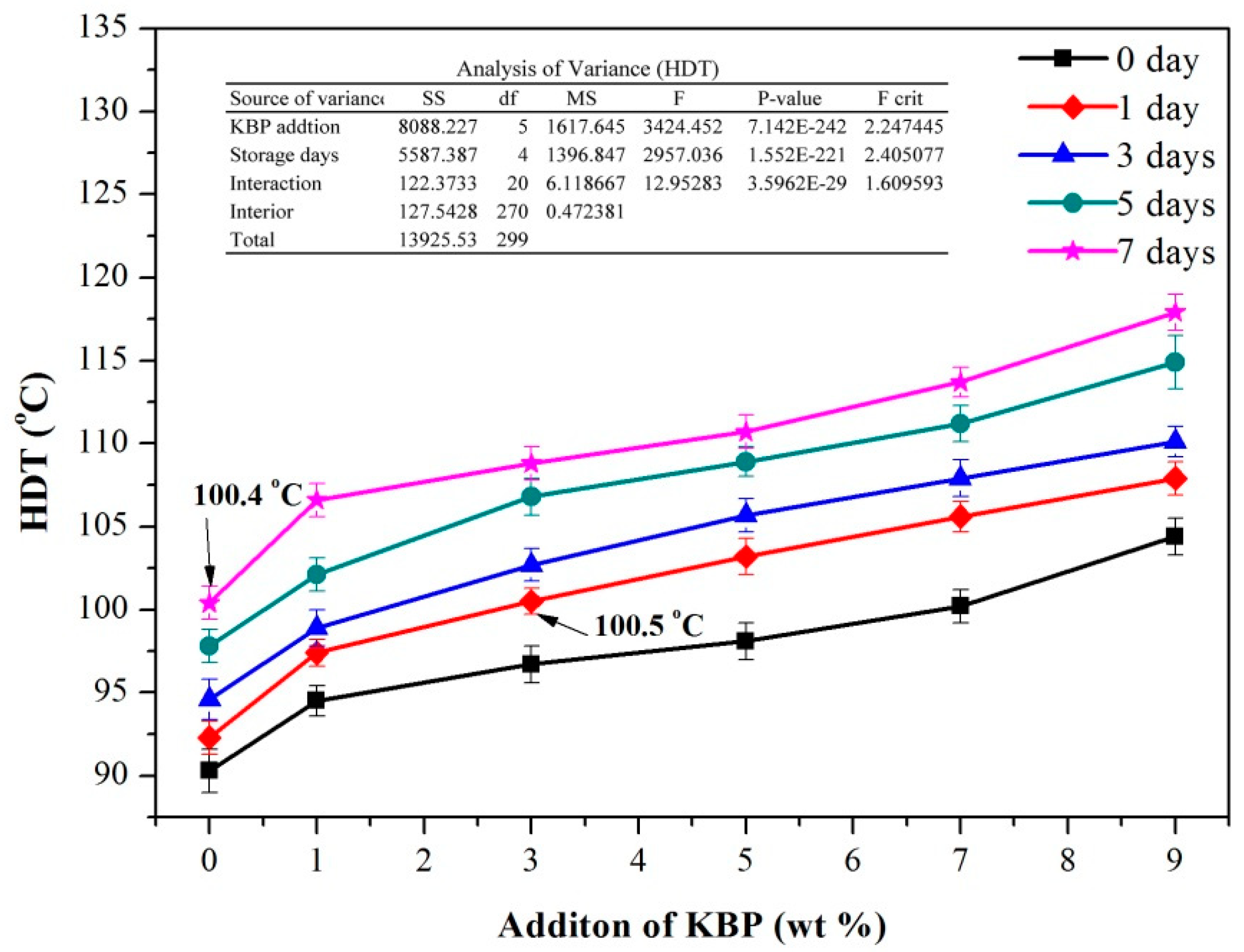
3.4. HDT of iPB/KBP Composites
3.5. Crystallization Behavior of iPB/KBP Composites
3.6. SEM of iPB/KBP Composites
4. Conclusions
Author Contributions
Fundings
Conflicts of Interest
References
- Su, F.; Li, X.; Zhou, W.; Wei, C.; Li, H.; Cong, Y.; Hong, Z.; Qi, Z.; Li, L. Accelerating crystal–crystal transition in poly(1-butene) with two-step crystallization: An in-situ microscopic infrared imaging and microbeam X-ray diffraction study. Polymer 2013, 54, 3408–3416. [Google Scholar] [CrossRef]
- He, A.; Xu, C.; Shao, H.; Yao, W.; Huang, B. Effect of molecular weight on the polymorphic transformation of isotactic poly(1-butene). Polym. Degrad. Stabil. 2010, 95, 1443–1448. [Google Scholar] [CrossRef]
- Afrifah, K.A.; Hickok, R.A.; Matuana, L.M. Polybutene as a matrix for wood plastic composites. Compos. Sci. Technol. 2010, 70, 167–172. [Google Scholar] [CrossRef]
- Fowler, J.N.; Chapman, B.R.; Green, D.L. Impact of plasticizers and tackifiers on the crystallization of isotactic poly(1-butene). Eur. Polym. J. 2010, 46, 568–577. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Vollaro, P.; Resconi, L.; Guidotti, S.; Camurati, I. Crystallization Behavior of Propylene? Butene Copolymers: The Trigonal Form of Isotactic. Polypropylene and Form I of Isotactic Poly(1-butene). Macromolecules 2011, 44, 540–549. [Google Scholar] [CrossRef]
- Maruyama, M.; Sakamoto, Y.; Nozaki, K.; Yamamoto, T.; Kajioka, H.; Toda, A.; Yamada, K. Kinetic study of the II–I phase transition of isotactic polybutene-1. Polymer 2010, 51, 5532–5538. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Androsch, R.; Righetti, M.C. The irreversible Form II to Form I transformation in random butene-1/ethylene copolymers. Eur. Polym. J. 2015, 67, 264–273. [Google Scholar] [CrossRef]
- Ji, Y.; Su, F.; Cui, K.; Huang, N.; Qi, Z.; Li, L. Mixing Assisted Direct Formation of Isotactic Poly(1-butene) Form I‘ Crystals from Blend Melt of Isotactic Poly(1-butene)/Polypropylene. Macromolecules 2016, 49, 1761–1769. [Google Scholar] [CrossRef]
- Su, F.; Li, X.; Zhou, W.; Zhu, S.; Ji, Y.; Wang, Z.; Qi, Z.; Li, L. Direct Formation of Isotactic Poly(1-butene) Form I Crystal from Memorized Ordered Melt. Macromolecules 2013, 46, 7399–7405. [Google Scholar] [CrossRef]
- Lei, L.; Liu, T.; Zhao, L. Direct melt-crystallization of isotactic poly-1-butene with form I′ using high-pressure CO2. Polymer 2011, 52, 5659–5668. [Google Scholar] [CrossRef]
- Xing-Xing, Z.; Yan-Kai, L.; Zhao-Yan, S. Acceleration of crystal transformation from crystal form II to form I in Polybutene-1 induced by nanoparticles. Polymer 2018, 150, 119–129. [Google Scholar]
- Zhou, X.X.; Lin, Q.J.; Chen, L.H. Mechanical Properties and Rheological Behavior of Injection Moulded Foaming Bamboo Powder–Polypropylene Composites. Adv. Mater. Res. 2011, 287–290, 1980–1986. [Google Scholar] [CrossRef]
