Application of Protein-Based Films and Coatings for Food Packaging: A Review
Abstract
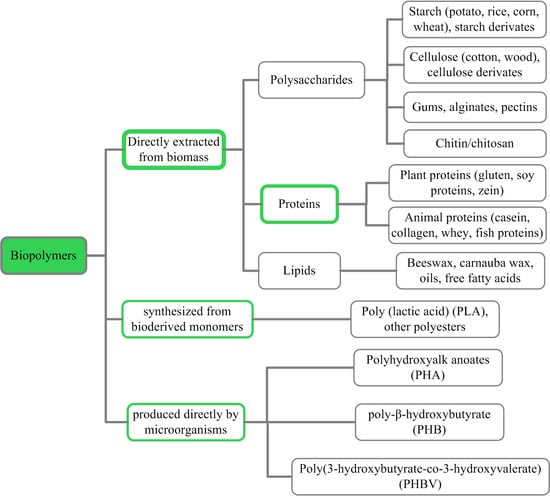
:1. Introduction
2. Proteins for Biodegradable Films
2.1. Gluten
2.2. Soy Proteins
2.3. Zein
2.4. Casein
2.5. Whey
2.6. Gelatin
3. Protein-Based Biopolymers Applications for Foods-Packaging
3.1. Fruits and Vegetables
3.2. Dairy Products
3.3. Meat and Products
3.4. Frozen Foods
4. Protein–Based Active Materials
4.1. Release Models Applied to Active Packaging
4.2. Antimicrobial Protein-Based Films
4.3. Antioxidant Protein-Based Films
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Haosagul, S.; Boonyawanich, S.; Pisutpaisal, N. Biomethane Production from co-fermentation of agricultural wastes. Int. J. Hydrogen Energy 2019, 44, 5355–5364. [Google Scholar] [CrossRef]
- Siracusa, V.; Lotti, N. Biobased Plastics for Food Packaging. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Petersen, K.; Væggemose Nielsen, P.; Bertelsen, G.; Lawther, M.; Olsen, M.B.; Nilsson, N.H.; Mortensen, G. Potential of biobased materials for food packaging. Trends Food Sci. Technol. 1999, 10, 52–68. [Google Scholar] [CrossRef]
- Zhang, H.; Mittal, G. Biodegradable protein-based films from plant resources: A review. Environ. Prog. Sustain. Energy 2010, 29, 203–220. [Google Scholar] [CrossRef]
- Tharanathan, R.N. Biodegradable films and composite coatings: Past, present and future. Trends Food Sci. Technol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Popović, S.Z.; Lazić, V.L.; Hromiš, N.M.; Šuput, D.Z.; Bulut, S.N. Chapter 8—Biopolymer Packaging Materials for Food Shelf-Life Prolongation. In Biopolymers for Food Design; Grumezescu, A.M., Holban, A.M., Eds.; AcademicPress: New York, NY, USA, 2018; pp. 223–277. [Google Scholar]
- Majid, I.; Thakur, M.; Nanda, V. Biodegradable Packaging Materials. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Robertson, G. 1-State-of-the-art biobased food packaging materials. In Environmentally Compatible Food Packaging; Chiellini, E., Ed.; Woodhead Publishing: Sawston, UK, 2008; pp. 3–28. [Google Scholar]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Rita, H.; Helén, H.; Talja, R.A.; Hyvönen, L.; Tenkanen, M. Effect of Polysaccharide Structure on Mechanical and Thermal Properties of Galactomannan-Based Films. Biomacromolecules 2007, 8, 3198–3205. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Osako, K.; Benjakul, S.; Tongdeesoontorn, W.; Rawdkuen, S. Biodegradable Protein-based Films and Their Properties: A Comparative Study. Packag. Technol. Sci. 2016, 29, 77–90. [Google Scholar] [CrossRef]
- Bourtoom, T. Edible protein films: Properties enhancement. Int. Food Res. J. 2011, 16, 1–9. [Google Scholar]
- Schmid, M.; Müller, K. Chapter 11—Whey Protein-Based Packaging Films and Coatings. In Whey Proteins; Deeth, H.C., Bansal, N., Eds.; Academic Press: New York, NY, USA, 2019; pp. 407–437. [Google Scholar]
- Caleb, O.J.; Ahajan, P.V.; Al-Said, F.A.; Opara, U.L. Transpirationrate and quality of omegranate arils as affected by storage conditions. Cyta J. Food 2013, 11, 199–207. [Google Scholar] [CrossRef]
- González, A.; AlvarezIgarzabal, C.I. Nanocrystal-reinforced soy protein films and their application as active packaging. Food Hydrocoll. 2015, 43, 777–784. [Google Scholar] [CrossRef]
- Krishna, M.; Nindo, C.I.; Min, S.C. Development of fish gelatin edible films using extrusion and compression molding. J. Food Eng. 2012, 108, 337–344. [Google Scholar] [CrossRef]
- Pérez, L.M.; Piccirilli, G.N.; Delorenzi, N.J.; Verdini, R.A. Effect of different combinations of glycerol and/or trehaloseon physical and structural properties of whey protein concentrate-based edible films. Food Hydrocoll. 2016, 56, 352–359. [Google Scholar] [CrossRef]
- Cao, N.; Fu, Y.; He, J. Preparation and physical properties of soy protein isolate and gelatin composite films. Food Hydrocoll. 2007, 21, 1153–1162. [Google Scholar] [CrossRef]
- Maryam Adilah, Z.A.; Jamilah, B.; NurHanani, Z.A. Functional and antioxidant properties of protein-based films incorporated with mango kernel extract for active packaging. Food Hydrocoll. 2018, 74, 207–218. [Google Scholar] [CrossRef]
- Murrieta-Martínez, C.L.; Soto-Valdez, H.; Pacheco-Aguilar, R.; Torres-Arreola, W.; Rodríguez-Felix, F.; MárquezRíos, E. Edible protein films: Sources and behavior. Packag. Technol. Sci. 2018, 31, 113–122. [Google Scholar] [CrossRef]
- Zavareze, E.; ElHalal, S.; Silva, R.; Dias, A.; Prentice-Hernandez, C. Mechanical, barrier and morphological properties of biodegradable films based on muscle and waste proteins from the white mouth croaker. J. Food Process. Preserv. 2014, 38, 1973–1981. [Google Scholar] [CrossRef]
- Letendre, M.; D’Aprano, G.; Lacroix, M.; Salmieri, S.; St-Gelais, D. Physicochemical properties and bacterial resistance of biodegradable milk protein films containing agar and pectin. J. Agric. Food Chem. 2002, 50, 6017–6022. [Google Scholar] [CrossRef]
- Guerrero, P.; Caba, K.D.L. Protein-Based Films and Coatings. Contemp. Food Eng. 2016, 81–120. [Google Scholar]
- Vieira, M.G.A.; DaSilva, M.A.; DosSantos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Brault, D.; Daprano, G.; Lacroix, M. Formation of free-standing sterilized edible films from irradiated caseinates. J. Agric. Food Chem. 1997, 45, 2964–2969. [Google Scholar] [CrossRef]
- Jenkins, A.D. Fundamental Principles of Polymeric Materials. Biomaterials 1982, 3, 253. [Google Scholar] [CrossRef]
- Swain, S.N.; Biswal, S.M.; Nanda, P.K.; Nayak, P.L. Biodegradable Soy-Based Plastics: Opportunities and Challenges. J. Polym. Environ. 2004, 12, 35–42. [Google Scholar] [CrossRef]
- Ananey-Obiri, D.; Matthews, L.; Azahrani, M.H.; Ibrahim, S.A.; Galanakis, C.M.; Tahergorabi, R. Application of protein-based edible coatings for fat uptake reduction in deep-fatfried foods with an emphasis on muscle food proteins. Trends Food Sci. Technol. 2018, 80, 167–174. [Google Scholar] [CrossRef]
- Cuq, B.; Gontard, N.; Cuq, J.-L.; Guilbert, S. Selected Functional Properties of Fish Myofibrillar Protein-Based Films As Affected by Hydrophilic Plasticizers. J. Agric. Food Chem. 1997, 45, 622–626. [Google Scholar] [CrossRef]
- Ou, S.; Wang, Y.; Tang, S.; Huang, C.; Jackson, M.G. Role of ferulicacid in preparing edible films from soy protein isolate. J. Food Eng. 2005, 205–210. [Google Scholar] [CrossRef]
- Karbowiak, T.; Hervet, H.; Léger, L.; Champion, D.; Debeaufort, F.; Voilley, A. Effect of Plasticizers (Water and Glycerol) on the Diffusion of a Small Moleculein Iota-Carrageenan Biopolymer Films for Edible Coating Application. Biomacromolecules 2006, 7, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, J.H. Mechanical and thermal characteristics of pea starch films plasticized with monosaccharides and polyols. J. Food Sci. 2006, E109–E118. [Google Scholar] [CrossRef]
- Kristo, E.; Biliaderis, C.G. Water sorption and thermo-mechanical properties of water/sorbitol – plasticized composite biopolymer films: Caseinate-pullulan bilayers and blends. Food Hydrocoll. 2006, 20, 1057–1071. [Google Scholar] [CrossRef]
- Cao, N.; Yang, X.; Fu, Y. Effects of various plasticizers on mechanical and water vapor barrier properties of gelatin films. Food Hydrocoll. 2009, 23, 729–735. [Google Scholar] [CrossRef]
- Suyatma, N.E.; Tighzert, L.; Copinet, A.; Coma, V. Effects of hydrophilic plasticizers on mechanical, thermal, and surface properties of chitosan films. J. Agric. Food Chem. 2005, 53, 3950–3957. [Google Scholar] [CrossRef] [PubMed]
- Audic, J.-L.; Chaufer, B. Influence of plasticizers and crosslinking on the properties of biodegradable films made from sodium caseinate. Eur. Polym. J. 2005, 41, 1934–1942. [Google Scholar] [CrossRef]
- Jangchud, A.; Chinnan, M.S. Properties of Peanut Protein Film: Sorption Isotherm and Plasticizer Effect. Food Sci. Technol. 1999, 32, 89–94. [Google Scholar] [CrossRef]
- Smits, A.L.M.; Kruiskamp, P.H.; VanSoest, J.J.G.; Vliegenthart, J.F.G. Interaction between dry starch and plasticizers glycerol or ethylene glycol, measured by differential scanning calorimetry and solid state NMR spectroscopy. Carbohydr. Polym. 2003, 53, 409–416. [Google Scholar] [CrossRef]
- Honary, S.; Orafai, H. The Effect of Different Plasticizer Molecular Weights and Concentrations on Mechanical and Thermomechanical Properties of Free Films. Drug Dev. Ind. Pharm. 2002, 711–715. [Google Scholar] [CrossRef]
- Talja, R.A.; Helén, H.; Roos, Y.H.; Jouppila, K. Effect of various polyols and polyolcontents on physical and mechanical properties of potato starch-based films. Carbohydr. Polym. 2007, 67, 288–295. [Google Scholar] [CrossRef]
