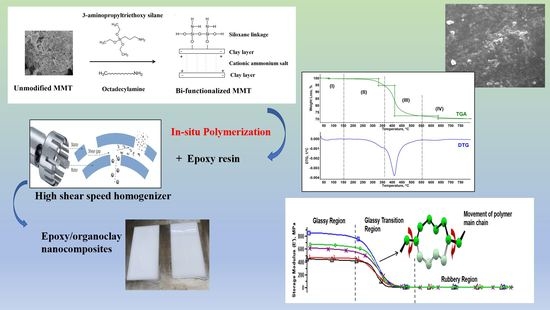
3.1. Structural and Morphological
Due to the fact that pristine MMTs are hydrophilic and the stacks of clay platelets are held tightly together by electrostatic forces, this led to a poor dispersion of nanoclay within the epoxy resin [
7]. Surface modification is often employed to change its hydrophilicity characteristic to hydrophobicity. In this present work, a commercial bi-functionalized modified MMT was used to prepare the epoxy/OMMT nanocomposites. According to the technical datasheet, the inner galleries of MMT are treated with octadecylamine (ODA) while 3-aminopropyltriethoxy silane (APTES) is used to treat the surface and edges of the clay layers to enhance dispersion in polymer resin.
Figure 3 illustrates the possible interaction between the unmodified MMT, cationic surfactant and silane coupling agent. The alkylammonium salt ions can be readily exchanged with the ions situated between the layers leading to the swelling and expansion of the interlayer space by increasing the d spacing of the clay layer [
29,
30]. On the other hand, a strong siloxane linkage may be formed from the reaction between the silane coupling agent with the hydroxyl groups around the surface or edges of the clay layers [
31].
In this work, epoxy/OMMT nanocomposites containing 0.5, 1.0, 2.0 and 4 wt % nanoclay were prepared by using a high shear speed homogenizer. The morphology and state of dispersion of the nanoclay in epoxy resin were investigated using WAXS and the findings were further confirmed with FESEM. The positively charged ammonium salt is attracted to the negatively charged clay sheets and the long carbon chains of the ammonium salt will extent away from the stacking clay layers. As a result of this, the electrostatic force between the silicate layers were reduced, thus facilitating the diffusion of the epoxy monomer into the clay galleries which induces intercalated or exfoliated morphology (
Figure 4) [
14]. Besides that, the interfacial adhesion between epoxy and the clay layer is improved due to the formation of strong siloxane linkage around the surface and edges of clay layers. The amine group (–NH
2) of APTES can react with the oxirane ring of the monomer of DGEBA (
Figure 5).
Figure 6a,b shows the WAXS pattern and FESEM image of the OMMT powder. From the WAXS, it shows a single sharp peak at 4.34° of
value, which corresponds to the 001 basal reflection peak of the interlayers. The interlayer distance of the OMMT powders calculated from Equation (1) is 2.038 nm and this matches with the technical specification in
Table 1. From the FESEM image, OMMT powder appears to be a soft plate like structure, consisting of two tetrahedral silica layers sandwiching an octahedral alumina layer.
The WAXS pattern of the epoxy/OMMT nanocomposite with a different concentration of OMMT is shown in
Figure 7. The WAXS for pristine epoxy was also displayed for comparative purposes. In the range of 3° to 5° of
value, the author observed peaks for all types of epoxy/OMMT nanocomposites. However, no peak was observed on pure epoxy, suggesting the existence of the OMMT in the epoxy matrix. The
peak of all nanocomposites and its corresponding d spacing are tabulated in
Table 2.
The author observed that the characteristic peak of OMMT in the prepared nanocomposites had shifted to a lower angle for nanocomposites with 0.5 wt % and 1.0 wt % OMMT. The d spacing increases from 2.04 nm to 2.48 nm and 2.54 nm, respectively. The increment of d spacing indicates that there is good interaction between epoxy and OMMT, suggesting intercalated nanocomposites have been formed. A similar observation has been reported by [
32,
33]. In contrast, nanocomposites with 2 wt % and 4 wt % of OMMT, the
peak was found to shift to higher with decreasing d spacing compared to OMMT. This may be attributed to the high loading of nanoclay leading to poor dispersion. Eventually agglomeration and stacking between the clay layer has occurred, resulting in smaller d spacing. The dispersion state of the nanoclay within the epoxy matrix were further confirmed with FESEM images (
Figure 8). The images confirmed that the nanoclay in epoxy/0.5% OMMT and epoxy/1.0% OMMT nanocomposites exhibit a more well dispersed state where less tactoids formation was observed. With a higher concentration of OMMT, bigger tactoids size and more agglomeration was observed in epoxy/2% OMMT and epoxy/4% OMMT nanocomposites, this correlates well with the findings from WAXS. From the structural and morphological study, epoxy/1% OMMT nanocomposite shows the best nanoclay dispersion state with the highest d spacing recorded among all the prepared nanocomposites, suggesting the formation of intercalated nanocomposites.