- Noor Leha, A.R.; Nordin, N.A. Effect of Filler Compositions on the Mechanical Properties of Bamboo Filled Polyester Composite. Adv. Mater. Res. 2014, 879, 90–95. [Google Scholar] [CrossRef]
- Xue, H.; Chen, Q.; Lin, J. Preparation and characterization of bamboo fibers coated with urushiol-ferric and its composite with polypropylene. J. Appl. Polym. Sci. 2012, 125, 439–447. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Y.; Zhu, S.; Lopez, C.A.F.; Sheng, K. Surface modification of bamboo-char and its reinforcement in PLA biocomposites. Polym. Compos. 2018, 39, E633–E639. [Google Scholar] [CrossRef]
- Colom, X.; Carrillo, F.; Caavate, J. Composites reinforced with reused tyres: Surface oxidant treatment to improve the interfacial compatibility. Compos. A Appl. Sci. Manuf. 2007, 38, 44–50. [Google Scholar] [CrossRef]
- Li, W.Y.; Cheng, H.T.; Zhang, S.B. Methods of Improving the Interfacial Compatibility of the Bamboo Fiber/Thermoplastic. Adv. Mater. Res. 2013, 602–604, 1130–1134. [Google Scholar] [CrossRef]
- Wang, Y.N.; Weng, Y.X.; Wang, L. Characterization of interfacial compatibility of polylactic acid and bamboo flour (PLA/BF) in biocomposites. Polym. Test. 2014, 36, 119–125. [Google Scholar] [CrossRef]
- Li, J. Effect of Silane Coupling Agent on the Tensile Properties of Carbon Fiber-Reinforced Thermoplastic Polyimide Composites. J. Macromol. Sci. D 2010, 49, 337–340. [Google Scholar] [CrossRef]
- Xin, F.; Li, L. The role of a silane coupling agent in carbon nanotube/polypropylene composites. J. Compos. Mater. 2012, 46, 3267–3275. [Google Scholar] [CrossRef]
- Park, S.J.; Jin, J.S. Effect of silane coupling agent on mechanical interfacial properties of glass fiber-reinforced unsaturated polyester composites. J. Polym. Sci. Polym. Phys. 2010, 41, 55–62. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.S.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. A Appl. Sci. Manuf. 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Kim, J.T.; Lee, D.Y.; Oh, T.S.; Lee, D.H. Characteristics of nitrile–butadiene rubber layered silicate nanocomposites with silane coupling agent. J. Appl. Polym. Sci. 2010, 89, 2633–2640. [Google Scholar] [CrossRef]
- Salon, M.C.B.; Gerbaud, G.; Abdelmouleh, M.; Bruzzese, C.C.; Boufi, S.; Belgacem, M.N. Studies of interactions between silane coupling agents and cellulose fibers with liquid and solid-state NMR. Magn. Reson. Chem. 2010, 45, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Gironès, J.; Méndez, J.A.; Boufi, S.; Vilaseca, F.; Mutjé, P. Effect of silane coupling agents on the properties of pine fibers/polypropylene composites. J. Appl. Polym. Sci. 2010, 103, 3706–3717. [Google Scholar] [CrossRef]
- Nakatani, H.; Iwakura, K.; Miyazaki, K.; Okazaki, N.; Terano, M. Effect of chemical structure of silane coupling agent on interface adhesion properties of syndiotactic polypropylene/cellulose composite. J. Appl. Polym. Sci. 2010, 119, 1732–1741. [Google Scholar] [CrossRef]