- Navarro-Tarazaga, M.L.; Sothornvit, R.; Perez-Gago, M.B. Effect of Plasticizer Type and Amount on Hydroxypropyl Methylcellulose–Beeswax Edible Film Properties and Postharvest Quality of Coated Plums (Cv. Angeleno). J. Agric. Food Chem. 2008, 56, 9502–9509. [Google Scholar] [CrossRef]
- Galdeano, M.C.; Mali, S.; Grossmann, M.V.E.; Yamashita, F.; García, M.A. Effects of plasticizers on the properties of oat starch films. Mater. Sci. Eng. C 2009, 29, 532–538. [Google Scholar] [CrossRef]
- Veiga-Santos, P.; Oliveira, L.M.; Cereda, M.P.; Scamparini, A.R.P. Sucrose and inverted sugar as plasticizer. Effect on cassavastarch–gelatin film mechanical properties, hydrophilicity and water activity. Food Chem. 2007, 103, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Pommet, M.; Redl, A.; Morel, M.-H.; Guilbert, S. Study of wheat gluten plasticization with fatty acids. Polymer 2003, 44, 115–122. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Fatty acids and their sucroseesters affect the properties of fish skin gelatin-based film. Eur. Food Res. Technol. 2006, 222, 650–657. [Google Scholar] [CrossRef]
- Santosa, F.X.B.; Padua, G.W. Tensile Properties and Water Absorption of Zein Sheets Plasticized with Oleic and Linoleic Acids. J. Agric. Food Chem. 1999, 47, 2070–2074. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Yu, J.; Ma, X. Ethanolamine as a novel plasticiser for thermo plastic starch. Polym. Degrad. Stab. 2005, 90, 501–507. [Google Scholar] [CrossRef]
- Galdeano, M.C.; Grossmann, M.V.E.; Mali, S.; Bello-Perez, L.A.; Garcia, M.A.; Zamudio-Flores, P.B. Effects of production process and plasticizers on stability of films and sheets of oat starch. Mater. Sci. Eng. C: Biomim. Mater. Sens. Syst. 2009, 29, 492–498. [Google Scholar] [CrossRef]
- Stein, T.M.; Gordon, S.H.; Greene, R.V. Amino acids as plasticizers II. Use of quantitative structure—Property relationships to predict the behavior of monoammonium monocarboxylate plasticizers in starch-glycerol blends. Carbohydr. Polym. 1999, 39, 7–16. [Google Scholar] [CrossRef]
- Kim, S.-J.; Ustunol, Z. Solubility and Moisture Sorption Isotherms of Whey-Protein-Based Edible Films as Influenced by Lipid and Plasticizer Incorporation. J. Agric. Food Chem. 2001, 49, 4388–4391. [Google Scholar] [CrossRef]
- Sobral, P.J.D.A.; Santos, J.S.D.; García, F.T. Effect of protein and plasticizer concentrations in film forming solutions on physical properties of edible films based on muscle proteins of a Thai Tilapia. J. Food Eng. 2004, 70, 93–100. [Google Scholar] [CrossRef]
- Galietta, G.; Gioia, L.D.; Guilbert, S.; Cuq, B. Mechanical and thermomechanical properties offilms based on whey proteins as affected by plasticizer and crosslinking agents. J. Dairy Sci. 1998, 81, 3123–3130. [Google Scholar] [CrossRef]
- Bergo, P.; Sobral, P.J.A. Effects of plasticizer on physical properties of pig skin gelatin films. Food Hydrocoll. 2007, 21, 1285–1289. [Google Scholar] [CrossRef]
- Sobral, P.J.A.; Menegalli, F.C.; Hubinger, M.D.; Roques, M.A. Mechanical, water vapor barrier and thermal properties of gelatin based edible films. Food Hydrocoll. 2001, 15, 423–432. [Google Scholar] [CrossRef]
- Bertan, L.C.; Tanada-Palmu, P.S.; Siani, A.C.; Grosso, C.R.F. Effect of fatty acids and ’Brazilianelemi’ on composite films based on gelatin. Food Hydrocoll. 2005, 19, 73–82. [Google Scholar] [CrossRef]
- Carvalho, R.A.; Ferreira Grosso, C.R.; Sobral, P.J.A. Effect of chemical treatment on the mechanical properties, water vapour permeability and sorption isotherms of gelatin-based films. Packag. Technol. Sci. 2008, 21, 165–169. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Zarate-Ramírez, L.S.; Romero, A.; Bengoechea, C.; Partal, P.; Guerrero, A. Bioplastics based on wheat gluten processed by extrusion. J. Clean. Prod. 2019, 239, 117994. [Google Scholar] [CrossRef]
- Gao, C.; Stading, M.; Wellner, N.; Parker, M.; Noel, T.; Mills, E.; Belton, P. Plasticization of a protein-based film by glycerol: A spectroscopic, mechanical, and thermal study. J. Agric. Food Chem. 2006, 54, 4611–4616. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.F.; Brodkorb, A. The casein micelle: Historical aspects, current concepts and significance. Int. Dairy J. 2008, 18, 677–684. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Reinas, I.; Silva, S.I.; Fernandes, J.C.; Cerqueira, M.A.; Pereira, R.N.; Vicente, A.A.; Poças, M.F.; Pintado, M.E.; Malcata, F.X. Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocoll. 2013, 30, 110–122. [Google Scholar] [CrossRef]
- Wieser, H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Cuq, B.; Gontard, N.; Guilbert, S. Proteins as agricultural polymers for packaging production. Cereal Chem. 1998, 75, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mangavel, C.; Rossignol, N.; Perronnet, A.; Barbot, J.; Popineau, Y.; Guéguen, J. Properties and microstructure of thermo-pressed wheat gluten films: A comparison with cast films. Biomacromolecules 2004, 5, 1596–1601. [Google Scholar] [CrossRef] [Green Version]
- Gällstedt, M.; Mattozzi, A.; Johansson, E.; Hedenqvist, M.S. Transport and tensile properties of compression-molded wheat gluten films. Biomacromolecules 2004, 5, 2020–2028. [Google Scholar] [CrossRef]
- Zuo, M.; Song, Y.; Zheng, Q. Preparation and properties of wheat gluten/methylcellulose binary blend film casting from aqueous ammonia: A comparison with compression molded composites. J. Food Eng. 2009, 91, 415–422. [Google Scholar] [CrossRef]
- Gennadios, A.; Brandenburg, A.H.; Weller, C.L.; Testin, R.F. Effect of pH on properties of wheat gluten and soy protein isolate films. J. Agric. Food Chem. 1993, 41, 1835–1839. [Google Scholar] [CrossRef]
- Arnon-Rips, H.; Poverenov, E. Improving food products’ quality and storability by using Layer by Layer edible coatings. Trends Food Sci. Technol. 2018, 75, 81–92. [Google Scholar] [CrossRef]
- Krochta, J.; Mulder-Johnston, C. Edible and Biodegradable Polymer Films: Challenges and Opportunities. Food Technol. 1997, 51, 61–74. [Google Scholar]
- Gennadios, A.; Weller, C.; Testin, R. Modification of physical and barrier properties of edible wheat gluten-based films. Cereal Chem. 1993, 70, 426–429. [Google Scholar]
- Ansorena, M.R.; Zubeldía, F.; Marcovich, N.E. Active wheat gluten films obtained by thermo plastic processing. Lwt Food Sci. Technol. 2016, 69, 47–54. [Google Scholar] [CrossRef]
- Baldwin, E.; Hagenmaier, R.; Bai, J. Edible Coatings and Films to Improve Food Quality; CRC Press: Boca Raton, FL, USA, 1994; Volume 33, pp. 86–87. [Google Scholar]
- Tian, H.; Guo, G.; Fu, X.; Yao, Y.; Yuan, L.; Xiang, A. Fabrication, properties and applications of soy-protein-based materials: A review. Int. J. Biol. Macromol. 2018, 120, 475–490. [Google Scholar] [CrossRef]
- Mariniello, L.; DiPierro, P.; Esposito, C.; Sorrentino, A.; Masi, P.; Porta, R. Preparation and mechanical properties of edible pectin-soy flour films obtained in the absence or presence of transglutaminase. J. Biotechnol. 2003, 102, 191–198. [Google Scholar] [CrossRef]
- SeungYong, C.; Chul, R. Mechanical properties and water vapor permeability of edible films made from fractionated soy proteins with ultra filtration. Lebensm. Wiss. Und-Technol. 2004, 37, 833–839. [Google Scholar]
- Wu, L.C.; Bates, R.P. Soy protein-lipid films. 1. Studies on the Film Formation Phenomenon. J. Food Sci. 1972, 31, 36–39. [Google Scholar]
- Ghorpade, V.M.; Hanna, M.A. Mechanical Properties of Soy Protein-polyethylene Ribbon and Film Extrudates. Trans. Asabe 1996, 39, 611–615. [Google Scholar] [CrossRef]
- Rampon, V.; Robert, P.; Nicolas, N.; Dufour, E. Protein structure and network orientationin edible films prepared by spinning process. J. Food Sci. 1999, 64, 313–316. [Google Scholar] [CrossRef]
- Ogale, A.A.; Cunningham, P.; Dawson, P.L.; Acton, J.C. Viscoelastic, thermal, and microstructural characterization of soy protein isolate films. J. Food Sci. 2000, 65, 672–679. [Google Scholar] [CrossRef]
- Rayner, M.; Ciolfi, V.; Maves, B.; Stedman, P.; Mittal, G.S. Development and application of soy-protein films to reduce fat intake in deep-fried foods. J. Sci. Food Agric. 2000, 80, 777. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Rhi, M. Sodium dodecyl sulfate treatment improves properties of cast films soy protein isolate. Ind. Crop. Prod. 2002, 15, 199–205. [Google Scholar]
- Sabato, S.; Ouattara, B.; Yu, H.; D’Aprano, G.; Tien, C.L.; Mateescu, M.; Lacroix, M. Mechanical and barrier properties of cross-linked soy and whey protein based films. J. Agric. Food Chem. 2001, 49, 1397–1403. [Google Scholar] [CrossRef]
