Enhancement of Solvent Resistance of Polyimide Electrospun Mat via the UV-Assisted Electrospinning and Photosensitive Varnish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PSPI resin
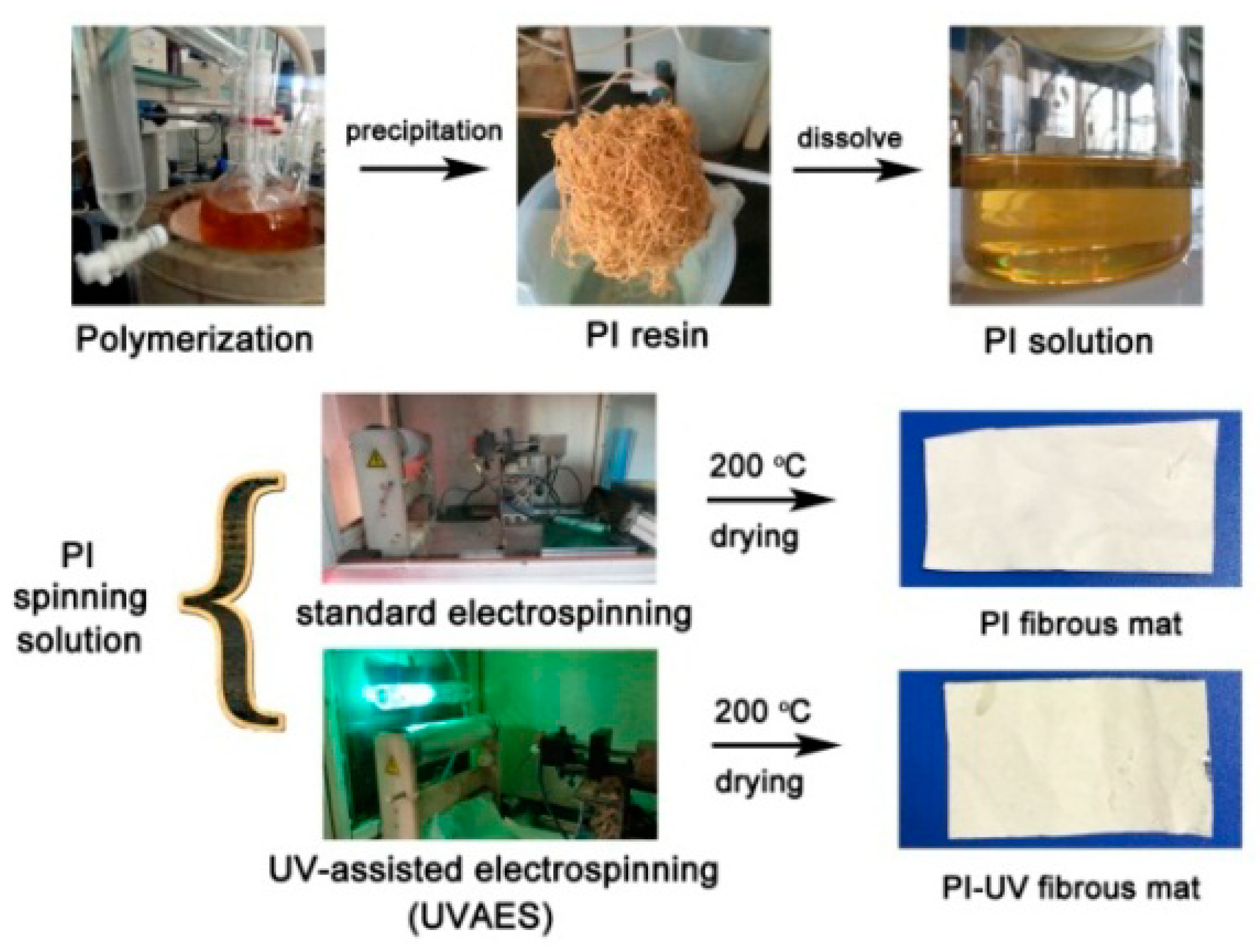
2.3. Fabrication of PSPI Electrospun Fibrous Mats
2.3.1. Standard Electrospinning
2.3.2. UV-assisted Electrospinning (UVAES)
2.4. Characterization Methods
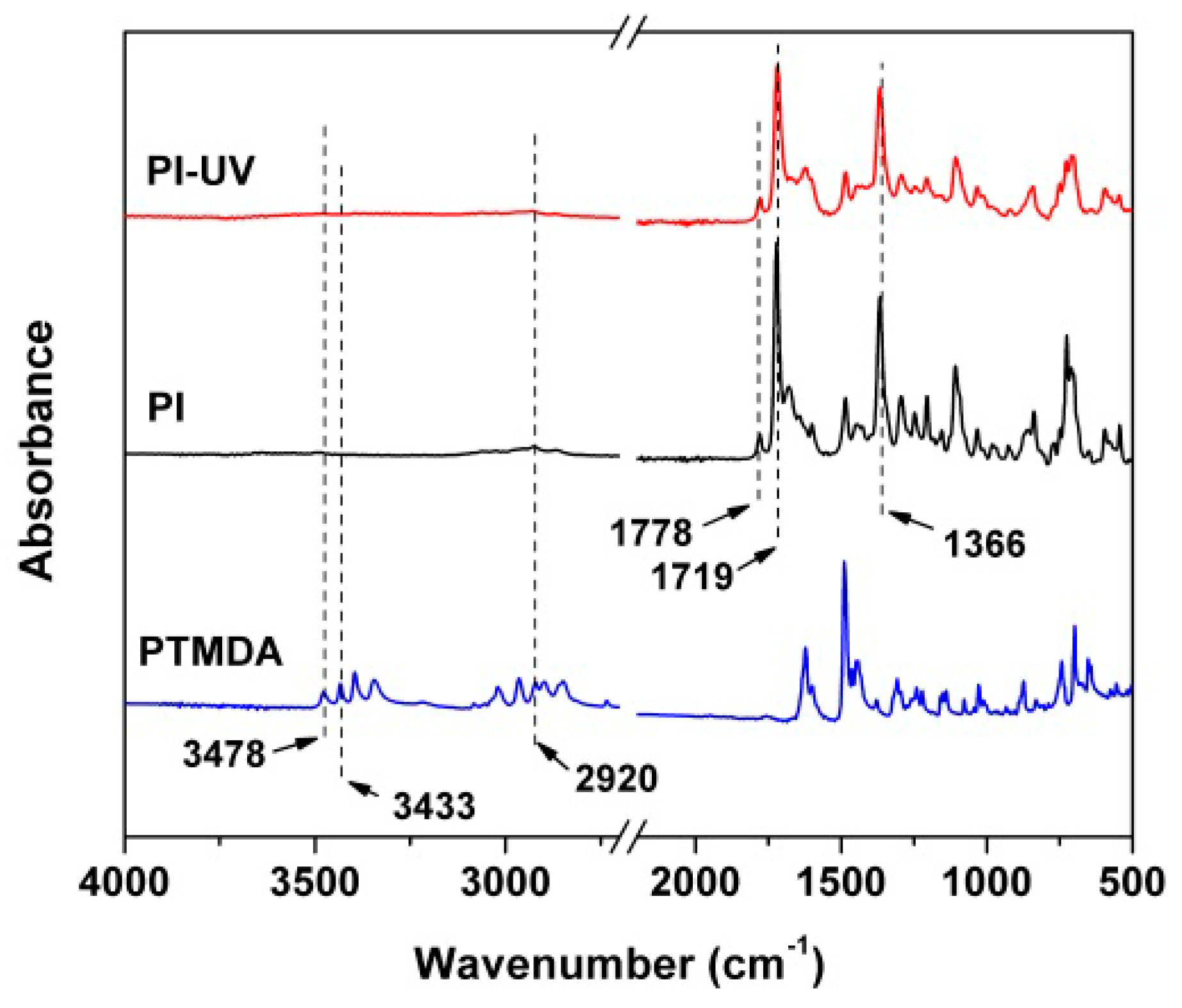
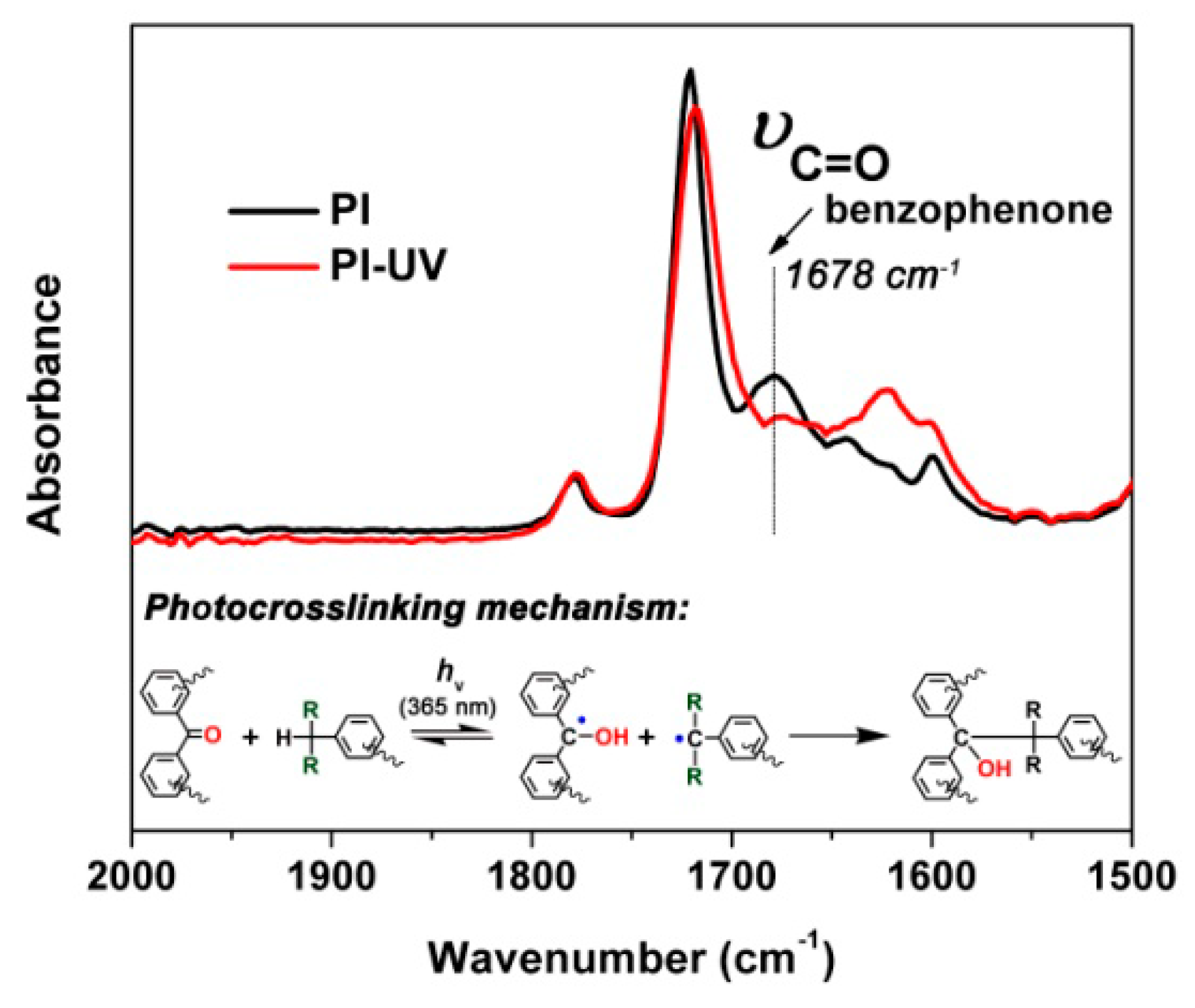
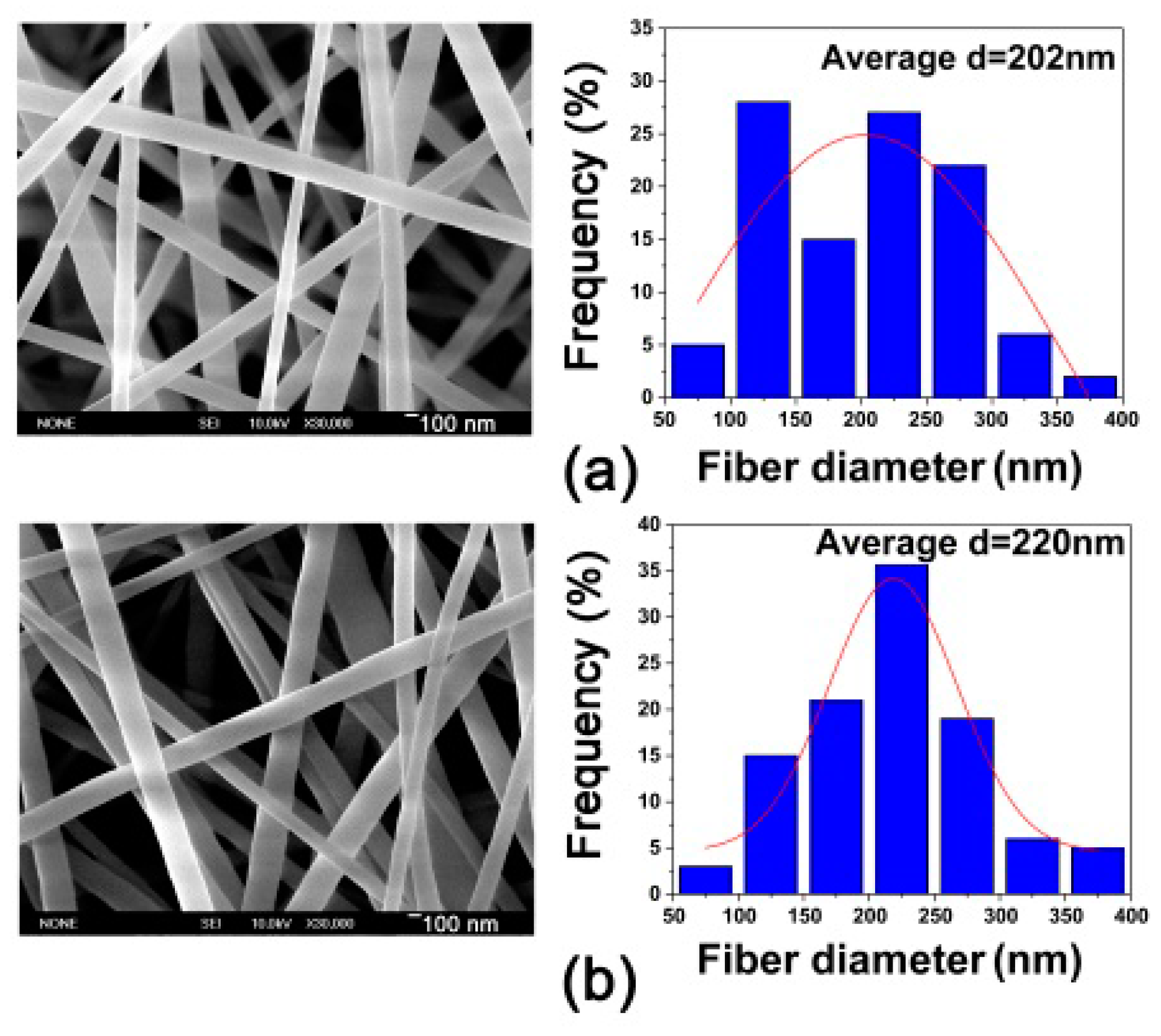
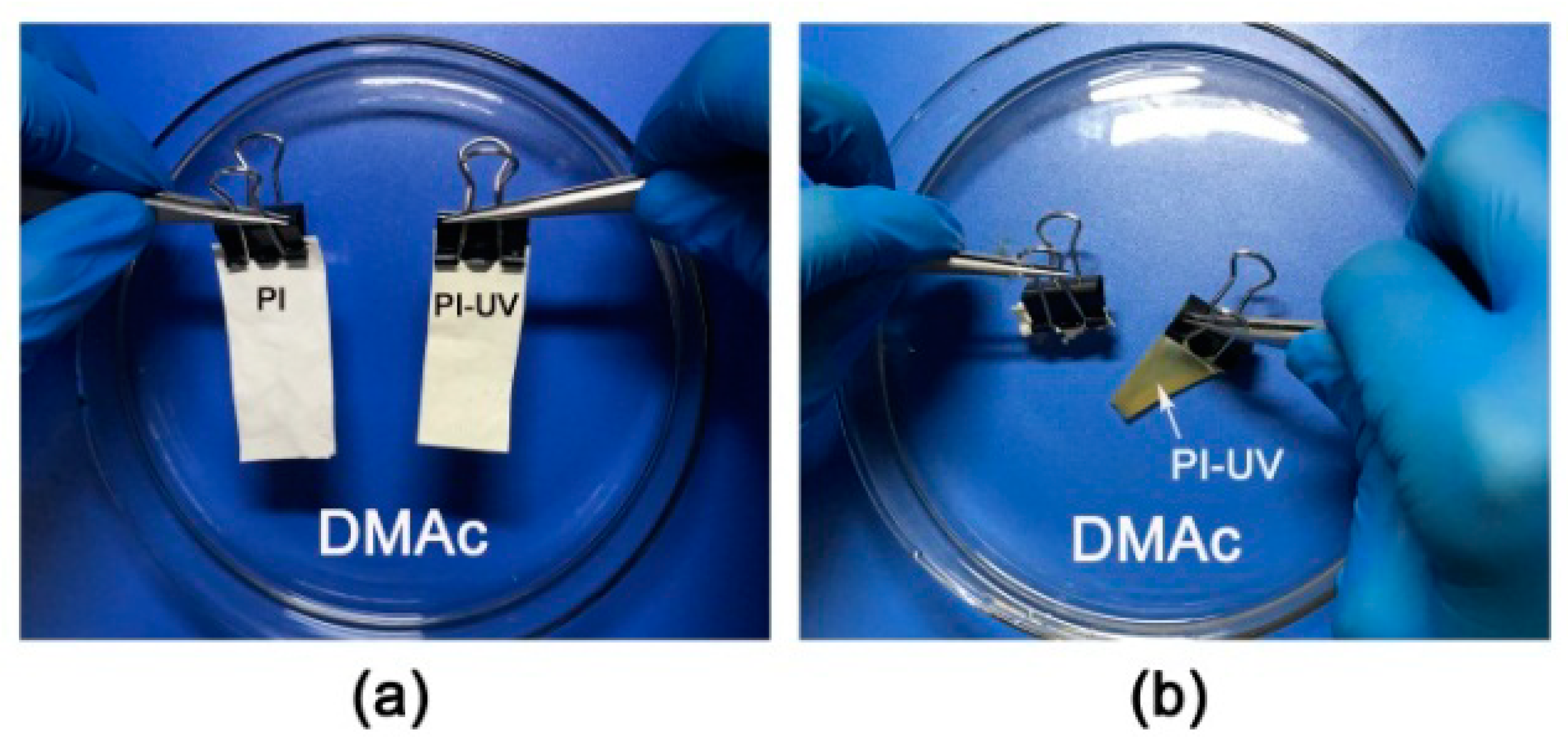
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.S.; Li, C.W.; Nguyen, N.C.; Nguyen, H.T. A comprehensive review: Electrospinning technique for fabrication and surface modification of membranes for water treatment application. RSC Adv. 2016, 6, 85495–85514. [Google Scholar] [CrossRef]
- Ding, Y.; Hou, H.; Zhao, Y.; Zhu, Z.; Fong, H. Electrospun polyimide nanofibers and their applications. Prog. Polym. Sci. 2016, 61, 67–103. [Google Scholar] [CrossRef]
