Investigation on Reaction Sequence and Group Site of Citric Acid with Cellulose Characterized by FTIR in Combination with Two-Dimensional Correlation Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabric Treatment
2.3. FTIR and 2Dcos
2.4. Anti-Wrinkle Properties
2.5. Gaussian Calculation
3. Results
3.1. Effects of Temperature on Peak Intensities
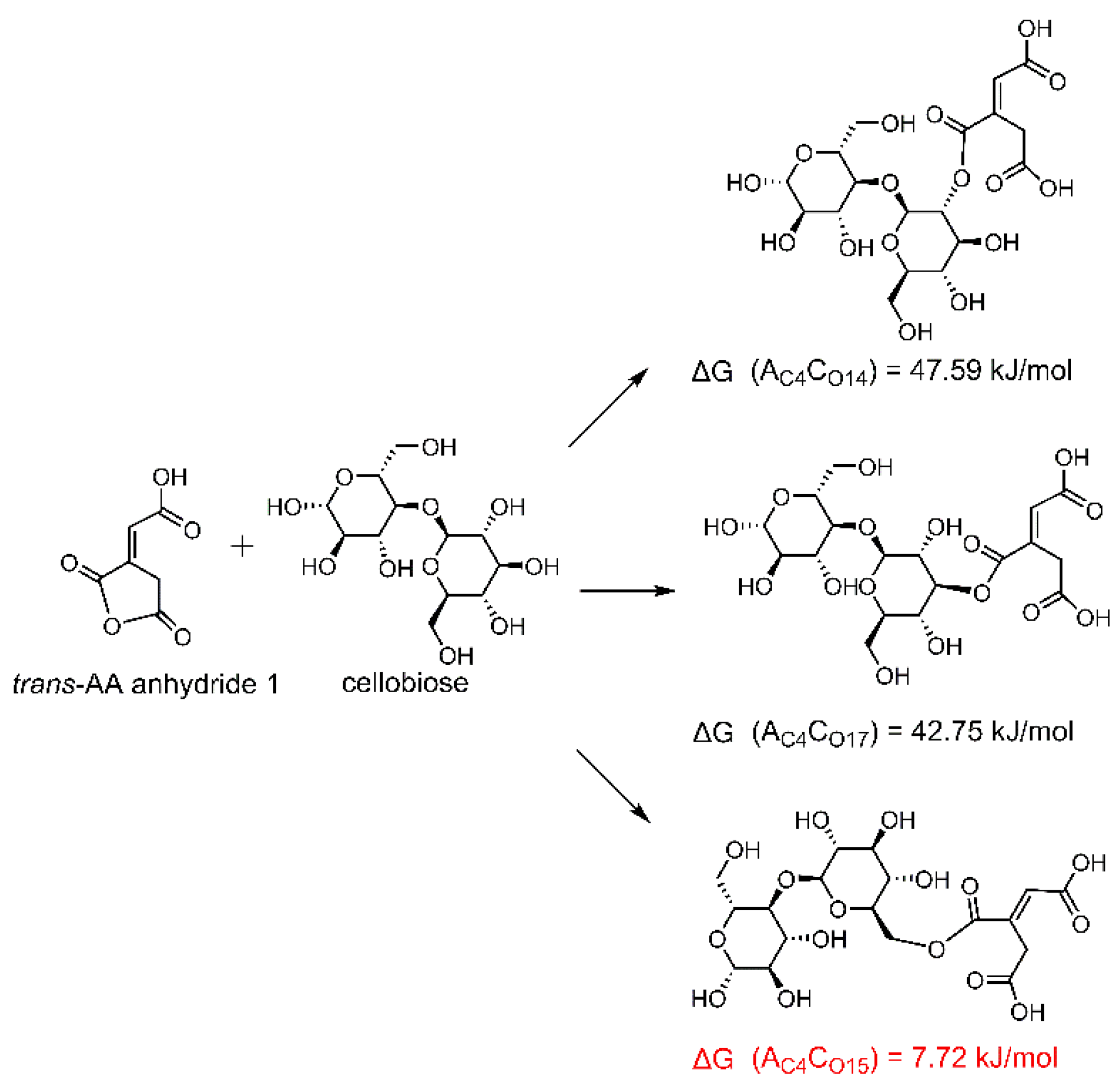
3.2. Reaction Mechanism
3.3. Theoretical Calculations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Chiu, F.-C. Cellulose nanocrystals reinforced k-carrageenan based UV resistant transparent bionanocomposite films for sustainable packaging applications. Carbohydr. Polym. 2019, 211, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Liu, Y.-K.; Chiu, F.-C. Fabrication of cellulose nanocrystal/silver/alginate bionanocomposite films with enhanced mechanical and barrier properties for food packaging application. Nanomaterials 2019, 9, 1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Hsiao, B.S. Efficient removal of UO22+ from water using carboxycellulose nanofibers prepared by the nitro-oxidation method. Ind. Eng. Chem. Res. 2017, 56, 13885–13893. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Antoine, R.; Hsiao, B.S. Efficient removal of arsenic using zinc oxide nanocrystal-decorated regenerated microfibrillated cellulose scaffolds. ACS Sustain. Chem. Eng. 2019, 7, 6140–6151. [Google Scholar] [CrossRef]
- Salmeia, K.; Jovic, M.; Ragaisiene, A.; Rukuiziene, Z.; Milasius, R.; Mikucioniene, D.; Gaan, S. Flammability of cellulose-based fibers and the effect of structure of phosphorous compounds on their flame retardancy. Polymers 2016, 8, 293. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wu, X.; Li, J.; Hao, X.; Lin, J.; Cheng, D.; Lu, Y. Thermosensitive behavior and anti-bacterial activity of cotton fabric modified with a chitosan-poly(N-isopropylacrylamide) interpenetrating polymer network hydrogel. Polymers 2016, 8, 110. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Xing, Z.; Yan, J.; Lu, Y.; Xiong, X.; Zheng, L. Thermosensitive behavior and super-antibacterial properties of cotton fabrics modified with a sercin-NIPAAm-AgNPs interpenetrating polymer network hydrogel. Polymers 2018, 10, 818. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Fang, K.; Ren, Y.; Tang, Z.; Wang, R.; Chen, W.; Xie, R.; Shi, Z.; Hao, L. Inkjet printable and self-curable disperse dyes/P(St-BA-MAA) nanosphere inks for both hydrophilic and hydrophobic fabrics. Polymers 2018, 10, 1402. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.Q.; Wang, X. Infrared spectroscopy studies of the cyclic anhydride as the intermediate for the ester crosslinking of cotton cellulose by polycarboxylic acids. II. Comparison of different polycarboxylic acids. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 1573–1580. [Google Scholar] [CrossRef]