- Soares, R.M.D.; Scremin, F.F.; Soldi, V. Thermal Stability of Biodegradable Films Based on Soy Protein and Corn Starch. Macromol. Symp. 2005, 229, 258–265. [Google Scholar] [CrossRef]
- Rhim, J.; Handa, A.; Weller, C.; Hanna, M.; Gennadios, A. Solubility, tensile, and color properties of modified soy protein isolate films. J. Agric. Food Chem. 2000, 48, 4937–4941. [Google Scholar] [CrossRef]
- Shukla, R.; Cheryan, M. Zein: The industrial protein from corn. Ind. Crop. Prod. 2001, 13, 171–192. [Google Scholar] [CrossRef]
- Beck, M.I.; Tomka, I.; Waysek, E. Physico-chemical characterization of zein as a film coating polymer A direct comparison with ethylcellulose. Int. J. Pharm. 1996, 141, 137–150. [Google Scholar] [CrossRef]
- Soliman, E.; Eldin, M.M.; Furuta, M. Biodegradable zein-based films: Influence of gamma-irradiation on structural and functional properties. J. Agric. Food Chem. 2009, 57, 2529–2535. [Google Scholar] [CrossRef] [PubMed]
- Dickey, L.C.; Parris, N.; Craig, J.C.; Kurantz, M.J. Ethanolic extraction of zein from maize. Ind. Crop. Prod. 2001, 13, 67–76. [Google Scholar] [CrossRef]
- Dickey, L.C.; Parris, N.; Craig, J.C.; Kurantz, M.J. Serial batch extraction of zein from milled maize. Ind. Crop. Prod. 2002, 15, 33–42. [Google Scholar] [CrossRef]
- Landry, J. Comparison of Extraction Methods for Evaluating Zein Conten tof Maize Grain. Cereal Chem. 1997, 74, 188–189. [Google Scholar] [CrossRef]
- Wang, Q.; Geil, P.; Padua, G. Role of Hydrophilic and Hydrophobic Interactionsin Structure Development of Zein Films. J. Polym. Environ. 2004, 12, 197–202. [Google Scholar] [CrossRef]
- Kim, S.; Sessa, D.J.; Lawton, J.W. Characterization of zein modified with a mild cross-linking agent. Ind. Crop. Prod. 2004, 20, 291–300. [Google Scholar] [CrossRef]
- Wang, Y.; Padua, G.W. Water Sorption Properties of Extruded Zein Films. J. Agric. Food Chem. 2004, 52, 3100–3105. [Google Scholar] [CrossRef]
- Kleen, D.; Padua, G.; Engeseth, N. Stabilization of lipids in a biodegradable zein-oleate film by incorporation of antioxidants. Cereal Chem. 2002, 79, 687–694. [Google Scholar] [CrossRef]
- Wang, Y.; Rakotonirainy, A.; Padua, G. Thermal behavior of zein-based biodegradable films. Starch-Starke 2003, 55, 25–29. [Google Scholar] [CrossRef]
- Khwaldia, K.; Perez, C.; Banon, S.; Desobry, S.; Hardy, J. Milk protein for edible films and coating. Crit. Rev. Food Sci. Nutr. 2004, 44, 239–251. [Google Scholar] [CrossRef]
- Parris, N.; Coffin, D.R. Composition Factors Affecting the Water Vapor Permeability and Tensile Properties of Hydrophilic Zein Films. J. Agric. Food Chem. 1997, 45, 1596–1599. [Google Scholar] [CrossRef]
- Wang, Q.; Padua, G.W. Properties of Zein Films Coated with Drying Oils. J. Agric. Food Chem. 2005, 53, 3444–3448. [Google Scholar] [CrossRef] [PubMed]
- Audic, J.; Chaufer, B.; Daufin, G. Non-food applications of milk components and dairy co-products: A review. Le Lait 2003, 83, 417–438. [Google Scholar] [CrossRef]
- Chiralt, A.; González-Martínez, C.; Vargas, M.; Atarés, L. 18—Edible films and coatings from proteins. In Proteins in Food Processing, 2nd ed.; Yada, R.Y., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 477–500. [Google Scholar]
- MendesdeSouza, P.; Fernández, A.; López-Carballo, G.; Gavara, R.; Hernández-Muñoz, P. Modified sodium caseinate films as releasing carriers of lysozyme. Food Hydrocoll. 2010, 24, 300–306. [Google Scholar] [CrossRef]
- Livney, Y.D. Milk proteins as vehicles for bioactives. Curr. Opin. Colloid Interface Sci. 2010, 15, 73–83. [Google Scholar] [CrossRef]
- Belyamani, I.; Prochazka, F.; Assezat, G. Production and characterization of sodium caseinate edible films made by blown-film extrusion. J. Food Eng. 2014, 121, 39–47. [Google Scholar] [CrossRef]
- Bonnaillie, M.L.; Zhang, H.; Akkurt, S.; Yam, L.K.; Tomasula, M.P. Casein Films: The Effects of Formulation, Environmental Conditions and the Addition of Citric Pectin on the Structure and Mechanical Properties. Polymers 2014, 6, 2018–2036. [Google Scholar] [CrossRef]
- Chevalier, E.; Assezat, G.; Prochazka, F.; Oulahal, N. Development and characterization of a novel edible extruded sheet based on different casein sources and influence of the glycerol oncentration. Food Hydrocoll. 2018, 75, 182–191. [Google Scholar] [CrossRef]
- Sohail, S.S.; Wang, B.; Biswas, M.A.S.; Oh, J.-H. Physical, Morphological, and Barrier Properties of Edible Casein Films with Wax Applications. J. Food Sci. 2006, 71, C255–C259. [Google Scholar] [CrossRef]
- Diak, O.A.; Bani-Jaber, A.; Amro, B.; Jones, D.; Andrews, G.P. The Manufacture and Characterization of Casein Films as Novel Tablet Coatings. Food Bioprod. Process. 2007, 85, 284–290. [Google Scholar] [CrossRef]
- Farhan, A.; Hani, N.M. Characterization of edible packaging films based on semi-refined kappa-carrageenan plasticized with glycerol and sorbitol. Food Hydrocoll. 2017, 64, 48–58. [Google Scholar] [CrossRef]
- Cao, N.; Fu, Y.; He, J. Mechanical properties of gelatin films cross-linked, respectively, by ferulic acid and tannin acid. Food Hydrocoll. 2007, 21, 575–584. [Google Scholar] [CrossRef]
- Ghosh, A.; Ali, M.A.; Dias, G.J. Effect of cross-linking on microstructure and physical performance of casein protein. Biomacromolecules 2009, 10, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- KeesdeKruif, C.G.; Anema, S.G.; Zhu, C.; Havea, P.; Coker, C. Water holding capacity and swelling of casein hydrogels. Food Hydrocoll. 2015, 44, 372–379. [Google Scholar]
- Song, F.; Zhang, L.; Shi, J.; Li, N. Novel casein hydrogels: Formation, structure and controlled drug release. Colloids Surf. B-Biointerfaces 2010, 79, 142–148. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, R.; Chen, F.; He, L.; Hu, B.; Zeng, X. Cross-Linking of Interfacial Casein Layer with Genipin Prevented pH-Induced Structural Instability and Lipase Digestibility of the Fat Droplets. J. Agric. Food Chem. 2015, 63, 2033–2040. [Google Scholar]
- Silva, N.N.; Saint-Jalmes, A.; Carvalho, A.F.D.; Gaucheron, F. Development of Casein Microgels from Cross-Linking of Casein Micelles by Genipin. Langmuir 2014, 30, 10167–10175. [Google Scholar] [CrossRef]
- Song, F.; Zhang, L.; Yang, C.; Yan, L. Genipin-crosslinked casein hydrogels for controlled drug delivery. Int. J. Pharm. 2009, 373, 41–47. [Google Scholar] [CrossRef]
- Picchio, M.L.; Linck, Y.G.; Monti, G.A.; Gugliotta, L.M.; Minari, R.J.; AlvarezIgarzabal, C.I. Casein films crosslinked by tannic acid for food packaging applications. Food Hydrocoll. 2018, 84, 424–434. [Google Scholar] [CrossRef]
- Fabra, M.J.; Talens, P.; Chiralt, A. Effect of alginate and λ-carrageenan on tensile properties and water vapour permeability of sodium caseinate–lipid based films. Carbohydr. Polym. 2008, 74, 419–426. [Google Scholar] [CrossRef]
- Pereda, M.; Aranguren, M.I.; Marcovich, N.E. Characterization of chitosan/caseinate films. J. Appl. Polym. Sci. 2008, 107, 1080–1090. [Google Scholar] [CrossRef]
- Chick, J.; Hernandez, R.J. Physical, Thermal, and Barrier Characterization of Casein-Wax-Based Edible Films. J. Food Sci. 2002, 67, 1073–1079. [Google Scholar] [CrossRef]
- Fabra, M.J.; Talens, P.; Chiralt, A. Microstructure and optical properties of sodium caseinate films containing oleicacid–beeswax mixtures. Food Hydrocoll. 2009, 23, 676–683. [Google Scholar] [CrossRef]
- Avena-Bustillos, R.J.; Krochta, J.M. Water Vapor Permeability of Caseinate-Based Edible Films as Affected by pH, Calcium Crosslinking and Lipid Content. J. Food Sci. 1993, 58, 904–907. [Google Scholar] [CrossRef]
- Baroli, B. Photopolymerization of biomaterials: Issues and potentialities in drug delivery, tissue engineering, and cellencapsulation applications. J. Chem. Technol. Biotechnol. 2006, 81, 491–499. [Google Scholar] [CrossRef]
- Wihodo, M.; Moraru, C.I. Effect of Pulsed Light treatment on the functional properties of casein films. Lwt Food Sci. Technol. 2015, 64, 837–844. [Google Scholar] [CrossRef] [Green Version]
- Jauregi, P.; Welderufael, F.T. Added-value protein products from whey: Extraction, fractionation, separation, purification. Nutrafoods 2010, 9, 13–23. [Google Scholar] [CrossRef]
- Shimada, K.; Claude Cheftel, J. Sulfhydryl group/disulfide bond interchange reactions during heat-induced gelation of whey protein isolate. J. Agric. Food Chem. 2002, 37, 161–168. [Google Scholar] [CrossRef]
- McHugh, T.H.; Krochta, J.M. Water vapor permeability properties of edible whey protein-lipid emulsion films. J. Am. Oil Chem. Soc. 1994, 71, 307–312. [Google Scholar] [CrossRef]
