Cellulose-Cyclodextrin Co-Polymer for the Removal of Cyanotoxins on Water Sources
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cellulose Nanofibril Characterization
2.2.1. Dry Content Determination
2.2.2. Charge Density and pH Measurements
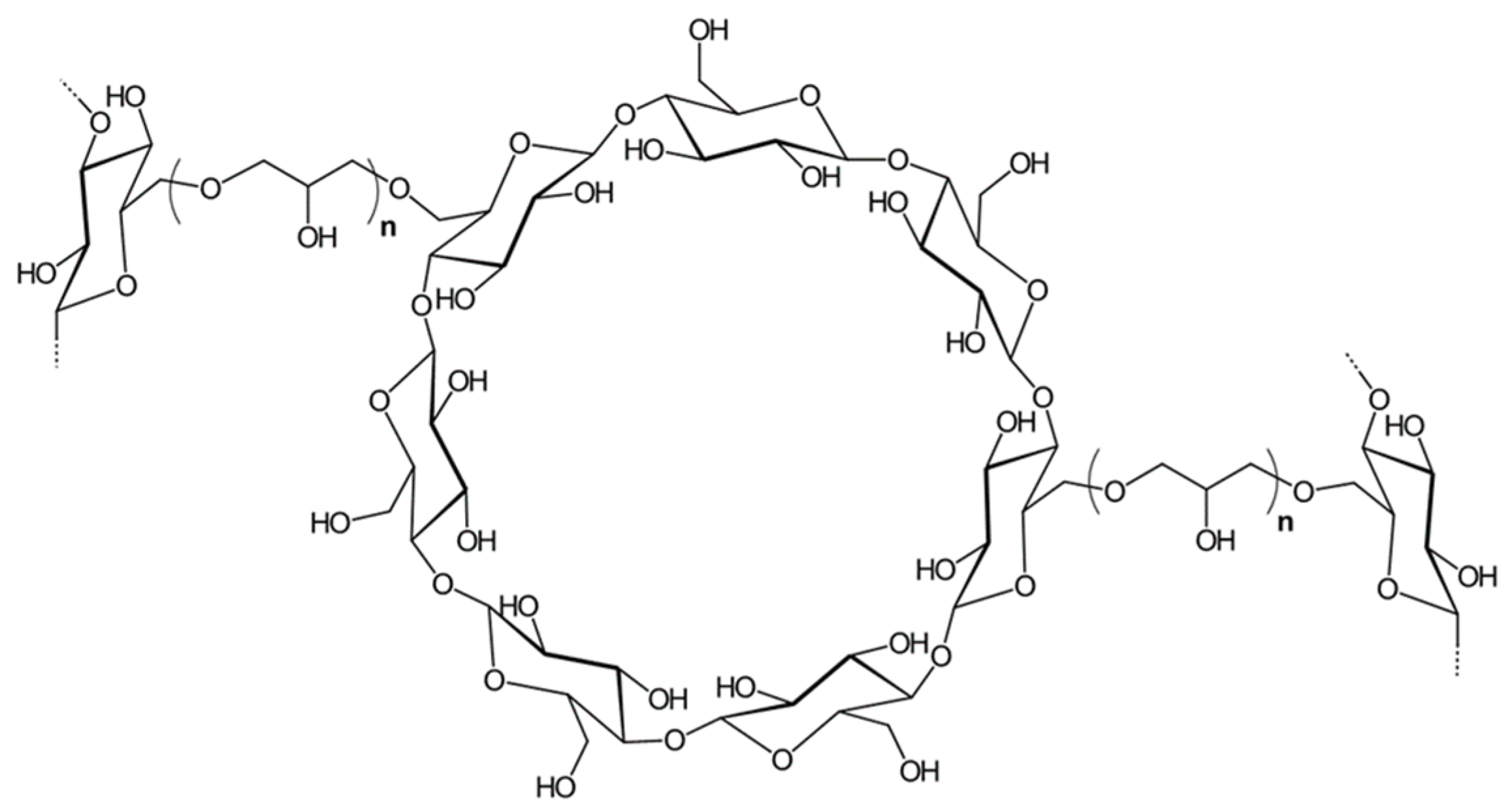
2.3. Polymerization of β-Cyclodextrin
2.4. Preparation of CNF/β-Cyclodextrin Co-Polymers
2.4.1. Synthesis of CNF-CD
2.4.2. Synthesis of CNF-PCD
2.5. Characterization Techniques
2.5.1. Fourier-Transform Infrared Spectroscopy with Attenuated Total Reflectance (FTIR-ATR)
2.5.2. X-Ray Photoelectron Spectroscopy (XPS)
2.5.3. Thermogravimetric Analysis (TGA)
2.5.4. Atomic Force Microscopy (AFM)
2.6. Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D)
3. Results and Discussions
3.1. Characterization of CNF and the Modified Polysaccharides
3.1.1. CNF Suspensions
3.1.2. Synthesis of Poly(β-Cyclodextrin) (PCD)
3.1.3. Synthesis of CNF-CD and CNF-PCD
3.2. Formation of Surfaces in Situ on QCM-D and Adsorption of Microcystin-LR
3.2.1. Surface Generation and Characterization
3.2.2. Capture of MC Followed by QCM-D
3.2.3. Mass Modeling from QCM-D Data
4. Conclusions
5. Future Work
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gomez-Maldonado, D.; Vega Erramuspe, I.B.; Peresin, M.S. Natural Polymers as Alternative Adsorbents and Treatment Agents for Water Remediation. BioResources 2019, 14, 10093–10160. [Google Scholar]
- Olivera, S.; Muralidhara, H.B.; Venkatesh, K.; Guna, V.K.; Gopalakrishna, K.; Kumar, Y. Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: A review. Carbohydr. Polym. 2016, 153, 600–618. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, J.; Mao, J.; Zhang, L.; He, C.; Chen, G.; Parkin, I.P.; Lai, Y. In vivo and in vitro efficient textile wastewater remediation by Aspergillus niger biosorbent. Nanoscale Adv. 2019, 1, 168–176. [Google Scholar] [CrossRef]
- Mao, J.; Li, S.; He, C.; Tang, Y.; Chen, Z.; Huang, J.; Lai, Y. Robust amphiprotic konjac glucomannan cross-linked chitosan aerogels for efficient water remediation. Cellulose 2019, 26, 6785–6796. [Google Scholar] [CrossRef]
- Filpponen, I.; Kontturi, E.; Nummelin, S.; Rosilo, H.; Kolehmainen, E.; Ikkala, O.; Laine, J. Generic method for modular surface modification of cellulosic materials in aqueous medium by sequential “click” reaction and adsorption. Biomacromolecules 2012, 13, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.Y.; de Assis Barros, L.; Yan, N.; Sain, M.; Qing, Y.; Wu, Y. Nanocellulose composites with enhanced interfacial compatibility and mechanical properties using a hybrid-toughened epoxy matrix. Carbohydr. Polym. 2017, 177, 249–257. [Google Scholar] [CrossRef]