- Wang, Q.; Song, W.L.; Wang, L.; Song, Y.; Shi, Q.; Fan, L.Z. Electrospun polyimide-based fiber membranes as polymer electrolytes for lithium-ion batteries. Electrochim. Acta 2014, 132, 538–544. [Google Scholar] [CrossRef]
- Shayapat, J.; Chung, O.H.; Park, J.S. Electrospun polyimide-composite separator for lithium-ion batteries. Electrochim. Acta 2015, 170, 110–121. [Google Scholar] [CrossRef]
- Miao, Y.E.; Zhu, G.N.; Hou, H.; Xia, Y.Y.; Liu, T.X. Electrospun polyimide nanofiber-based nonwoven separators for lithium-ion batteries. J. Power Sour. 2013, 226, 82–86. [Google Scholar] [CrossRef]
- Cao, L.; An, P.; Xu, Z.; Huang, J. Performance evaluation of electrospun polyimide non-woven separators for high power lithium-ion batteries. J. Electroanal. Chem. 2016, 767, 34–39. [Google Scholar] [CrossRef]
- Li, S.; Wu, D.; Yan, X.; Guan, Y. Acetone-activated polyimide electrospun nanofiber membrane for thin-film microextraction and thermal desorption-gas chromatography-mass spectrometric analysis of phenols in environmental water. J. Chromatogr. A 2015, 1411, 1–8. [Google Scholar] [CrossRef]