- Mchugh, T.; Aujard, J.; Krochta, J. Plasticized whey protein edible films: Water vapor permeability properties. J. Food Sci. 1994, 59, 416–419. [Google Scholar] [CrossRef]
- Lent, L.E.; Vanasupa, L.S.; Tong, P.S. Whey protein edible films tructures determined by atomic force microscope. J. Food Sci. 1998, 63, 824–827. [Google Scholar] [CrossRef]
- Pérez-Gago, M.B.; Krochta, J.M. Water vapor permeability of whey protein emulsion films as affected by pH. J. Food Sci. 1999, 64, 695–698. [Google Scholar] [CrossRef]
- Banerjee, R.; Chen, H. Functional properties of edible films using whey protein concentrate. J. Dairy Sci. 1995, 78, 1673–1683. [Google Scholar] [CrossRef]
- Díaz, O.; Candia, D.; Cobos, Á. Effects of ultraviolet radiation on properties of films from whey protein concentratetreated before or after film formation. Food Hydrocoll. 2016, 55, 189–199. [Google Scholar] [CrossRef]
- Bourtoom, T.; Chinnan, M.S.; Jantawat, P.; Sanguandeekul, R. Effect of select parameters on the properties of edible film from water-soluble fish proteins insurimi wash-water. Lwt Food Sci. Technol. 2006, 39, 406–419. [Google Scholar] [CrossRef]
- Quinn, G.; Monahan, F.J.; O’Riordan, E.D.; O’Sullivan, M.; Longares, A. Role of Covalent and Noncovalent Interactions in the Formation of Films from Unheated Whey Protein Solutions Following pH Adjustment. J. Food Sci. 2003, 68, 2284–2288. [Google Scholar] [CrossRef]
- Díaz, O.; Candia, D.; Cobos, Á. Whey protein film properties as affected by ultraviolet treatment under alkaline conditions. Int. Dairy J. 2017, 73, 84–91. [Google Scholar] [CrossRef]
- Banerjee, R.; Chen, H.; Wu, J. Milk protein-based edible film mechanical strength changes due to ultrasound process. J. Food Sci. 1996, 61, 824–828. [Google Scholar] [CrossRef]
- Javanmard, M.; Golestan, L. Effect of oliveoil and glycerol on physical properties of whey protein concentrate films. J. Food Process Eng. 2008, 31, 628–639. [Google Scholar] [CrossRef]
- Shaw, N.B.; Monahan, F.J.; O’Riordan, E.D.; O’Sullivan, M. Effect of soya oil and glycerol on physical properties of composite WPI films. J. Food Eng. 2002, 51, 299–304. [Google Scholar] [CrossRef]
- Kokoszka, S.; Debeaufort, F.; Lenart, A.; Voilley, A. Liquid and vapour water transfer through whey protein/lipide mulsion films. J. Sci. Food Agric. 2010, 90, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Soazo, M.; Pérez, L.M.; Rubiolo, A.C.; Verdini, R.A. Effect of freezing on physical properties of whey protein emulsion films. Food Hydrocoll. 2013, 31, 256–263. [Google Scholar] [CrossRef]
- Pérez-Gago, M.B.; Krochta, J.M. Lipid Particle Size Effect on Water Vapor Permeability and Mechanical Properties of Whey Protein/Beeswax Emulsion Films. J. Agric. Food Chem. 2001, 49, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Janjarasskul, T.; Rauch, D.J.; McCarthy, K.L.; Krochta, J.M. Barrier and tensile properties of whey protein–candelilla wax film/sheet. Lwt Food Sci. Technol. 2014, 56, 377–382. [Google Scholar] [CrossRef]
- Fernández, L.; DeApodaca, E.D.; Cebrián, M.; Villarán, M.C.; Maté, J.I. Effect of the unsaturation degreeand concentration offatty acids on the properties of WPI-based edible films. Eur. Food Res. Technol. 2007, 224, 415–420. [Google Scholar] [CrossRef]
- Anker, M.; Berntsen, J.; Hermansson, A.-M.; Stading, M. Improved water vapor barrier of whey protein films by addition of an acetylated monoglyceride. Innov. Food Sci. Emerg. Technol. 2002, 3, 81–92. [Google Scholar] [CrossRef]
- Ta, K. Handbook of Engineering Biopolymers; Homopolymers, Blends, and Composites; Sci-Tech News Carl Hanser Verlag GmbH & CO. KG: Munich, Germany, 2007; Volume 61, p. 64. [Google Scholar]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Prodpran, T.; Tanaka, M. Characterization of edible films from skin gelatin of brown stripered snapper and big eye snapper. Food Hydrocoll. 2006, 20, 492–501. [Google Scholar] [CrossRef]
- Menegalli, F.C.; Sobral, P.J.; Roques, M.A.; Laurent, S. Characteristics of gelatin biofilms in relation to drying process conditions near melting. Dry. Technol. 1999, 17, 1697–1706. [Google Scholar] [CrossRef]
- Fraga, A.N.; Williams, R.J.J. Thermal properties of gelatin films. Polymer 1985, 26, 113–118. [Google Scholar] [CrossRef]
- Andreuccetti, C.; Carvalho, R.A.; Galicia-García, T.; Martinez-Bustos, F.; González-Nuñez, R.; Grosso, C.R.F. Functional properties of gelatin-based films containing Yuccaschidigera extract produced via casting, extrusion and blown extrusion processes: Apreliminary study. J. Food Eng. 2012, 113, 33–40. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Properties and antioxidant activity of fish skin gelatin film incorporated with citrus essential oils. Food Chem. 2012, 134, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Benjakul, S.; Prodpran, T.; Agustini, T.W. Physico-mechanical and antimicrobial properties of gelatin film from the skin of unicorn leather jacket incorporated with essential oils. Food Hydrocoll. 2012, 28, 189–199. [Google Scholar] [CrossRef]
- Kavoosi, G.; Dadfar, S.M.M.; Purfard, A.M. Mechanical, physical, antioxidant, and antimicrobial properties of gelatin films incorporated with thymol for potential use as nano wound dressing. J. Food Sci. 2013, 78, E244–E250. [Google Scholar] [CrossRef] [PubMed]
- Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Edible coatings with antibrowning agents to maintain sensory quality and antioxidant properties of fresh-cut pears. Postharvest Biol. Technol. 2008, 50, 87–94. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- O’Sullivan, M.G. Chapter6—Shelf Life and Sensory Quality of Foods and Beverages. In A Handbook for Sensory and Consumer-Driven New Product Development; O’Sullivan, M.G., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 103–123. [Google Scholar]
- Westall, S.; Filtenborg, O. Spoilage yeasts of decorated soft cheese packed in modified atmosphere. Food Microbiol. 1998, 15, 243–249. [Google Scholar] [CrossRef]
- DeGruson, M.L. Biobased Packaging. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Han, J.-W.; Kim, B.; Li, J.; Meyyappan, M. Carbon Nanotube Based Humidity Sensoron Cellulose Paper. J. Phys. Chem. C 2012, 116, 22094–22097. [Google Scholar] [CrossRef]
- Shayanfar, S. Modified atmosphere packaging for fresh produce. Glob. Saf. Fresh Prod. 2014, 42, 175–186. [Google Scholar]
- Robertson, G.L. Food Packaging: Principles and Practice; Taylor&Francis/CRCPress: NewYork, NY, USA, 1993. [Google Scholar]
- Floros, J. Controlled and Modified Atmospheres in Food Packaging and Storage. Chem. Eng. Prog. 1990, 86, 25–32. [Google Scholar]
- Park, H.J.; Chinnan, M.S.; Shewfelt, R.L. Edible Coating Effects on Storage Life and Quality of Tomatoes. J. Food Sci. 1994, 59, 568–570. [Google Scholar] [CrossRef]
- Park, H.J.; Rhim, J.W.; Lee, H.Y. Edible Coating Effects on Respiration Rate and Storage Life of “Fuji” Apples and “Shingo” Pears. Food Sci. Biotechnol. 1996, 5, 59–63. [Google Scholar]
- Paul, S.K. Edible Films and Coatings for Fruits and Vegetables. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Wang, X.; Kong, D.; Ma, Z.; Zhao, R. Effect of carrot puree edible films on quality preservation of fresh-cut carrots. Ir. J. Agric. Food Res. 2015, 54, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Licodiedoff, S.; Koslowski, L.; Scartazzini, L.; Monteiro, A.; Ninow, J.; Borges, C. Conservation of physalis by ediblecoating of gelatin and calciumchloride. Int. Food Res. J. 2016, 23, 1629–1634. [Google Scholar]
- Zhang, B.; Feng, X.; Han, P.; Duan, X. Effect of propolis/nano-silica composite coating on activities of ripening and senescence related enzymesin cherry tomato fruits. J. Chin. Inst. Food Sci. Technol. 2016, 16, 159–165. [Google Scholar]
- Poverenov, E.; Zaitsev, Y.; Arnon, H.; Granit, R.; Alkalai-Tuvia, S.; Perzelan, Y.; Weinberg, T.; Fallik, E. Effects of a composite chitosan–gelatin edible coating on postharvest quality and storability of red bellpeppers. Postharvest Biol. Technol. 2014, 96, 106–109. [Google Scholar] [CrossRef]
- Andrade, R.; Skurtys, O.; Osorio, F. Drop impact of gelatin coating formulated with cellulose nanofibers on banana and eggplant epicarps. LwtFood Sci. Technol. 2015, 61, 422–429. [Google Scholar] [CrossRef]