- Ji, B.; Zhao, C.; Yan, K.; Sun, G. Effects of acid diffusibility and affinity to cellulose on strength loss of polycarboxylic acid crosslinked fabrics. Carbohydr. Polym. 2016, 144, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.L.; Kan, C.W.; Yuen, C.W.M.; Au, C.H. Fabric objective measurement of the plasma-treated cotton fabric subjected to wrinkle-resistant finishing with BTCA and TiO2 system. Fibers. Polym. 2011, 12, 626–634. [Google Scholar] [CrossRef]
- Bosetti, C.; McLaugnlin, J.K.; Tarone, R.E.; Pira, E.; La Vecchia, C. Formaldehyde and cancer risk: A quantitative review of cohort studies through 2006. Ann. Oncol. 2008, 19, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Hauptmann, M.; Stewart, P.A.; Lubin, J.H.; Beane Freeman, L.E.; Hornung, R.W.; Herrick, R.F.; Hoover, R.N.; Fraumeni, J.F.; Blair, A.; Hayes, R.B. Morality from lymphohematopoitic malignancies and brain cancer among embalmers exposed to formaldehyde. J. Natl. Cancer. Inst. 2009, 101, 1696–1708. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Qi, H.; Yan, K.; Sun, G. Catalytic actions of alkaline salts in reactions between 1,2,3,4-butanetetracarboxylic acid and cellulose: I. Anhydride formation. Cellulose 2016, 23, 259–267. [Google Scholar] [CrossRef]
- Ji, B.; Tang, P.; Yan, K.; Sun, G. Catalytic actions of alkaline salts in reactions between 1,2,3,4-butanetetracarboxylic acid and cellulose: II. Esterification. Carbohydr. Polym. 2015, 132, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Wang, B.; Ye, T.; Yang, Y. Durable press finishing of cotton fabrics with citric acid: Enhancement of whiteness and wrinkle recovery by polyol extenders. Ind. Eng. Chem. Res. 2013, 52, 16118–16127. [Google Scholar] [CrossRef]
- Ye, T.; Wang, B.; Liu, J.; Chen, J.; Yang, Y. Quantitative analysis of citric acid/sodium hypophosphite modified cotton by HPLC and conductometric titration. Carbohydr. Polym. 2015, 121, 92–98. [Google Scholar] [CrossRef]
- Yang, C.Q.; Wang, X.; Kang, I.-S. Ester crosslinking of cotton fabrics by polymeric carboxylic acids and citric acid. Text. Res. J. 1997, 67, 334–342. [Google Scholar] [CrossRef]
- Hou, A.; Sun, G. Multifunctional finishing of cotton with 3,3′,4,4′-benzophenone tetracarboxylic acid: Functional performance. Carbohydr. Polym. 2013, 96, 435–439. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, G. Catalytic actions of sodium salts in direct esterification of 3,3′,4,4′-benzophenone tetracarboxylic acid with cellulose. Ind. Eng. Chem. Res. 2015, 54, 10553–10559. [Google Scholar] [CrossRef]