- Carpenter, A.W.; de Lannoy, C.-F.; Wiesner, M.R. Cellulose Nanomaterials in Water Treatment Technologies. Environ. Sci. Technol. 2015, 49, 5277–5287. [Google Scholar] [CrossRef]
- Kim, S.; Yun, Y.S.; Choi, Y.E. Development of waste biomass based sorbent for removal of cyanotoxin microcystin-LR from aqueous phases. Bioresour. Technol. 2018, 247, 690–696. [Google Scholar] [CrossRef]
- Hitzfeld, B.C.; Hoger, S.J.; Dietrich, D.R. Cyanobacterial Toxins: Removal during Drinking Water Treatment, and Human Risk Assessment Cyanobacteria. Environ. Health Perspect. 2000, 108, 113–122. [Google Scholar]
- Walls, J.T.; Wyatt, K.H.; Doll, J.C.; Rubenstein, E.M.; Rober, A.R. Hot and toxic: Temperature regulates microcystin release from cyanobacteria. Sci. Total Environ. 2018, 610, 786–795. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Boufi, S. Nanocellulose as a novel nanostructured adsorbent for environmental remediation: A review. Cellulose 2017, 24, 1171–1197. [Google Scholar] [CrossRef]
- Ali, I.; Gupta, V.K. Advances in water treatment by adsorption technology. Nat. Protoc. 2006, 1, 2661. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-Y.; Tian, N.; Li, J.-T.; Broadwell, I.; Sun, S.-G. Nanomaterials of high surface energy with exceptional properties in catalysis and energy storage. Chem. Soc. Rev. 2011, 40, 4167–4185. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Thielemans, W. Thermodynamics of Adsorption on Nanocellulose Surfaces; Springer Netherlands: Dordrecht, The Netherlands, 2019; Volume 26, ISBN 0123456789. [Google Scholar]
- Archimandritis, A.S.; Papadimitriou, T.; Kormas, K.A.; Laspidou, C.S.; Yannakopoulou, K.; Lazarou, Y.G. Theoretical investigation of microcystin-LR, microcystin-RR and nodularin-R complexation with α-, β-, and γ-cyclodextrin as a starting point for the targeted design of efficient cyanotoxin traps. Sustain. Chem. Pharm. 2016, 3, 25–32. [Google Scholar] [CrossRef]
- Sinha, A.; Jana, N.R. Separation of microcystin-LR by cyclodextrin-functionalized magnetic composite of colloidal graphene and porous silica. ACS Appl. Mater. Interfaces 2015, 7, 9911–9919. [Google Scholar] [CrossRef]
- Saenger, W.; Steiner, T. Cyclodextrin Inclusion Complexes: Host ± Guest Interactions and Hydrogen-Bonding Networks. Acta Crystallogr. Sect. A Found. Crystallogr. 1998, 54, 798–805. [Google Scholar] [CrossRef]
- Pinto, L.M.A.; Fraceto, L.F.; Santana, M.H.A.; Pertinhez, T.A.; Oyama, S.; De Paula, E. Physico-chemical characterization of benzocaine-β-cyclodextrin inclusion complexes. J. Pharm. Biomed. Anal. 2005, 39, 956–963. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. Synthesis, characterization and application of Epichlorohydrin-β-cyclodextrin polymer. Colloids Surf. B Biointerfaces 2014, 114, 130–137. [Google Scholar] [CrossRef]