- Poverenov, E.; Rutenberg, R.; Danino, S.; Horev, B.; Rodov, V. Gelatin-Chitosan Composite Films and Edible Coatings to Enhance the Quality of Food Products: Layer-by-Layervs. Blended Formulations. Food Bioprocess Technol. 2014, 7, 3319–3327. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Casari, A.C.A.; Mariano, M.; Yamashita, F.; Mei, L.H.I.; Soldi, V.; Martelli, S.M. Effect of a gelatin-based edible coating containing cellulos enanocrystals(CNC) on the quality and nutrient retention of fresh strawberries during storage. In Proceedings of the 2nd International Conference on Structural Nano Composites (Nanostruc 2014), IOP Conference Series: Materials Science and Engineering, Madrid, Spain, 20–21 May 2014. [Google Scholar]
- Bizura Hasida, M.R.; NurAida, M.P.; Zaipun, M.Z.; Hairiyah, M. Quality evaluation of fresh-cut ‘josapine’ pineapple coated with hydrocolloid based edible coating using gelatin. Vii Int. Postharvest Symp. 2013, 1012, 1037–1041. [Google Scholar] [CrossRef]
- Feng, D.; Zhengguang, W.; Yimei, Z.; Xiang, Z.; Meng, G.; Xu, Y.; Bi, Y. Effect of chitosan composite coating on Chinese blueberry fruit (Vaccinium uliginosum L.). Ii Int. Symp. Discov. Dev. Innov. Strateg. Postharvest Dis. Manag. 2014, 1053, 207–211. [Google Scholar]
- NevesJunior, A.C.V.; Coneglian, R.C.C.; Soares, A.G.; Freitas, D.G.C.; Fonseca, M.J.O.; Barreira, F.R.; Miranda, A.F.M.D.; BECantwell, M.I.; Almeida, D.P.F. Physical and sensory characterization of edible coatings applied to minimally processed persimmon. Acta Hortic. 2012, 934, 537–542. [Google Scholar] [CrossRef]
- Fox, P.F.; McSweeney, P.L.H. Chapter1—Cheese: An Overview. In Cheese, 4th ed.; Springer US: New York, NY, USA, 2017; pp. 5–21. [Google Scholar]
- Pantaleão, I.; Pintado, M.M.E.; Poças, M.F.F. Evaluation of two packaging systems for regional cheese. Food Chem. 2007, 102, 481–487. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Sousa-Gallagher, M.J.; Macedo, I.; Rodriguez-Aguilera, R.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Use of galactomannan edible coating application and storage temperature for prolonging shelf-life of “Regional” cheese. J. Food Eng. 2010, 97, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Unalan, I.U.; Arcan, I.; Korel, F.; Yemenicioglu, A. Application of active zein-based films with controlled release properties to control Listeria monocytogenes growth and lipid oxidation in fresh Kashar cheese. Innov. Food Sci. Emerg. Technol. 2013, 20, 208–214. [Google Scholar] [CrossRef] [Green Version]
- DiPierro, P.; Sorrentino, A.; Mariniello, L. Chitosan/whey protein film as active coating to extend Ricotta cheese shelf-life. LwtFood Sci. Technol. 2011, 44, 2324–2327. [Google Scholar]
- Wagh, Y.R.; Pushpadass, H.A.; Emerald, F.M.E.; Nath, B.S. Preparation and characterization of milk protein films and their application for packaging of Cheddar cheese. J Food Sci Technol 2014, 51, 3767–3775. [Google Scholar] [CrossRef] [Green Version]
- Cao-Hoang, L.; Chaine, A.; Grégoire, L.; Waché, Y. Potential of nisin-incorporated sodium caseinate films to control Listeriain artificially contaminated cheese. Food Microbiol. 2010, 27, 940–944. [Google Scholar] [CrossRef]
- Moreira, M.D.R.; Pereda, M.; Marcovich, N.E.; Roura, S.I. Antimicrobial Effectiveness of Bioactive Packaging Materials from Edible Chitosan and Casein Polymers: Assessmenton Carrot, Cheese, and Salami. J. Food Sci. 2011, 76, M54–M63. [Google Scholar] [CrossRef]
- Ture, H.; Eroglu, E.; Banu, O.; Soyer, F. Effect of biopolymers containing natamycin against Aspergillus niger and Penicillium roquefortii on fresh kashar cheese. Int. J. Food Sci. Technol. 2011, 46, 154–160. [Google Scholar] [CrossRef]
- Bartkowski, L.; Dryden, F.D.; Marchello, J.A. Quality Changes of Beef Steaks Stored in Controlled Gas Atmospheres Containing High or Low Levels of Oxygen. J. Food Prot. 1982, 45, 41–45. [Google Scholar] [CrossRef]
- Andersen, H.J.; Bertelsen, G.; Boegh-Soerensen, L.; Shek, C.K.; Skibsted, L.H. Effect of light and packaging conditions on the colour stability of sliced ham. Meat Sci. 1988, 22, 283–292. [Google Scholar] [CrossRef]
- Andersen, H.J.; Bertelsen, G.; Ohlen, A.; Skibsted, L.H. Modified packaging as protection against photo degradation of the colour of pasteurized, sliced ham. Meat Sci. 1990, 28, 77–83. [Google Scholar] [CrossRef]
- Andersen, H.J.; Skibsted, L.H. Kinetics and mechanism of thermal oxidation and photooxidation of nitrosylmyoglobin in aqueous solution. J. Agric. Food Chem. 1992, 40, 1741–1750. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Kerry, J.P.; Hopkins, D.L. Meat packaging solutions to current industry challenges: A review. Meat Sci. 2018, 144, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Khosrozadeh, A.; Darabi, M.A.; Xing, M.; Wang, Q. Flexible Electrode Design: Fabrication of Freestanding Polyaniline-Based Composite Films for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 11379–11389. [Google Scholar] [CrossRef] [PubMed]
- Badr, K.R.; Ahmed, Z.S.; ElGamal, M.S. Evaluation of the Antimicrobial Action of Whey Protein Edible Films Incorporated with Cinnamon, Cumin and Thyme Against Spoilage Flora of FreshBeef. Int. J. Agric. Res. 2014, 9, 242–250. [Google Scholar]
- Alparslan, Y.; Baygar, T.; Baygar, T.; Yapıcı, H.; Metin, C. Effects of Gelatin-Based Edible Films Enriched with Laurel Essential Oil on the Quality of Rainbow Trout (Oncorhynchus mykiss) Fillets during Refrigerated Storage. Food Technol. Biotechnol. 2014, 52, 325–333. [Google Scholar]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P. Effects of bio-nanocomposite films from tilapia and squid skin gelatins incorporated with ethanolic extract from coconut husk on storage stability of mackerel meat powder. Food Packag. Shelf Life 2015, 6, 42–52. [Google Scholar] [CrossRef]
- Emiroğlu, Z.K.; Yemiş, G.P.; Coşkun, B.K.; Candoğan, K. Antimicrobial activity of soy edible films incorporated with thyme and oregano essential oils on fresh ground beef patties. Meat Sci. 2010, 86, 283–288. [Google Scholar] [CrossRef]
- Coşkun, B.K.; Çalikoğlu, E.; Emiroğlu, Z.K.; Candoğan, K. Antioxidant Active Packaging with Soy Edible Films and Oregano or Thyme Essential Oils for Oxidative Stability of Ground Beef Patties. J. Food Qual. 2014, 37, 203–212. [Google Scholar] [CrossRef]
- Zinoviadou, K.G.; Koutsoumanis, K.P.; Biliaderis, C.G. Physical and thermo-mechanical properties of whey protein isolate films containing antimicrobials, and their effect against spoilage flora of fresh beef. Food Hydrocoll. 2010, 24, 49–59. [Google Scholar] [CrossRef]
- Thaker, M.; Hanjabam, M.D.; Gudipati, V.; Kannuchamy, N. Protective Effect of Fish Gelatin-Based Natural Antimicrobial Coatings on Quality of Indian Salmon Fillets during Refrigerated Storage. J. Food Process Eng. 2017, 40. [Google Scholar] [CrossRef]
- Shokri, S.; Ehsani, A.; Jasour, M.S. Efficacy of Lactoperoxidase System - Whey Protein Coating on Shelf-life Extension of Rainbow Trout Fillets During Cold Storage (4 °C). Food Bioprocess Technol. 2015, 8, 54–62. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, Y.; Wei, X.; Xie, H.; Hider, R.; Zhou, T. Edible Antimicrobial Coating Incorporating a Polymeric Iron Chelator and Its Application in the Preservation of Surimi Product. Food Bioprocess Technol. 2016, 9, 1031–1039. [Google Scholar] [CrossRef]
- Christophersen, A.G.; Bertelsen, G.; Andersen, H.; Knuthsen, P.; Skibsted, L. Storage life of frozen salmonoids Effect of light and packaging conditions on carotenoid oxidation and lipid oxidation. Z. Unters. Nahr. Genußmittelsowie Gebrauchsgegenstände 1992, 194, 115–119. [Google Scholar] [CrossRef]
- Bak, L.S.; Andersen, A.B.; Andersen, E.M.; Bertelsen, G. Effect of modified atmosphere packaging on oxidative changes in frozen stored cold water shrimp (Pandalus borealis). Food Chem. 1999, 64, 169–175. [Google Scholar] [CrossRef]
- Aguilera Barraza, F.A.; León, R.A.Q.; Álvarez, P.X.L. Kinetics of protein and textural changes in Atlantic salmon under frozen storage. Food Chem. 2015, 182, 120–127. [Google Scholar] [CrossRef]
- Rodriguez-Turienzo, L.; Cobos, A.; Diaz, O. Effects of edible coatings based on ultrasound-treated whey proteins in quality attributes of frozen Atlantic salmon (Salmo salar). Innov. Food Sci. Emerg. Technol. 2012, 14, 92–98. [Google Scholar] [CrossRef]
- Rodriguez-Turienzo, L.; Cobos, A.; Moreno, V.; Caride, A.; Vieites, J.M.; Diaz, O. Whey protein-based coatings on frozen Atlantic salmon (Salmo salar): Influence of the plasticiser and themoment of coating on quality preservation. Food Chem. 2011, 128, 187–194. [Google Scholar] [CrossRef]
- Rodriguez-Turienzo, L.; Cobos, A.; Diaz, O. Effects of microbial transglutaminase added edible coatings based on heated or ultrasound-treated whey proteins in physical and chemical parameters of frozen Atlantic salmon (Salmo salar). J. Food Eng. 2013, 119, 433–438. [Google Scholar] [CrossRef]