- Oktay, B.; Toker, R.D.; Kayaman-Apohan, N. Superhydrophobic behavior of polyimide-siloxane mats produced by electrospinning. Polym. Bull. 2015, 72, 2831–2842. [Google Scholar] [CrossRef]
- Gu, J.; Lv, Z.; Wu, D.; Guo, Y.; Tian, L.; Qiu, H.; Li, W.; Zhang, Q. Dielectric thermally conductive boron nitride/polyimide composite with outstanding thermal stabilities via in-situ polymerization-electrospinning-hot press method. Compos. Part A Appl. Sci. Manuf. 2017, 94, 209–216. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, C.; Hsu, P.C.; Zhang, C.; Liu, N.; Zhang, J.; Cui, Y. Nanofiber air filters with high-temperature stability for efficient PM2. 5 removal from the pollution sources. Nano Lett. 2016, 16, 3642–3649. [Google Scholar] [CrossRef]
- Khalid, B.; Bai, X.; Wei, H.; Huang, Y.; Wu, H.; Cui, Y. Direct blow-spinning of nanofibers on a window screen for highly efficient PM2. 5 removal. Nano Lett. 2017, 17, 1140–1148. [Google Scholar] [CrossRef]
- Jiang, S.; Hou, H.; Agarwal, S.; Greiner, A. Polyimide nanofibers by “Green” electrospinning via aqueous solution for filtration applications. ACS Sustain. Chem. Eng. 2016, 4, 4797–4804. [Google Scholar] [CrossRef]
- Gautam, A.K.; Lai, C.; Fong, H.; Menkhaus, T.J. Electrospun polyimide nanofiber membranes for high flux and low fouling microfiltration applications. J. Membr. Sci. 2014, 466, 142–150. [Google Scholar] [CrossRef]
- Guo, C.Y.; Wu, X.; Liu, J.G.; Zhang, Y.; Zhang, X.M. Preparation and Properties of High-whiteness Polyimide Ultrafine Fabrics by Electrospinning from Organo-soluble Semi-alicyclic Polyimide Resins. J. Photopolym. Sci. Technol. 2018, 31, 27–36. [Google Scholar] [CrossRef]
- Guo, C.Y.; Liu, J.G.; Yin, L.M.; Huangfu, M.G.; Zhang, Y.; Wu, X.; Zhang, X.M. Preparation and Characterization of Electrospun Polyimide Microfibrous Mats with High Whiteness and High Thermal Stability from Organo-soluble Polyimides Containing Rigid-rod Moieties. Fibers Polym. 2018, 19, 1706–1714. [Google Scholar] [CrossRef]