Figure 9 presented the 2D SAXS (small angle X-ray scattering) signal of epoxy/OMMT nanocomposites. The 2D plots provides insight about the polymer structure. The isotropic sample typically displays a complete ring, while the oriented sample often displays two or more bright maxima [
34,
35]. The 2D SAXS signal of all epoxy/OMMT nanocomposites shows a ring pattern, indicating the samples are isotropic with no preffered orientation of the nanostructure within the plane of the samples.
3.2. Thermal Stability Properties
Thermal stability of the OMMT powder and epoxy/OMMT nanocomposites were accessed by TGA under nitrogen atmosphere. The curves and data evaluation were done by METTLER TOLEDO STARe software (Mettler Toledo, Greifensee, Switzerland). The TGA and DTG (derivative thermo gravimetric) curves of OMMT powder are shown in
Figure 10. The details of the corresponding weight loss step are tabulated in
Table 3. According to the DTG curve of OMMT, the decomposition profile of OMMT reveals four weight loss steps. The first weight loss step takes place in the temperature region of 30–150 °C with weight loss of 0.41%, which can be related to the moisture evaporation. From the TGA curve, the major decomposition was observed to happen in the temperature range of 200–550 °C. However, from the DTG curve it reveals there is a relatively small weight loss step observed in the temperature range of 150–358 °C with weight loss of 5% (weight loss step (II)), followed by the major weight loss in the temperature range of 358–550 °C with weight loss of 22% (weight loss step (III)). The second weight loss step can be attributed to the decomposition of APTES and third weight loss step attributed to the decomposition of ODA [
36]. According to data published by the PubChem-National Center for Biotechnology Information, the decomposition temperature for APTES is around 217 °C [
37] while ODA decomposes at around 350 °C [
38]. In addition, the percentage of weight loss also correlates well with the composition of the surface modifier as per technical specification in
Table 1. At higher temperatures of around 650 °C, another small weight loss of 2.12% was observed which can correlate to the dehydroxylation of the clay layers [
20,
39]. The reaction process may be shown chemically as below [
40]:
The thermal decomposition profile of epoxy nanocomposites with 0.5, 1.0, 2 and 4 wt % of OMMT is shown in
Figure 11. The thermal decomposition profile of pristine epoxy also is shown for comparison purposes. The initial decomposition (
TIDT), maximum decomposition (
TMAX), and final decomposition (
TFDT) temperature of the composites are summarized in
Table 4. The initial decomposition temperature of the nanocomposites was found lower compared to pristine epoxy. The
TIDT of epoxy is 365 °C, while the
TIDT for nanocomposites with 0.5, 1, 2 and 4 wt % of OMMT are 353, 356, 347 and 336 °C, respectively. The reduction in thermal stability at a low temperature may be due to the existence of the organic surface modifier on clay which exhibits lower thermal stability. In addition, the ammonium salt that attached to the clay may act as a catalyzer towards the degradation of the polymer matrix through the Hoffman degradation mechanism; a similar observation has been reported by several researches [
33,
41,
42,
43]. The
TIDT of the epoxy nanocomposites decreases as the concentration of the OMMT increases. This may be due to the increased amount of surface modifier in the epoxy matrix leading to the acceleration of the decomposition reaction of the polymer matrix.
The presence of the nanoclay has improved the thermal stability of the epoxy nanocomposites at a higher temperature range compared to pristine epoxy as illustrated in
Figure 11. The optimum performance was observed on epoxy/1% OMMT nanocomposite with the highest
TMAX and
TFDT among all the composites. With increasing OMMT concentration, the
TMAX and
TFDT of the corresponding nanocomposites decrease, yet it is still higher compared to pristine epoxy. In addition, the char formation of epoxy nanocomposites was also found to be higher compared to pristine epoxy. The decomposition temperature at different weight loss percentages of 10%, 30%, 50%, 80% and 90% and char residue at 800 °C are reported in
Table 5. The improvement of the thermal stability at a high temperature may be attributed to the silicate layer acting as a protective barrier to hinder the moving out of volatile products generated during the decomposition process. In addition, the charring capability induced by clay minerals through its catalytic effects and reinforcement of char structure is also an important characteristic to improve the thermal stability of the nanocomposites by reducing the mass transport and permeability of oxygen [
44,
45]. Thus, epoxy/1% OMMT nanocomposites reveal the best thermal stability performance due the best intercalation morphology obtained among all the nanocomposites. As filler loading increases, this induces more agglomeration and reduces the effectiveness of forming intercalation morphology composites. This may lead to reduction of char building capability which cannot effectively protect the polymer matrix. This can be proven by the char residue for 2% and 4% OMMT nanocomposites showing relatively low increments in char residue despite the high loading of OMMT.