- Seyfzadeh, M.; Motalebi, A.A.; Kakoolaki, S.; Gholipour, H. Chemical, microbiological and sensory evaluation of guttedkilka coated with whey protein based edible film incorporated with sodium alginate during frozen storage. Iran. J. Fish. Sci. 2013, 12, 140–153. [Google Scholar]
- Min, B.J.; Oh, J.-H. Antimicrobial Activity of Catfish Gelatin Coating Containing Origanum (Thymus capitatus) Oil against Gram-Negative Pathogenic Bacteria. J. Food Sci. 2009, 74, M143–M148. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ortega, I.; García-Almendárez, B.E.; Santos-López, E.M.; Amaro-Reyes, A.; Barboza-Corona, J.E.; Regalado, C. Antimicrobial Edible Films and Coatings for Meat and Meat Products Preservation. Sci. World J. 2014, 2014, 248935. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable Polymers and Green-based Antimicrobial Packaging Materials: Amini-review. Adv. Ind. Eng. Polym. Res. 2019. [Google Scholar] [CrossRef]
- Bourtoom, T. Factors affecting the properties of edible film prepared from mungbean proteins. Int. Food Res. J. 2010, 16, 167–180. [Google Scholar]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V. Edible bio-nano-hybrid coatings for food protection based on pectins and LDH-salicylate: Preparation and analysis of physical properties. Lwt Food Sci. Technol. 2016, 69, 139–145. [Google Scholar] [CrossRef]
- Wihodo, M.; Moraru, C.I. Physical and chemical methods used to enhance the structure and mechanical properties of protein films: A review. J. Food Eng. 2013, 114, 292–302. [Google Scholar] [CrossRef]
- Monedero, F.M.; Hambleton, A.; Talens, P.; Debeaufort, F.; Chiralt, A.; Voilley, A. Study of the retention and release of n-hexanal incorporated into soy protein isolate–lipid composite films. J. Food Eng. 2010, 100, 133–138. [Google Scholar] [CrossRef]
- Fabra, M.J.; Talens, P.; Chiralt, A. Tensile properties and water vapor permeability of sodium caseinate films containing oleicacid–beeswax mixtures. J. Food Eng. 2008, 85, 393–400. [Google Scholar] [CrossRef]
- Hu, C.; Chen, M.; Wang, Z. Release of Thymol, Cinnamaldehyde and Vanillin from Soy Protein Isolate Films into Olive Oil. Packag. Technol. Sci. 2012, 25, 97–106. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Z.; Hu, C.; Wang, J. Effects of Temperature on Release of Eugenol and Isoeugenol from Soy Protein Isolate Films into Simulated Fatty Food. Packag. Technol. Sci. 2012, 25, 485–492. [Google Scholar] [CrossRef]
- Liu, F.; Avena-Bustillos, R.J.; Chiou, B.-S.; Li, Y.; Ma, Y.; Williams, T.G.; Wood, D.F.; McHugh, T.H.; Zhong, F. Controlled-release of tea polyphenol from gelatin films incorporated with different ratios of free/nanoencapsulated tea polyphenolsin to fatty food simulants. Food Hydrocoll. 2017, 62, 212–221. [Google Scholar] [CrossRef]
- Kashiri, M.; Cerisuelo, J.P.; Domínguez, I.; López-Carballo, G.; Hernández-Muñoz, P.; Gavara, R. Novel antimicrobial zein film for controlled release of lauroyl arginate (LAE). Food Hydrocoll. 2016, 61, 547–554. [Google Scholar] [CrossRef] [Green Version]
- Calva-Estrada, S.J.; Jiménez-Fernández, M.; Lugo-Cervantes, E. Protein-Based Films: Advances in the Development of Biomaterials Applicable to Food Packaging. Food Eng. Rev. 2019, 11, 78–92. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Lara, H.H.; Ayala-Nuñez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of antiviral action of silver nano particles against HIV-1. J. Nanobiotechnology 2010, 8, 1–10. [Google Scholar] [CrossRef]
- Nocchetti, M.; Donnadio, A.; Ambrogi, V.; Andreani, P.; Bastianini, M.; Pietrella, D.; Latterini, L. Ag/Ag Clnano particle decorated layered double hydroxides: Synthesis, characterization and antimicrobial properties. J. Mater. Chem. B 2013, 1, 2383–2393. [Google Scholar] [CrossRef]
- Dutta, R.K.; Nenavathu, B.P.; Gangishetty, M.K.; Reddy, A.V.R. Studies on antibacterial activity of ZnO nanoparticles by ROS induced lipid peroxidation. Colloids Surf. B: Biointerfaces 2012, 94, 143–150. [Google Scholar] [CrossRef]
- Gadang, V.P.; Hettiarachchy, N.S.; Johnson, M.G.; Owens, C. Evaluation of antibacterial activity of whey protein isolate coating incorporated with nisin, grapeseed extract, malicacid, and EDTA ona Turkey frank furter system. J. Food Sci. 2008, 73, M389–M394. [Google Scholar] [CrossRef]
- Ku, K.; Song, K.B. Physical properties of nisin-incorporated gelatin and corn zein films and antimicrobial activity against Listeria monocytogenes. J. Microbiol. Biotechnol. 2007, 17, 520–523. [Google Scholar] [PubMed]
- Pintado, C.M.B.S.; Ferreira, M.A.S.S.; Sousa, I. Control of pathogenic and spoilage microorganisms from cheese surface by whey protein films containing malicacid, nisin and natamycin. Food Control 2010, 21, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Sivarooban, T.; Hettiarachchy, N.S.; Johnson, M.G. Physical and antimicrobial properties of grapeseed extract, nisin, and EDTA incorporated soy protein edible films. Food Res. Int. 2008, 41, 781–785. [Google Scholar] [CrossRef]
- Zinoviadou, K.G.; Koutsoumanis, K.P.; Biliaderis, C.G. Physico-chemical properties of whey protein isolate films containing oregano oil and their antimicrobial action against spoilage flora of fresh beef. Meat Sci. 2009, 82, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, K.L.; Han, I.Y.; Dawson, P.L. Antimicrobial Effects of Corn Zein Films Impregnated with Nisin, Lauric Acid, and EDTA. J. Food Prot. 2001, 64, 885–889. [Google Scholar] [CrossRef]
- Kristo, E.; Koutsoumanis, K.P.; Biliaderis, C.G. Thermal, mechanical and water vapor barrier properties of sodium caseinate films containing antimicrobials and their inhibitory actionon Listeria monocytogenes. Food Hydrocoll. 2008, 22, 373–386. [Google Scholar] [CrossRef]
- Pintado, C.M.B.S.; Ferreira, M.A.S.S.; Sousa, I. Properties of Whey Protein-Based Films Containing Organic Acids and Nisin to Control Listeria monocytogenes. J. Food Prot. 2009, 72, 1891–1896. [Google Scholar] [CrossRef] [Green Version]
- Seacheol, M.; Krochta, J.M. Ascorbic acid-containing whey protein film coatings for control of oxidation. J. Agric. Food Chem. 2007, 55, 2964–2969. [Google Scholar]
- Park, S.I.; Daeschel, M.A.; Zhao, Y. Functional properties of antimicrobial lysozyme-chitosan composite films. J. Food Sci. 2004, 69, M215–M221. [Google Scholar] [CrossRef]
- Shokri, S.; Ehsani, A. Efficacy of whey protein coating incorporated with lactoperoxidase and α-tocopherol in shelflife extension of Pike-Perch fillets during refrigeration. Lwt Food Sci. Technol. 2017, 85, 225–231. [Google Scholar] [CrossRef]
- Al-Sahlany, S.T.G. Production of Biodegradable Film from Soy Protein and Essential Oil of Lemon Peeland Useitas Cheese Preservative. Basrah J. Agric. Sci. 2017, 30, 27–35. [Google Scholar] [CrossRef]
- Kashiri, M.; Cerisuelo, J.P.; Domínguez, I.; López-Carballo, G.; Muriel-Gallet, V.; Gavara, R.; Hernández-Muñoz, P. Zein films and coatings as carriers and release systems of Zataria multiflora Boiss. essential oil for antimicrobial food packaging. Food Hydrocoll. 2017, 70, 260–268. [Google Scholar]
- Alparslan, Y.; Yapıcı, H.H.; Metin, C.; Baygar, T.; Günlü, A.; Baygar, T. Quality assessment of shrimps preserved with orange leaf essential oil incorporated gelatin. Lwt Food Sci. Technol. 2016, 72, 457–466. [Google Scholar] [CrossRef]
- Bahram, S.; Rezaei, M.; Soltani, M.; Kamali, A.; Ojagh, S.M.; Abdollahi, M. Whey Protein Concentrate Edible Film Activated with Cinnamon Essential Oil. J. Food Process. Preserv. 2014, 38, 1251–1258. [Google Scholar] [CrossRef]
- Bahram, S.; Rezaie, M.; Soltani, M.; Kamali, A.; Abdollahi, M.; Ahmadabad, M.K.; Nemati, M. Effect of whey Protein Concentrate Coating Cinamon Oil on Quality and ShelfLife of Refrigerated Beluga Sturegeon (Huso huso). J. Food Qual. 2016, 39, 743–749. [Google Scholar] [CrossRef]
- Gómez-Heincke, D.; Martínez, I.; Partal, P.; Guerrero, A.; Gallegos, C. Development of antimicrobial active packaging materials based on gluten proteins. J. Sci. Food Agric. 2016, 96, 3432–3438. [Google Scholar] [CrossRef]
- Kavas, G.; Kavas, N.; Saygili, D. The Effects of Thymeand Clove Essential Oil Fortified Edible Films on the Physical, Chemical and Microbiological Characteristics of Kashar Cheese. J. Food Qual. 2015, 38, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Won, M.; Song, K.B. Physical properties and antimicrobial activities of porcine meat and bone meal protein films containing corianderoil. Lwt Food Sci. Technol. 2015, 63, 700–705. [Google Scholar] [CrossRef]
- Martucci, J.F.; Gende, L.B.; Neira, L.M.; Ruseckaite, R.A. Oregano and lavender essential oils as antioxidant and antimicrobial additives of biogenic gelatin films. Ind. Crop. Prod. 2015, 71, 205–213. [Google Scholar] [CrossRef]