- Tang, P.; Ji, B.; Sun, G. Whiteness improvement of citric acid crosslinked cotton fabrics: H2O2 bleaching under alkaline condition. Carbohydr. Polym. 2016, 147, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Sun, G. Generation of hydroxyl radicals and effective whitening of cotton fabrics by H2O2 under UVB irradiation. Carbohydr. Polym. 2017, 160, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Shao, D.; Xu, C.; Wang, Q.; Gao, W. An eco-friendly way to whiten yellowish anti-wrinkle cotton fabrics using TBCC-activated peroxide low-temperature post-bleaching. Cellulose 2019, 26, 3575–3588. [Google Scholar] [CrossRef]
- Noda, I. Two-dimensional infrared spectroscopy. J. Am. Chem. Soc. 1989, 111, 8116–8118. [Google Scholar] [CrossRef]
- Hou, L.; Wu, P. Two-dimensional correlation infrared spectroscopy of heat-induced esterification of cellulose with 1,2,3,4-butantetracarboxylic acid in the presence of sodium hypophosphite. Cellulose 2019, 26, 2759–2769. [Google Scholar] [CrossRef]
- Watanabe, A.; Morita, S.; Ozaki, Y. Temperature-dependent changes in hydrogen bonds in cellulose I α studied by infrared spectroscopy in combination with perturbation-correlation moving-window two-dimensional correlation spectroscopy: Comparison with cellulose I β. Biomacromolecules 2007, 8, 2969–2975. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, P. Investigation of the hydrogen-bond structure of cellulose diacetate by two-dimensional infrared correlation spectroscopy. Carbohydr. Polym. 2008, 74, 509–513. [Google Scholar] [CrossRef]
- Sun, S.; Wu, P. On the thermally reversible dynamic hydration behavior of oligo (ethylene glycol) methacrylate-based polymers in water. Macromolecules 2013, 46, 236–246. [Google Scholar] [CrossRef]
- Housseinian, A.; Vessally, E.; Babazadeh, M.; Edjlali, L.; Es’haghi, M. Adsorption properties of chloropicrin on pristine and borazine-doped nanographene: A theoretical study. J. Phys. Chem. Solids 2018, 115, 277–282. [Google Scholar] [CrossRef]
- Luo, Q.; Shi, Z.; Li, D.; Chen, C.; Wang, M. DFT study on the ionic cyclization mechanism of copolymers of acrylonitrile-itaconic acid: Direct or autocatalytic? Chem. Phys. Lett. 2017, 687, 158–162. [Google Scholar] [CrossRef]
- Ji, B.; Tang, P.; Hu, C.; Yan, K. Catalytic and ionic cross-linking actions of L-glutamate salt for the modification of cellulose by 1,2,3,4-butanetetracarboxylic acid. Carbohydr. Polym. 2019, 207, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kin, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef] [PubMed]