3.3. Dynamic Mechanical Properties
The effect of the organoclay loading on the viscoelastic behavior of the nanocomposites as a function of temperature and frequency under an oscillating force was studied by the DMA. The storage modulus (E′), loss modulus (E″), and tan delta curves of the pristine epoxy and epoxy nanocomposites are shown in
Figure 12, and the obtained DMA parameters data are summarized in
Table 6 and
Table 7. The ability of the material to store or return energy is represented by E′ and provides valuable insight on the load bearing capacity [
46], stiffness [
23], degree of crosslinking [
47], and fiber/matrix interfacial bonding [
48]. The E′ decreases as the temperature increases with a steep change in modulus was observed in the temperature range of 60–90 °C. This can be denoted as the glass transition (
Tg) region or α relaxation of the polymer whereby it represents the movement of the polymer main chain. Below the glass transition region, the movement of the polymer chain was restricted due to the low mobility of the frozen and closely packed molecules arrangement. As a result, the E′ is high in the glassy state. With temperature increases, the closely packed molecule arrangement collapses resulting in high molecular mobility and increases the free volume components, thus resulting in a drastic fall of modulus and moving into the rubbery region of the material.
An increasing loading of OMMT increases the E′ in both glassy and rubbery regions as shown in
Table 6. The storage modulus in the glassy state is represented by E′ at 25 °C while the storage modulus in rubbery region is represented by È at (
Tg + 30) °C. With 0.5 and 1.0 wt % of OMMT loading, the E′ at the glassy region improved by 50% and 87% respectively compared to pristine epoxy. With filler loading at 2 wt %, we observed a decrease in modulus with E′ recorded at 615MPa and a further reduction in E′ was observed on epoxy/4% OMMT nanocomposites with the recorded E′ of 466 MPa. A similar trend was also observed on the storage modulus in the rubbery region. The improvement of the E′ in both glassy and rubbery region can be attributed to the incorporation of the OMMT limiting the movement of the epoxy polymer chain as they intercalated in between the clay layers of OMMT [
49]. The decrease in modulus at a higher clay content (≥2 wt % ) is due to the poorly dispersed nanoclay in the epoxy resin with the aggregation of particles [
50]. On the other hand, the cross link density can be calculated from the kinetic theory of rubber elasticity from the DMA data according to Equation (2) [
47].
where
is the crosslink density (mol/m
3),
is the storage modulus at
T = (
Tg + 30) °C, R is the gas constant (8.3145 J/K mol) and T is temperature in Kelvin. It is evident that the increasing loading of the OMMT improved the crosslink density compared to pristine epoxy. The crosslink density of epoxy raised from 6.1 × 10
−4 to 10.2 × 10
−4 (mol/m
3) (
Table 6). The obtained results are in line with other research findings for functionalized soybean oil modified toughened epoxy/organoclay [
51]. As discussed earlier, silane coupling agent induced better interfacial bonding between organoclay and epoxy matrix thus resulting in a high crosslink density. As a result, the flexibility of the material is being reduced due to the segmental mobility of the polymer molecules being constrained. However, a decrease in crosslink density is observed on Epoxy/2% OMMT and Epoxy/4% OMMT. This can be attributed to the nanoclay agglomeration which affected the curing reaction and reduces when the cross link occurs. A similar argument is also reported by [
52].
The obtained loss modulus (E″) was summarized in
Table 7. E″ describes the viscous response of a material where the energy dissipated during the stress cycle was measured. In the glass transition region, the E″ exhibits a distinct peak indicating high losses of energy caused by the internal friction and non-elastic deformation in the molecular segmental motion. Improvement of loss modulus was also observed from the plot of loss modulus vs. temperature as the filler loading of OMMT increases. The loss modulus curve for epoxy nanocomposites appears to be broader and increased in peak height compared to pristine epoxy. A similar observation also reported by other researchers is that the incorporation of nanoclay induces internal friction and leads to high energy dissipation [
49,
53]. Epoxy/1% OMMT nanocomposites marks the highest peak of loss modulus followed by epoxy/0.5% OMMT and epoxy/2.0% OMMT nanocomposite. Epoxy/4% OMMT nanocomposite exhibits the lowest peak of E″ among all epoxy nanocomposites. Besides that, all epoxy nanocomposites of E″ display a broader peak shape compared to pristine epoxy which indicates the free volume and chain segments of the polymer matrix were increased with the addition of nanoclay.