- Clarke, D.; Molinaro, S.; Tyuftin, A.; Bolton, D.; Fanning, S.; Kerry, J.P. Incorporation of commercially-derived antimicrobials into gelatin-based films and assessment of their antimicrobial activity and impact on physical film properties. Food Control 2016, 64, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Ibarguren, C.; Céliz, G.; Díaz, A.S.; Bertuzzi, M.A.; Daz, M.; Audisio, M.C. Gelatine based films added with bacteriocins and a flavonoid ester active against food-borne pathogens. Innov. Food Sci. Emerg. Technol. 2015, 28, 66–72. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Physicochemical properties of gelatin/silver nanoparticle antimicrobial composite films. Food Chem. 2014, 148, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Pereira, L.; Cruz, J.M.; Sendón, R.; Rodríguez Bernaldode Quirós, A.; Ares, A.; Castro-López, M.; Abad, M.J.; Maroto, J.; Paseiro-Losada, P. Development of antioxidant active films containing tocopherols to extend the shelflife of fish. Food Control 2013, 31, 236–243. [Google Scholar] [CrossRef]
- Fabra, M.J.; Hambleton, A.; Talens, P.; Debeaufort, F.; Chiralt, A. Effect of ferulicacid and α-tocopherol antioxidants on properties of sodium caseinate edible films. Food Hydrocoll. 2011, 25, 1441–1447. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Physical properties and antioxidant capacity of starch–sodium caseinate films containing lipids. J. Food Eng. 2013, 116, 695–702. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Chambin, O.; Assifaoui, A.; Al-Assaf, S.; Karbowiak, T.; Debeaufort, F. Release of coumarin incorporated into chitosan-gelatin irradiated films. Food Hydrocoll. 2016, 56, 266–276. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Osako, K.; Benjakul, S.; Suthiluk, P.; Rawdkuen, S. Shelflife extension for Bluefin tuna slices (Thunnus thynnus) wrapped with myofibrillar protein film incorporated with catechin-Kradon extract. Food Control 2017, 79, 333–343. [Google Scholar] [CrossRef]
- Ciannamea, E.M.; Stefani, P.M.; Ruseckaite, R.A. Properties and antioxidant activity of soy protein concentrate films incorporated with redgrape extract processed by casting and compression molding. LWT 2016, 74, 353–362. [Google Scholar] [CrossRef]
- Gallego, M.G.; Gordon, M.H.; Segovia, F.; Pablos, M.P.A. Gelatine-Based Antioxidant Packaging Containing Caesalpinia decapetala and Tara as a Coating for Ground Beef Patties. Antioxidants 2016, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Chen, S.; Ge, S.; Miao, J.; Li, J.; Zhang, Q. Preparation, properties and antioxidant activity of an active film from silver carp (Hypophthalmichthys molitrix) skin gelatin incorporated with greentea extract. Food Hydrocoll. 2013, 32, 42–51. [Google Scholar] [CrossRef]
- Yang, H.-J.; Lee, J.-H.; Won, M.; Song, K.B. Antioxidant activities of distiller dried grains with solubles as protein films containing tea extracts and their application in the packaging of pork meat. Food Chem. 2016, 196, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Ihl, M.; Bifani, V.; Silva, A.; Montero, P. Edible films made from tuna-fish gelatin with antioxidant extracts of two different murta ecotypes leaves (Ugni molinae Turcz). Food Hydrocoll. 2007, 21, 1133–1143. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; DiasdosSantos, S.M.; Pintado, M.E.; Pérez-Álvarez, J.A.; Fernández-López, J.; Viuda-Martos, M. Chemical composition and invitro antimicrobial, antifungal and antioxidant properties of essential oils obtained from some herbs widely used in Portugal. Food Control 2013, 32, 371–378. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. In Vitro antibacterial and antioxidant properties of chitosan edible films incorporated with Thymus moroderi or Thymus piperella essential oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Song, N.-B.; Lee, J.-H.; AlMijan, M.; Song, K.B. Development of a chicken feather protein film containing cloveoil and its application in smoked salmon packaging. Lwt Food Sci. Technol. 2014, 57, 453–460. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; RazaviRohani, S.M.; Mahmoudian, A. Antioxidant and antimicrobial effects of zein edible film impregnated with Zataria multiflor a Boiss. essential oil and monolaurin. Lwt Food Sci. Technol. 2016, 72, 37–43. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Lourenço, R.V.; Bittante, A.M.Q.B.; Moraes, I.C.F.; Sobral, P.J.D.A. Gelatin-based films reinforced with montmorillonite and activated with nanoemulsion of ginger essential oil for food packaging applications. Food Packag. Shelf Life 2016, 10, 87–96. [Google Scholar] [CrossRef]
- MaryamAdilah, Z.A.; NurHanani, Z.A. Active packaging of fish gelatin films with Morinda citrifolia oil. Food Biosci. 2016, 16, 66–71. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Pires, C.; Ramos, C.; Batista, I.; Saraiva, J.A.; Nunes, M.L. Characterization of fish protein films incorporated with essential oils of clove, garlic and origanum: Physical, antioxidant and antibacterial properties. Lwt Food Sci. Technol. 2014, 59, 533–539. [Google Scholar] [CrossRef]





| System of Application | Plasticizer | References |
|---|---|---|
| Zein | Oleic and linoleic acids | [47] |
| Whey protein | GLY and sorbitol | [51] |
| Wheat gluten | saturated fatty acids | [45] |
| Glycerin | [52] | |
| Caseinate-pullulan | Water and sorbitol | [34] |
| Whey protein/beeswax emulsion | GLY | [53] |
| Gelatin | GLY and sorbitol | [53] |
| Sucrose, oleic acid, citric acid, tartaric acid, malic acid, PEG of different molecular weights (300, 400, 600, 800, 1500, 4000,10,000 and 20,000), sorbitol, mannitol, EG, DEG, TEG, EA, di ethanol amine (DEA) and TEA | [35] | |
| Pigskin gelatin | GLY | [54] |
| Sorbitol | [55] | |
| Bovine gelatin | Fatty acids | [56] |
| Sorbitol | [55] | |
| GLY | [57] |
| Films | Plasticizers | Opacity (A.nm) | Mechanical Properties (TS in MPa) | Thermal Properties | Water Vaper Permeability | References |
|---|---|---|---|---|---|---|
| Wheat gluten | ||||||
| Gliadins | Gly 35% | ~34 | %E = ~390 TS = ~7 | NR | ~7 × 1011 [(gm)/(m2 s Pa)] | [58] |
| Glutenins | Gly 35% | ~101 | %E = ~250 TS = ~1 | NR | ~4 × 1011 [(g m)/(m2 s Pa)] | |
| Other Sources | ||||||
| Zein | Gly 40% | NR | %E = ~118 TS = ~4 | Tg = ~30 °C | ~4 (g mm/m2 h kPa) | [59] |
| Kafirin | Gly 40% | NR | %E = ~24 TS = ~1 | Tg = ~30 °C | ~8 (g mm/m2 h kPa) | |
| Avenin | Gly 40% | NR | %E = ~40 TS = ~4 | Tg = ~28 °C | ~3 (g mm/m2 h kPa) | |
| Milk | ||||||
| Casein | Gly 50% | NR | %E = ~65 TS = ~2.5 | NR | ~7 (g mm/m2 h kPa) | [60] |
| Whey fraction | ||||||
| WPI | Gly 40% | NR | %E = ~33 TS = ~0.9 | Tg = ~50 °C | ~8 (g mm/m2 d kPa) | [61] |
| WPC | Gly 40% | NR | %E = ~18 TS = ~0.7 | Tg = ~43 °C | ~10 (g mm/m2 d kPa) | |
| Synthetic polymers | ||||||
| High Density Polyethylene (HDPE) | NR | NR | %E = ~600 TS = ~54 | Tg = ~80 °C | ~6 (g/m2 d) | [21] |
| Low Density Polyethylene (LDPE) | NR | NR | %E = ~300 TS = ~27 | Tg = ~−125 °C | ~18 (g/m2 d) | |
| Polypropylene (PP) | NR | NR | %E = ~150 TS = ~151 | Tg = ~−10 °C | ~8 (g/m2 d) | |
| Polyethylene Terephthalate (PET) | NR | NR | %E = 70 TS = 79 | Tg = ~76 °C | ~21 (g/m2 d) | |
| Group | Commodity | CO2 (%) a | O2 (%) a |
|---|---|---|---|
| 1 | Potatoes | 0 | 0 |
| Carrots | 0 | 0 | |
| Beets | 0 | 0 | |
| 2 | Tomatoes | 0 | 3–5 |
| Peppers | 0 | 3–5 | |
| Cucumbers | 0 | 3–5 | |
| Lettuce | 0 | 2–5 | |
| Celery | 0 | 2–4 | |
| Onions(dry) | 0 | 1–2 | |
| 3 | Pears | 0–5 | 1–3 |
| Lemons | 0–5 | 5 | |
| Apples | 1–5 | 2–3 | |
| Cauliflowers | 2–5 | 2–5 | |
| Artichokes | 3–5 | 2–3 | |
| Peaches | 5 | 1–2 | |
| 4 | Others | 5–15 | 1–5 |
| Product, Storage | Film | Added Values | Effects | References | |
|---|---|---|---|---|---|
| Fresh Kashar cheese, 4 °C, 8 weeks | Zein (Z)/carnauba wax (5%) composite films (ZW) | Lysozyme (0.7 mg/cm2) (L) | L. monocytogenes | C and F-Z did not change significantly counts in the first 28 days, but the counts of these controls increased between the 28th and 56th days All samples containing lysozyme showed significant reduction No significant increase occurred in counts of cheese samples AF-Z-L, AF-ZW-L, AF-Z-MIX, AF-ZW-MIX | [178] |
| Mixture of lysozyme (0.7 mg/cm2), catechin (3 mg/cm2) and gallic acid (3.0 mg/cm2) (MIX) | Lipid oxidation/TBARS | C > F-Z = AF-Z-L = AF-ZW-L (no significant effect) >AF-Z-MIX = AF-ZW-MIX (significantly lower) | |||