- Szejtli, J.; Zsadon, B.; Fenyvesi, É.; Tüdös, F. Sorbents of Cellulose Basis Capable of Forming Inclusion Complexes and a Process for the Preparation Thereof 1982. U.S. Patent 4,357,468, 2 November 1982. [Google Scholar]
- Sun, T.; Lindsay, J.D. Cyclodextrins Covalently Bound to Polysaccharides 2004. U.S. Patent 6,689,378, 10 February 2004. [Google Scholar]
- Abdolali, A.; Guo, W.S.; Ngo, H.H.; Chen, S.S.; Nguyen, N.C.; Tung, K.L. Bioresource Technology Typical lignocellulosic wastes and by-products for biosorption process in water and wastewater treatment: A critical review. Bioresour. Technol. 2014, 160, 57–66. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, W.; Sharma, S.; Tong, G.; Deng, Y. Synthesis of Cyclodextrin-functionalized Cellulose Nanofibril Aerogel as a Highly Effective Adsorbent for Phenol Pollutant Removal. BioResources 2015, 10, 7555–7568. [Google Scholar] [CrossRef]
- Jagur-Grodzinski, J. Nanostructured polyolefins/clay composites: Role of the molecular interaction at the interface. Polym. Adv. Technol. 2006, 17, 395–418. [Google Scholar] [CrossRef]
- Ji, B.; Tang, P.; Yan, K.; Sun, G. Catalytic actions of alkaline salts in reactions between 1,2,3,4-butanetetracarboxylic acid and cellulose: II. Esterification. Carbohydr. Polym. 2015, 132, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Parvinzadeh, M.; Alimohammadi, F.; Shamei, A. Surface & Coatings Technology Preparation of water-repellent cellulose fi bers using a polycarboxylic acid/hydrophobic silica nanocomposite coating. Surf. Coat. Technol. 2012, 206, 3208–3215. [Google Scholar]
- Medronho, B.; Andrade, R.; Vivod, V.; Ostlund, A.; Miguel, M.G.; Lindman, B.; Voncina, B.; Valente, A.J.M. Cyclodextrin-grafted cellulose: Physico-chemical characterization. Carbohydr. Polym. 2013, 93, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Alzate-Sánchez, D.M.; Ling, Y.; Li, C.; Frank, B.P.; Bleher, R.; Fairbrother, D.H.; Helbling, D.E.; Dichtel, W.R. β-Cyclodextrin Polymers on Microcrystalline Cellulose as a Granular Media for Organic Micropollutant Removal from Water. ACS Appl. Mater. Interfaces 2019, 11, 8089–8096. [Google Scholar] [CrossRef]
- Lahti, K.; Rapala, J.; Färdig, M.; Niemelä, M.; Sivonen, K. Persistence of cyanobacterial hepatotoxin, microcystin-LR in particulate material and dissolved in lake water. Water Res. 1997, 31, 1005–1012. [Google Scholar] [CrossRef]
- Meriluoto, J.; Codd, G.A. Cyanobacterial Monitoring and Cyanotoxin Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2005; ISBN 9517652593. [Google Scholar]
- World Health Organization. Guidelines for drinking-water quality fourth edition. WHO Chronicle 2011, 38, 104–108. [Google Scholar]