Lastly, the loss factor or Tan delta curve corresponds to the ratio of loss modulus to storage modulus and its maximum peak height can be denoted as the glass transition temperature (
Tg) of the material.
Table 7 summarized the peak height of tan delta and
Tg by the peak of tan delta. The tan delta curve of pristine epoxy appears to be a sharp and narrow peak with the highest tan delta value recorded at 1.15 with
Tg recorded at 77.8 °C. A high tan delta value indicates high damping behavior or a high degree of non-elastic deformation. On the contrary, a low value indicates that the material is more elastic. With the incorporation of organoclay, the tan delta curve of epoxy nanocomposites appears to be broader and decreases in peak intensity with the
Tg also found to be shifted to a higher temperature. The lower tan delta value and broad peak is evidence that the improving damping behavior is due to the presence of the intercalated layers of OMMT modifying the network structure, effecting the relaxation mechanisms and arresting the segmental motion near the organic-inorganic interface. These findings are in line with other research findings [
50,
51,
54,
55]. Additionally, the author observed that increasing crosslink density shifts the
Tg higher. The increase of
Tg can be attributed to the constraint of relaxation mobility in the polymer segments caused by the chemical bonding around the interface of the clay layer and epoxy matrix [
55]. Epoxy/1% OMMT nanocomposite reveal the highest crosslink density (10.2 × 10
−4 mol/m
3) with the highest
Tg (82.6 °C) among all composites. This is followed by the Epoxy/0.5% OMMT nanocomposite with a crosslink density of 9.2 × 10
−4 mol/m
3 and its
Tg recorded at 81 °C. However, lacking surrounding entanglements and reduced crosslink density at the interface decreases the
Tg. This trend was observed on epoxy nanocomposites with 2 and 4 wt % of OMMT loading.
3.4. Tensile Properties
The tensile properties of epoxy organoclay nanocomposites strongly depends on the structure or configuration of nanocomposites, i.e., conventional/intercalated/exfoliated and interfacial interaction between polymer and clay layers [
56]. The tensile properties of the pristine epoxy and the nanocomposites on the effect of different OMMT loading are shown in
Figure 13. From the analysis, it was observed that the addition of OMMT improved the tensile strength and modulus with increasing the clay loading up to 1 wt %. With 0.5 wt % and 1.0 wt % of OMMT have improved the tensile strength by 90% and 103%, respectively, while the tensile modulus has improved by 143% and 200%, respectively. The reinforcing effect of the clay layers on the tensile properties is mainly due to the inherently high moduli and high aspect ratio of the clay particles [
14,
55]. The high aspect ratio of organoclay induces more surface contact area with polymer matrix and it can act efficiently as a stress transfer agent in the composites, thus improving the tensile properties. This improvement is also evidence that the nanoscale dispersion is prevalent with the formation of the intercalated structure with the bi-functionalized organoclay through the ultra-high shear speed mixing. It has been reported extensively that surface modification on clay results in better polymer-clay interfacial adhesion with improvement of tensile properties (
Table 8).
Beyond 1 wt % of OMMT loading, the tensile properties of the nanocomposites start to decline. A similar phenomena is also reported elsewhere [
32,
45,
58]. Several possible reasons may contribute to this observation, such as the micro voids formation during sample preparation due to the weak boundaries between the particles and air-trapped bubbles [
59]. Another possible reason is the organoclay induces epoxy self-polymerization which increases the viscosity of epoxy/organoclay mixture as organoclay loading increases [
60]. The amine base organic modifier used in this work may partially contribute to curing during the mixing process of epoxy resin and organoclay which could lead to heterogeneity in the resultant samples [
55]. During the preparation of the nanocomposites, we do observe the viscosity of the epoxy/organoclay mixture become more viscous with higher loading of organoclay. Due to the high viscosity of the mixture, it is more difficult to cast the mixture into mould. This eventually lead to more air-trapped bubbles and void formation which can act as the weak point of the samples. As the curing is being accelerated by the organic surface modifier, the extra-gallery viscous force outweighs the inter-gallery elastic force between the clay layers thus leading to tactoids formation (
Figure 14) [
61]. This can be proven from the d spacing obtained from the WAXS study, the d spacing for epoxy/2% OMMT and epoxy 4%/OMMT nanocomposite reduces by 2.25 % (1.992 nm) and 5.05% (1.935 nm) respectively compared to pristine epoxy (2.038 nm). Moreover, tactoids formation leading to the agglomeration of nanoclay could give a stress concentration effect and reduce the tensile strength of nanocomposites.