| Unripened, creamy Ricotta cheese, MAP (40% CO2, 60% N2) at 4 °C, 30days | Chitosan/whey protein coating | pH | C = ACO (decrease, after 7, and remained relatively constant until 30 days) | [179] | |
| Titratable acidity | C (increased) > ACO (no significant differences) | ||||
| LAB | C > ACO | ||||
| Mesophilic acrobic bacteria | C > ACO | ||||
| Psychrotrophic bacteria | C > ACO | ||||
| Acidity | Delayed development by ACO | ||||
| Sensory quality | No effect of ACO | ||||
| Shelf-life | C < ACO | ||||
| Cheddar cheese, 5 ± 1 °C, 30 days | Casein (CS) Whey protein concentrate (WPC) films | Soluble nitrogen | C = F-CS = F-WPC (125.7–151.2 mgN2/100 g) | [180] | |
| TBARS | C (0.01–0.05) > F-CS = F-WPC (0.01–0.04) | ||||
| Titratable acidity | C > F-CS = F-WPC | ||||
| TVC | C = F-CS = F-WPC (7.8–8.1 log CFU/g) | ||||
| Yeast, mold | C (1.1–1.9 log CFU/g) >F-CS = F-WPC (1.1–1.8 log CFU/g) | ||||
| Sensory | No significant effect | ||||
| Semisoft, mini RedBabybel® cheese, 4 °C, 1 week | Sodium caseinate film | Nisin (1000 IU/cm2 surface area AF) | *Inoculated product was put on active film for analyses Listeria innocua | [181] | |
| Surface-contaminated cheese | C > AF (1.1 log) | ||||
| In-depth contaminated cheese, mm distance of film from contaminated spot | AF, 3 mm (0.25 log) > AF, 2 mm (0.9 log) > AF, 1 mm (1.1 log) | ||||
| Cheddar cheese, 65% RH, 10 °C, 5 days | Sodium caseinate (SC) | Psychrotrophic bacteria | C > F-SC = CO-SC > F-CH = CO-CH = F-SC/CH = CO-SC/CH | [182] | |
| Chitosan/sodium caseinate (SC/CH) films | Yeast | C > F-SC = CO-SC > F-CH = CO-CH = F-SC/CH = CO-SC/CH | |||
| Molds | C > F-SC = CO-SC > F-CH = CO-CH = F-SC/CH = CO-SC/CH | ||||
| Fresh Kashar cheese, 10 °C, 30 days | Wheat gluten (WG) methyl cellulose (MC) films | Natamycin 1.2 mg NA/10 g film solution 2.5 mg NA/10 g film solution 3.10 mg NA/10 g film solution 4.20 mg NA/10 g film solution | A. niger | C > F-MC (0.6 log) = AF-MC1 (no significant reduction) >AF-MC2 (2 log) = AF-MC3 = AF-MC4 C > F-WG (4.11 log) > AF-WG1 (completely inhibited) = AF-MC2 = AF-MC3 = AF-MC4 | [183] |
| Product, Storage | Films/Coatings | Added Value | Effect | References | |
|---|---|---|---|---|---|
| Fresh beef cuts: 5 °C, 12 days | Whey protein isolate | Cinnamon, cumin, thyme essential oil (TO) | TVC (shelf life) | C = F < AF-cinnamon (4–12 days) < AF-cumin (6–12 days) < AF with-thyme (8–>12 days) | [190] |
| Rainbow trout fillets vacuum: 4 °C, 26 days | Gelatin | LEO | TVC, psychrotrophic bacteria counts, Enterobacteriaceae, and LAB | C < F < AF 0.1% LEO < AF 1% LEO | [191] |
| Color, pH increase, TVB-N, free fatty acid, PV, and TBARS | Preservative effect followed increasing order: C < F < AF 0.1% LEO < AF 1% LEO | ||||
| Sensory shelf life | AF 1 % LEO (22 days) > AF 0.1% LEO = F (20 days) > C (15 days) | ||||
| Mackerel meat powder: 28–30 °C, 30% RH, 30 days | Gelatin with CNa lid sealed to aluminum cups | Coconut husk ethanol extract (CH) | Oxidation (PV, TBARS, and volatile compounds) | Decrease in AF–CNa–CH | [192] |
| Moisture absorption | Decrease in AF–CNa–CH | ||||
| Ground beef patties vacuum: 4 °C, 12 days | Isolated soy protein | Oreganum heracleoticum (OR), Thymus vulgaris L. (TH) essential oil OR+TH ratio of 1:1 | TBARS | No effect | [193,194] |
| PV and free fatty acids | Lower values were determined for AF-OR or AF-TH particularly at later stages of storage | ||||
| Color | Reduced, but acceptable, redness (a*) values | ||||
| TVC, LAB, and Staphylococcus spp. | No effect of films | ||||
| Coliform bacteria and Pseudomonas spp. | Reduced in AFs | ||||
| Fresh beef cuts: 5 °C, 12 days | Whey protein isolate | Sodium lactate (NaL); ε-polylysine (ε-PL) | TVC (shelf life) | C = F (6 days) <AF–ε–PL 0.25% = AF-NaL 1% (8–10 days) < AF-ɛ–PL 0.75% = AF-NaL 2% (10–12 days) V | [195] |
| Pseudomonades counts | C = F > AF- ɛ–PL 0.25% = AF-NaL 1% > AF-ɛ–PL 0.75% = AF-NaL 2% | ||||
| LAB counts | C = F = AF-NaL 1% = AF-NaL 2% > AF-ɛ–PL 0.25% > AF-ɛ–PL 0.75% | ||||
| Indian salmon fillets 6 °C, 16 days | Gelatin chitosan; T1: gelatin; T2: gelatin + chitosan + garlic extract; T3: gelatin + chitosan + lime juice | Lime extract; garlic extract | TBARS (shelf life) | C (8 days) < T2 < T1 = T3 (16 days) | [196] |
| TVB-N (shelf life) | No effect of coatings (between 8 and 12 days) | ||||
| pH increase | C = T1 > T3 > T2 | ||||
| TVC (shelf life) | C = T1 (8 days) < T3 (16 days) < T2 (above 16 days) | ||||
| Psychrophilic count | C = T1 > T3 (2 log) > T2 (3 log) | ||||
| Sensory shelf life | C (8–12 days) < T1 = T3 (12–16 days) < T2 (16 days) | ||||
| Rainbow Trout Fillets: 4 °C, 16 days | WPC | LPOS | TVB-N | Reduced | [197] |
| Bacterial growth | Reduced | ||||
| pH changes | Reduced | ||||
| Lipid oxidation | No effect | ||||
| Sensory shelf life | Extended by 4 days for ACO with 1.25% (v/w) LPOS; while 2.5%, 5%, and 7.5% LPOS ACOs showed moderate to high overall acceptability even until the 16th day of the storage period | ||||
| Grass carp fish balls: 4 °C, 20 days | Corn zein | Hexadentate 3-hydroxypyridinones (polymeric chelator) | Sensory properties | C < CO < ACO (similar till 10th day and than considerable differences) | [198] |
| TVB-N | C > CO > ACO | ||||
| TBARS | C > CO > ACO | ||||
| TVC | C > CO (2 log) > ACO (4 log) | ||||
| pH | ACO maintained stable pH during storage | ||||
| Shelf life | C (7 days) < CO (13 days) < ACO (19 days) | ||||
| Antimicrobial Agents | Microorganisms | Performance Impact of Protein-Based Films | References | |
|---|---|---|---|---|
| Bacteriocins | nisin | Listeria monocytogenes, Pseudomonas aeruginosa, Yarrowialipolytica, Penicillium commune, Penicillium chrysogenum | The strength was increased and the permeability was decreased. | [224,225,226,227] |
| ε-polylysine | Spoilage flora of fresh beef | The strength was decreased and the flexibility was increased. | [228] | |
| EDTA | L. monocytogenes, Escherichia coli, Salmonella typhimurium, and Salmonella enteritidis | There was a minimal effect on the mechanical properties. | [224,227,229] | |
| Acidulant agents | sodium lactate, potassium sorbate, and citric, acetic, malic, lactic, tartaric, sorbic and paminobenzoicacids | L. monocytogenes, E. coli, Salmonella gaminara, and Salmonella typhimurium | The water-content equilibrium, water vapor permeability, and extensibility that affected the glass-transition temperature of the film were increased. | [230,231] |
| Antimicrobial enzymes | Lacto Per Oxidase System (LPOS) and lysozyme | Shewanellaputrefaciens, Pseudomonas fluorescens, L. monocytogenes, Bacillus subtilis, E. coli, and Staphylococcus aureus | The film structure and integrity were weakened, but when the concentration of active compounds was low, the film’s properties would not be affected. | [102,232,233,234] |
| EOs | lemon peel, Zataria multiflora Boiss, orange leaves, cinnamon, thyme, clove and oregano | Pathogens and food-spoilage microorganisms | The permeability, water solubility, strength and extensibility were decreased. | [235,236,237,238,239,240,241,242,243] |
| Commercially derived antimicrobials | ArticoateDLP-02, Artimex 152/NL, sodium octanoate, and Auranta FV | E. coli, Bacillus cereus, P. fluorescens, S. aureus, and microflora from beef steaks [32] | The protein network was destabilized. | [244] |
| Ethyl-Nα-dodecanoyl-L-Arginate hydrochloride (LAE) | L. monocytogenes and E. coli | A barrier against carbon dioxide and oxygen was formed. | [218] | |
| Prunin Laurate ester (PL) | L. monocytogenes, S. aureus, and B. cereus | The functional properties were not affected. | [245] | |
| NPs | Silver Nano Particles (AgNP) | foodborne pathogens | The barrier and mechanical properties were enhanced, but there might be potential toxicity. | [246] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of Protein-Based Films and Coatings for Food Packaging: A Review. Polymers 2019, 11, 2039. https://doi.org/10.3390/polym11122039
Chen H, Wang J, Cheng Y, Wang C, Liu H, Bian H, Pan Y, Sun J, Han W. Application of Protein-Based Films and Coatings for Food Packaging: A Review. Polymers. 2019; 11(12):2039. https://doi.org/10.3390/polym11122039
Chicago/Turabian StyleChen, Hongbo, Jingjing Wang, Yaohua Cheng, Chuansheng Wang, Haichao Liu, Huiguang Bian, Yiren Pan, Jingyao Sun, and Wenwen Han. 2019. "Application of Protein-Based Films and Coatings for Food Packaging: A Review" Polymers 11, no. 12: 2039. https://doi.org/10.3390/polym11122039
APA StyleChen, H., Wang, J., Cheng, Y., Wang, C., Liu, H., Bian, H., Pan, Y., Sun, J., & Han, W. (2019). Application of Protein-Based Films and Coatings for Food Packaging: A Review. Polymers, 11(12), 2039. https://doi.org/10.3390/polym11122039