- Goponenko, A.V.; Hou, H.; Dzenis, Y.A. Avoiding fusion of electrospun 3,3′,4,4′-biphenyl- tetracarboxylic dianhydride-4,4′-oxydianiline copolymer nanofibers during conversion to polyimide. Polymer 2011, 52, 3776–3782. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Wujcik, E.K.; Qiu, B.; Rutman, D.; Zhang, X.; Guo, Z. Hydrophobic electrospun polyimide nanofibers for self-cleaning materials. Macromol. Mater. Eng. 2015, 300, 358–368. [Google Scholar] [CrossRef]
- Nielsen, L.E. Cross-linking–effect on physical properties of polymers. J. Macromol. Sci. Part C 1969, 3, 69–103. [Google Scholar] [CrossRef]
- Vanherck, K.; Koeckelberghs, G.; Vankelecom, I.F. Crosslinking polyimides for membrane applications: A review. Prog. Polym. Sci. 2013, 38, 874–896. [Google Scholar] [CrossRef]
- Rohde, O.; Smolka, P.; Falcigno, P.A.; Pfeifer, J. Novel auto-photosensitive polyimides with tailored properties. Polym. Eng. Sci. 1992, 32, 1623–1629. [Google Scholar] [CrossRef]
- Scaiano, J.C.; Netto--Ferreira, J.C.; Becknell, A.F.; Small, R.D. The mechanism of photocure of inherently photosensitive polyimides containing a benzophenone group. Polym. Eng. Sci. 1989, 29, 942–944. [Google Scholar] [CrossRef]
- Rich, D.C.; Sichel, E.K.; Cebe, P. Curing study of a preimidized photosensitive polyimide. Polym. Eng. Sci. 1996, 36, 2179–2187. [Google Scholar] [CrossRef]
- Qian, Z.G.; Pang, Z.Z.; Li, Z.X.; He, M.H.; Liu, J.G.; Fan, L.; Yang, S.Y. Photoimageable polyimides derived from α, α-(4-amino-3, 5-dimethylphenyl) phenylmethane and aromatic dianhydride. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3012–3020. [Google Scholar] [CrossRef]
- Lin, A.A.; Sastri, V.R.; Tesoro, G.; Reiser, A.; Eachus, R. On the crosslinking mechanism of benzophenone-containing polyimides. Macromolecules 1988, 21, 1165–1169. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horie, K. Photophysics, photochemistry, and optical properties of polyimides. Prog. Polym. Sci. 2001, 26, 259–335. [Google Scholar] [CrossRef]