- Moharram, M.A.; Mahmoud, O.M. FTIR spectroscopic study of the effect of microwave heating on the transformation of cellulose I into cellulose II during mercerization. J. Appl. Polym. Sci. 2008, 107, 30–36. [Google Scholar] [CrossRef]
- Ning, Y.C. Interpretation of Organic Spectra; Science Press: Beijing, China, 2010; pp. 368–375. ISBN 978-7-03-025859-5. [Google Scholar]
- Saund, S.S.; Sosa, V.; Henriquez, S.; Nguyen, Q.N.N.; Bianco, C.L.; Soeda, S.; Millikin, R.; White, C.; Le, H.; Ono, K.; et al. The chemical biology of hydropersulfides (RSSH): Chemical stability, reactivity and redox roles. Arch. Biochem. Biophys. 2015, 588, 15–24. [Google Scholar] [CrossRef] [Green Version]










| Wavenumber (cm−1) | Tentative Assignments [27,33,34,35] |
|---|---|
| 3544 | ν(O–H) (free) |
| 3446 | ν(O(2)–H(2)…O(6))(intrachain) |
| 3417 | ν(O(6)–H(6)…O(3)’) (interchain) |
| 3334 | ν(O(3)–H(3)…O(5)) (intrachain) |
| 3271 | ν(O–H) (weak hydrogen bond) |
| 1784 | ν(C=O) (anhydride) |
| 1725 | ν(C=O) (carboxyl) |
| 1701 | ν(C=O) (ester) |
| 1656 | ν(C=C) (unsaturated polycarboxylic acid) |
| Chemicals | HOMO (eV) | Location | LUMO (eV) | Location | ΔE (eV) 1 |
|---|---|---|---|---|---|
| CA | −7.1830 | O11 2, O13 | −0.2982 | C1, C8, H18 | 6.0832 |
| CA anhydride 1 | −7.6040 | O10, O12 | −1.1045 | C1, C4, C8 | 5.2769 |
| CA anhydride 2 | −7.8568 | O5, O11 | −1.1557 | C1, C4, C7 | 5.2257 |
| cis-AA | −7.5009 | O11, O12 | −3.3089 | C1, C2, C4, C7 | 3.0725 |
| trans-AA | −7.5311 | O11, O12 | −2.4831 | C1, C2, C3, C4 | 3.8983 |
| trans-AA anhydride 1 | −8.0307 | O5, O9, O11 | −3.0393 | C1, C2, C3, C4 | 3.3421 |
| trans-AA anhydride 2 | −8.0459 | O6, O7 | −3.0110 | C1l, C2, C3, C5 | 3.3704 |
| cellobiose | −6.3814 | O14, O15, O17 | 1.1595 | H36, H45 | 7.5409 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.; Ji, B.; Yan, K.; Zhu, Q. Investigation on Reaction Sequence and Group Site of Citric Acid with Cellulose Characterized by FTIR in Combination with Two-Dimensional Correlation Spectroscopy. Polymers 2019, 11, 2071. https://doi.org/10.3390/polym11122071
Cai Z, Ji B, Yan K, Zhu Q. Investigation on Reaction Sequence and Group Site of Citric Acid with Cellulose Characterized by FTIR in Combination with Two-Dimensional Correlation Spectroscopy. Polymers. 2019; 11(12):2071. https://doi.org/10.3390/polym11122071
Chicago/Turabian StyleCai, Zijing, Bolin Ji, Kelu Yan, and Quan Zhu. 2019. "Investigation on Reaction Sequence and Group Site of Citric Acid with Cellulose Characterized by FTIR in Combination with Two-Dimensional Correlation Spectroscopy" Polymers 11, no. 12: 2071. https://doi.org/10.3390/polym11122071