- Li, X.; Tabil, L.G.; Panigrahi, S. Chemical Treatments of Natural Fiber for Use in Natural Fiber-Reinforced Composites: A Review. J. Polym. Environ. 2007, 15, 25–33. [Google Scholar] [CrossRef]
- He, L.; Li, W.; Chen, D.; Zhou, D.; Gang, L.; Yuan, J. Effects of amino silicone oil modification on properties of ramie fiber and ramie fiber/polypropylene composites. Mater. Des. 2015, 77, 142–148. [Google Scholar] [CrossRef]
- Qiu, W.; Endo, T.; Hirotsu, T. Interfacial interaction, morphology, and tensile properties of a composite of highly crystalline cellulose and maleated polypropylene. J. Appl. Polym. Sci. 2006, 102, 3830–3841. [Google Scholar] [CrossRef]
- Wcj, Z.; Westzaan, C.; Huetink, J.; Gaymans, R.J. Toughening of polypropylene with calcium carbonate particles. Polymer 2003, 44, 261–275. [Google Scholar]
- Wang, B.; Yang, D.; Zhang, H.R.; Huang, C.; Xiong, L.; Luo, J.; Chen, X.D. Preparation of Esterified Bacterial Cellulose for Improved Mechanical Properties and the Microstructure of Isotactic Polypropylene/Bacterial Cellulose Composites. Polymers 2016, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Shieh, Y.T.; Lee, M.S.; Chen, S.A. Crystallization behavior, crystal transformation, and morphology of polypropylene/polybutene-1 blends. Polymer 2001, 42, 4439–4448. [Google Scholar] [CrossRef]









| Sample | iPB (g) | KBP (g) |
|---|---|---|
| iPB | 1000 | - |
| iPB/KBP1 | 1000 | 10 |
| iPB/KBP3 | 1000 | 30 |
| iPB/KBP5 | 1000 | 50 |
| iPB/KBP7 | 1000 | 70 |
| iPB/KBP9 | 1000 | 90 |
| Sample | Tend (°C) | Tp (°C) | ΔH (J/g) |
|---|---|---|---|
| iPB | 58.1 | 75.1 | 33.1 |
| iPB/KBP1 | 62.3 | 75.1 | 33.2 |
| iPB/KBP3 | 67.6 | 77.2 | 33.3 |
| iPB/KBP5 | 66.8 | 77.1 | 33.2 |
| iPB/KBP7 | 66.4 | 76.4 | 33.1 |
| iPB/KBP9 | 66.4 | 76.2 | 33.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Lin, F.-h.; Zhao, Y.-y.; Li, X.-y.; Liu, Y.-c.; Li, J.-b.; Han, X.-J.; Liu, S.-x.; Ji, X.-r.; Luo, J.; et al. Isotactic Polybutene-1/Bamboo Powder Composites with Excellent Properties at Initial Stage of Molding. Polymers 2019, 11, 1981. https://doi.org/10.3390/polym11121981
Wang B, Lin F-h, Zhao Y-y, Li X-y, Liu Y-c, Li J-b, Han X-J, Liu S-x, Ji X-r, Luo J, et al. Isotactic Polybutene-1/Bamboo Powder Composites with Excellent Properties at Initial Stage of Molding. Polymers. 2019; 11(12):1981. https://doi.org/10.3390/polym11121981
Chicago/Turabian StyleWang, Bo, Fu-hua Lin, Yu-ying Zhao, Xiang-yang Li, Yan-chao Liu, Jing-bo Li, Xiao-Jing Han, Si-xiao Liu, Xu-ran Ji, Jun Luo, and et al. 2019. "Isotactic Polybutene-1/Bamboo Powder Composites with Excellent Properties at Initial Stage of Molding" Polymers 11, no. 12: 1981. https://doi.org/10.3390/polym11121981
APA StyleWang, B., Lin, F.-h., Zhao, Y.-y., Li, X.-y., Liu, Y.-c., Li, J.-b., Han, X.-J., Liu, S.-x., Ji, X.-r., Luo, J., & Wei, Y.-h. (2019). Isotactic Polybutene-1/Bamboo Powder Composites with Excellent Properties at Initial Stage of Molding. Polymers, 11(12), 1981. https://doi.org/10.3390/polym11121981