- Liaw, D.J.; Liaw, B.Y.; Li, L.J.; Sillion, B.; Mercier, R.; Thiria, R.; Sekiguchi, H. Synthesis and characterization of new soluble polyimides from 3,3’,4,4’-benzhydrol tetracarboxylic dianhydride and various diamines. Chem. Mater. 1998, 10, 734–739. [Google Scholar] [CrossRef]
- Rivaton, A.; Gardette, J.L. Photo-oxidation of aromatic polymers. Angew. Makromol. Chem. 1998, 261, 173–188. [Google Scholar] [CrossRef]


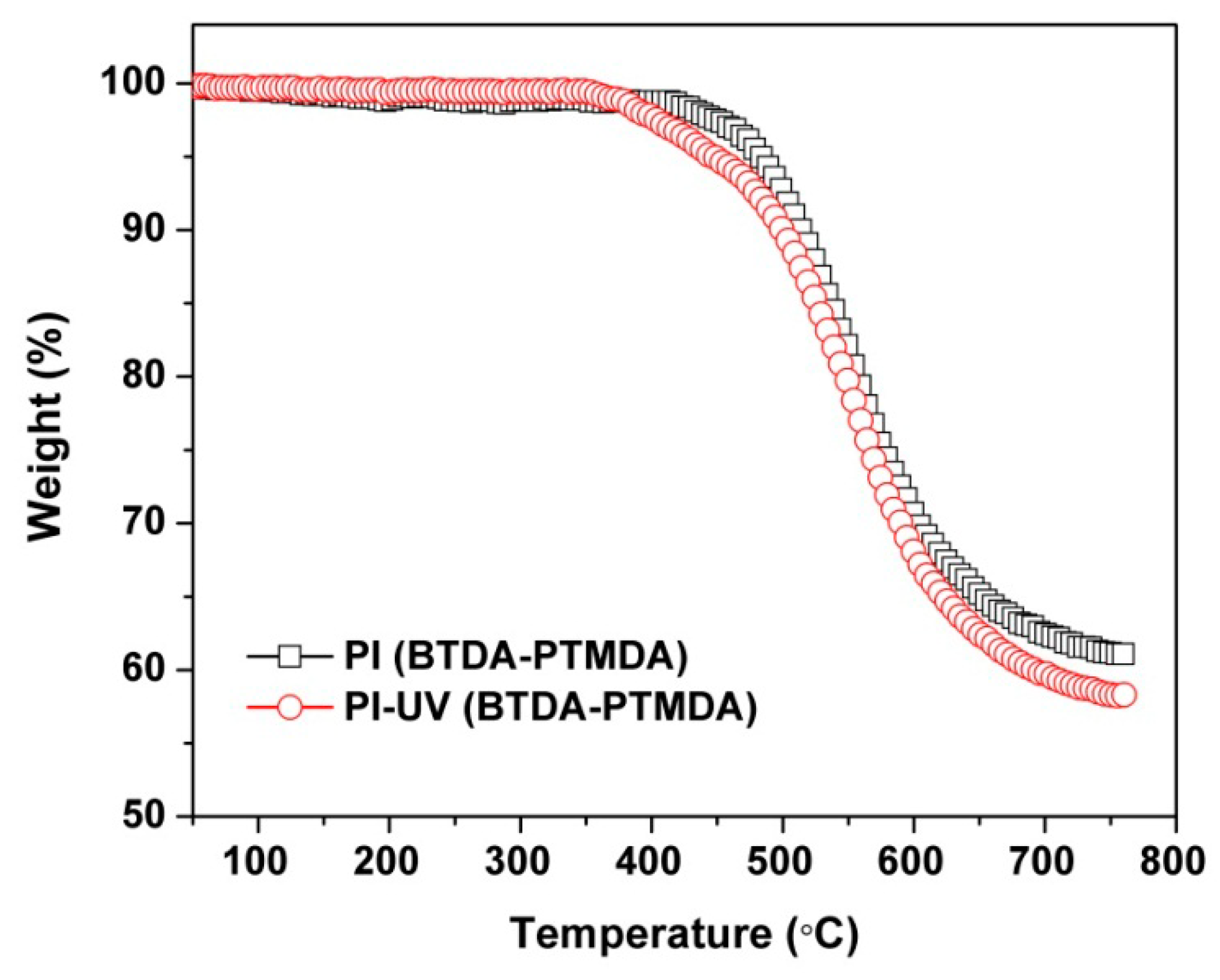
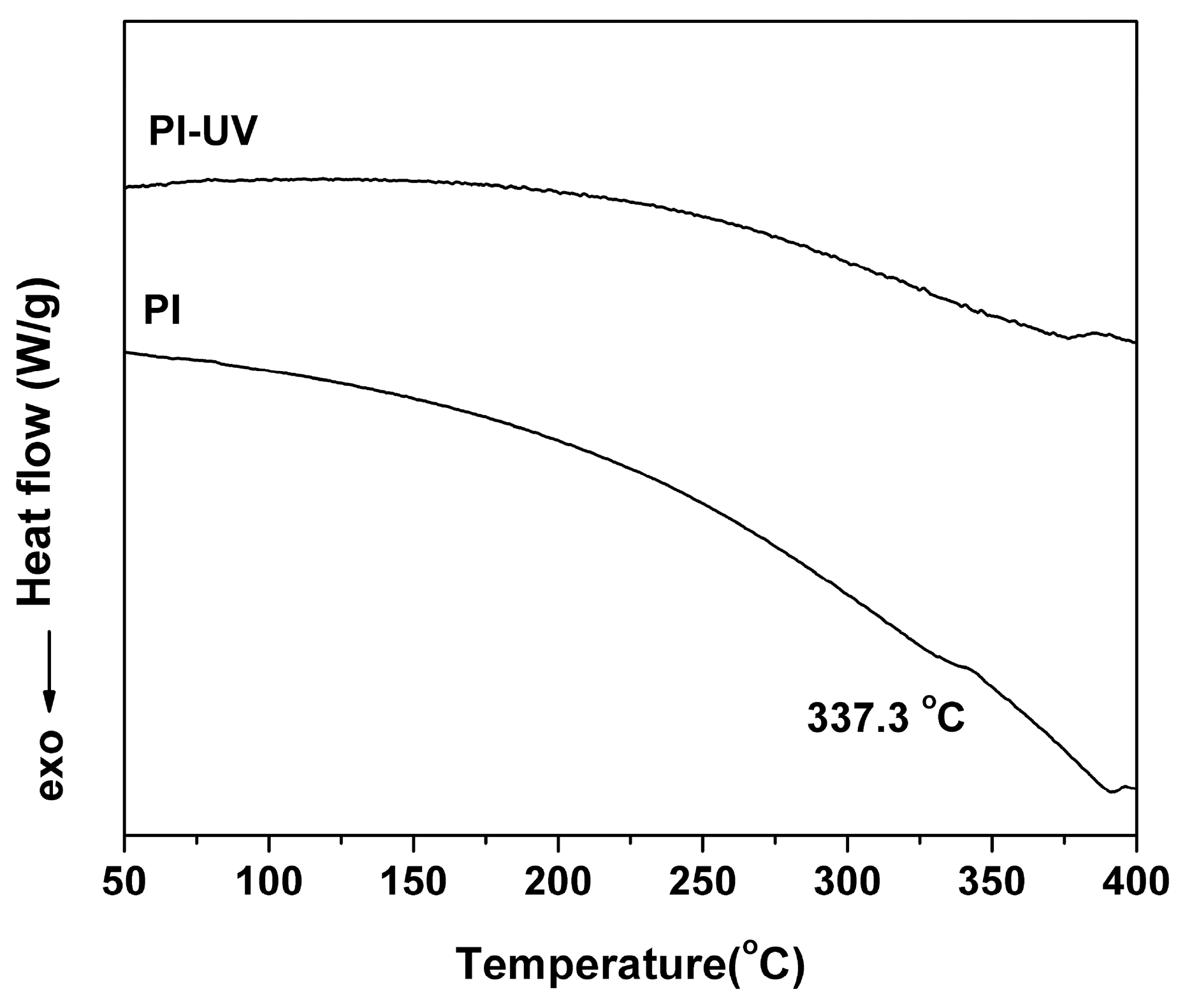

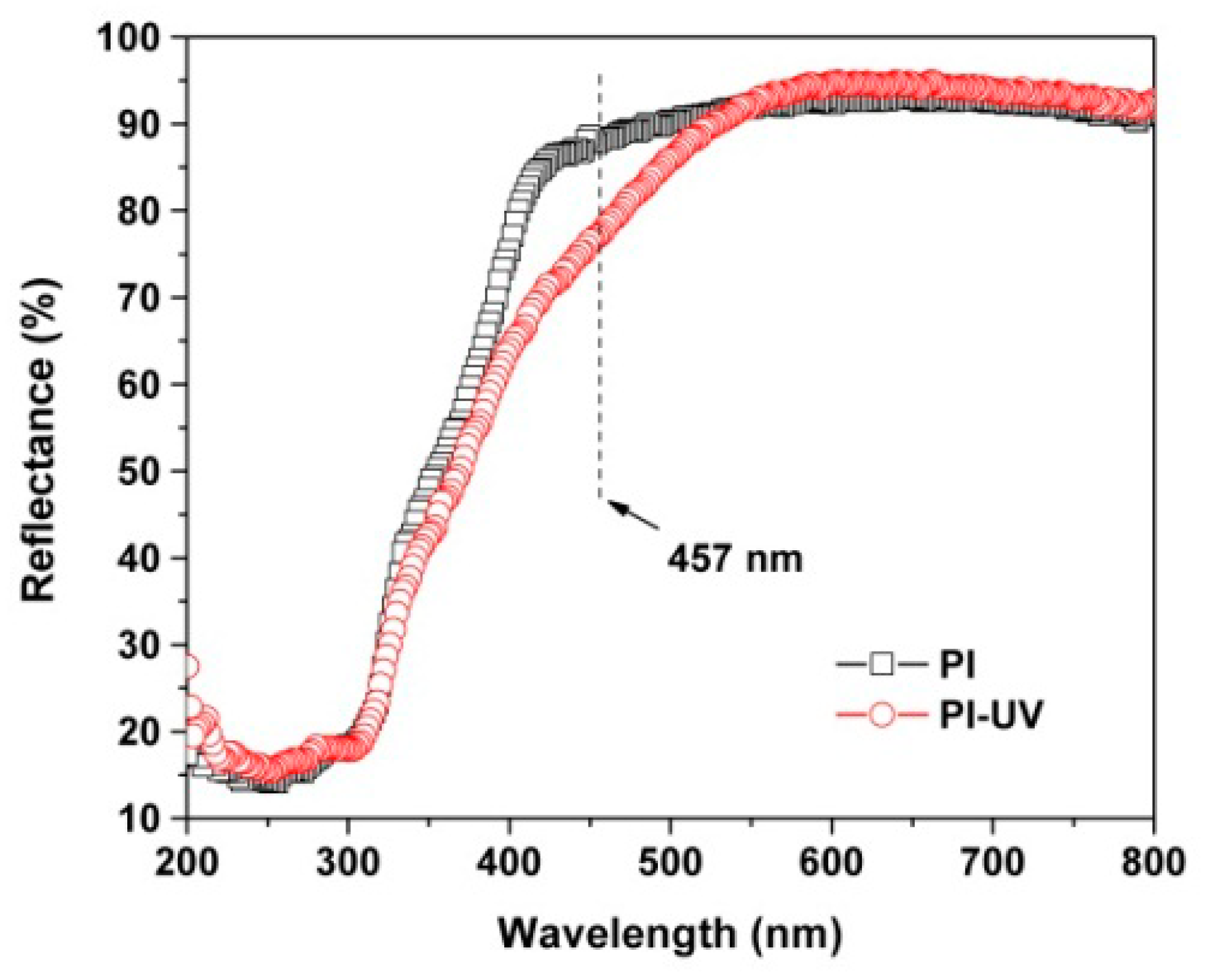
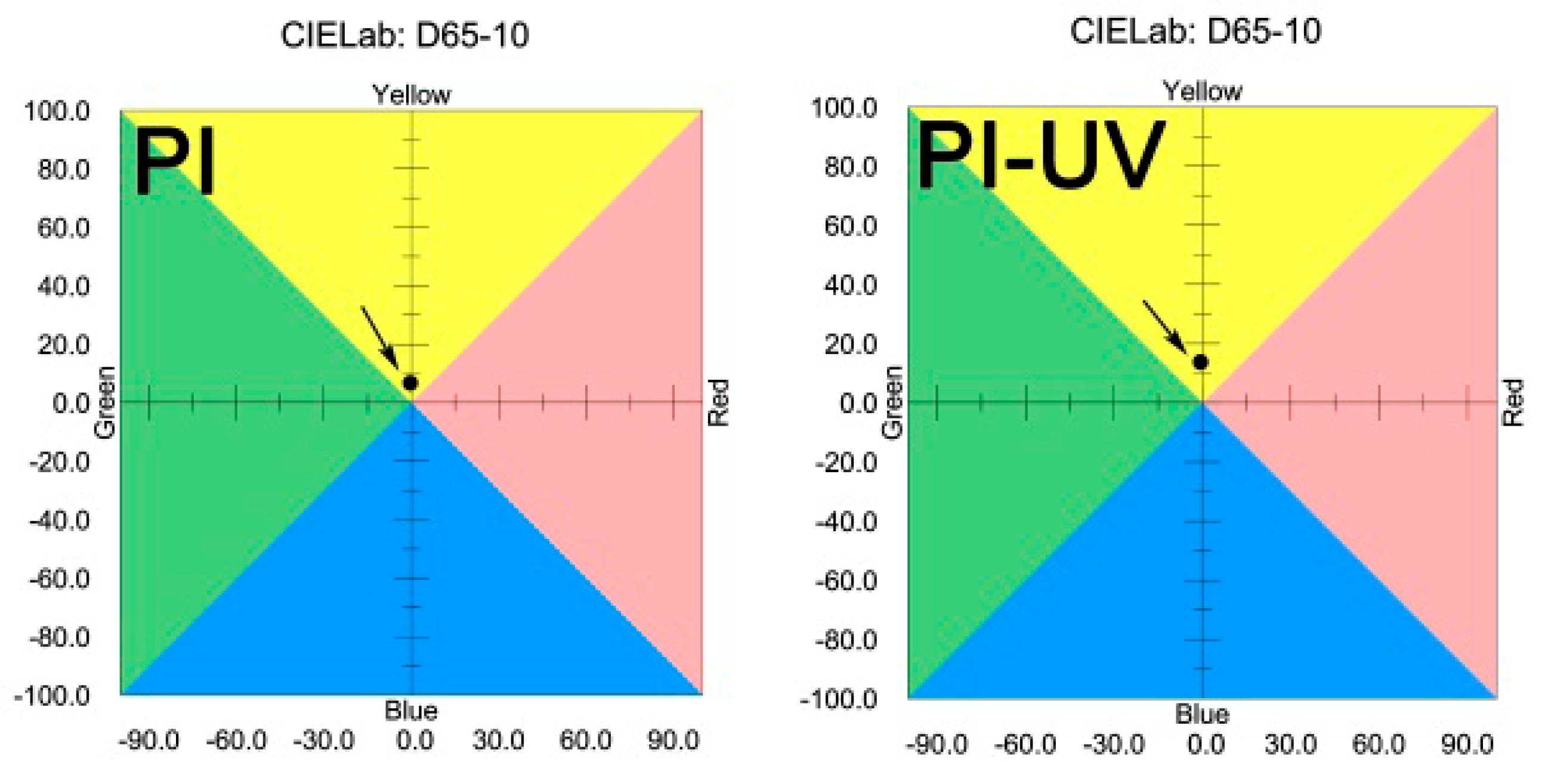
| PI | [η]inh a (dL/g) | Molecular Weight b | Thermal Properties c | Optical Properties d | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mn (g/mol) | Mw (g/mol) | PDI | Tg (°C) | T5% (°C) | Rw750 (%) | R457 (%) | L | a* | b* | WI (%) | ||||
| PI | 1.21 | 69628 | 136560 | 1.96 | 337.3 | 483.7 | 61.2 | 88.1 | 92.6 | −0.3 | 6.7 | 90.0 | ||
| PI-UV | ND e | ND | ND | ND | ND | 447.2 | 58.3 | 77.4 | 92.4 | −0.6 | 13.7 | 84.3 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, L.; Guo, C.-Y.; Huang Fu, M.-G.; Zhang, Y.; Yin, L.-m.; Wu, L.; Liu, J.-g.; Zhang, X.-m. Enhancement of Solvent Resistance of Polyimide Electrospun Mat via the UV-Assisted Electrospinning and Photosensitive Varnish. Polymers 2019, 11, 2055. https://doi.org/10.3390/polym11122055
Qi L, Guo C-Y, Huang Fu M-G, Zhang Y, Yin L-m, Wu L, Liu J-g, Zhang X-m. Enhancement of Solvent Resistance of Polyimide Electrospun Mat via the UV-Assisted Electrospinning and Photosensitive Varnish. Polymers. 2019; 11(12):2055. https://doi.org/10.3390/polym11122055
Chicago/Turabian StyleQi, Lin, Chen-Yu Guo, Meng-Ge Huang Fu, Yan Zhang, Lu-meng Yin, Lin Wu, Jin-gang Liu, and Xiu-min Zhang. 2019. "Enhancement of Solvent Resistance of Polyimide Electrospun Mat via the UV-Assisted Electrospinning and Photosensitive Varnish" Polymers 11, no. 12: 2055. https://doi.org/10.3390/polym11122055