- US. EPA—Office of Water. Human Health Recreational Ambient Water Quality Criteria or Swimming Advisories for Microcystins and Cylindrospermopsin Draft Human Health Recreational Ambient Water Quality Criteria or Swimming Advisories for Microcystins and Cylindrospermopsin; EPA: Washington, DC, USA, 2016. [Google Scholar]
- Bouhadiba, A.; Belhocine, Y.; Rahim, M.; Djilani, I.; Nouar, L.; Khatmi, D.E. Host-guest interaction between tyrosine and β-cyclodextrin: Molecular modeling and nuclear studies. J. Mol. Liq. 2017, 233, 358–363. [Google Scholar] [CrossRef]
- Lee, J.; Walker, H.W. Adsorption of microcystin-Lr onto iron oxide nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 373, 94–100. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, J.Y.; Baez, C.; Kitin, P.; Elder, T. Highly thermal-stable and functional cellulose nanocrystals and nanofibrils produced using fully recyclable organic acids. Green Chem. 2016, 18, 3835–3843. [Google Scholar] [CrossRef]
- Wang, R.; Chen, L.; Zhu, J.Y.; Yang, R. Tailored and integrated production of carboxylated cellulose nanocrystals (CNC) with nanofibrils (CNF) through maleic acid hydrolysis. ChemNanoMat 2017, 3, 328–335. [Google Scholar] [CrossRef]
- Determination of equilibrium moisture in pulp, paper and paperboard for chemical analysis; TAPPI: Atlanta, GA, USA, 1993; T550 om-08.
- Espinosa, E.; Tarrés, Q.; Delgado-Aguilar, M.; González, I.; Mutjé, P.; Rodríguez, A. Suitability of wheat straw semichemical pulp for the fabrication of lignocellulosic nanofibres and their application to papermaking slurries. Cellulose 2016, 23, 837–852. [Google Scholar] [CrossRef]
- Johansson, L.S.; Campbell, J.M. Reproducible XPS on biopolymers: Cellulose studies. Surf. Interface Anal. 2004, 36, 1018–1022. [Google Scholar] [CrossRef]
- Briggs, D.; Beamson, G. XPS Studies of the Oxygen 1s and 2s Levels in a Wide Range of Functional Polymers. Anal. Chem. 1993, 65, 1517–1523. [Google Scholar] [CrossRef]
- KSV Instruments Ltd. What Is a Quartz Crystal Microbalance—Qcm; KSV Instruments Ltd.: Espoo, Finland, 2002; pp. 1–10. [Google Scholar]
- Voinova, M.V.; Jonson, M.; Kasemo, B. “Missing mass” effect in biosensor’s QCM applications. Biosens. Bioelectron. 2002, 17, 835–841. [Google Scholar] [CrossRef]
- Application example – Surfactant Adsorption to Gold, SiO2 and Stainless Steel (AISI316) Surfaces; KSV Instruments Ltd.: Espoo, Finland, 1991; Dissipative QCM; pp. 1–4.
- Voinova, M.V.; Rodahl, M.; Jonson, M.; Kasemo, B. Viscoelastic acoustic response of layered polymer films at fluid-solid interfaces: Continuum mechanics approach. Phys. Scr. 1999, 59, 391. [Google Scholar] [CrossRef]
- Turon, X.; Rojas, O.J.; Deinhammer, R.S. Enzymatic kinetics of cellulose hydrolysis: A QCM-D study. Langmuir 2008, 24, 3880–3887. [Google Scholar] [CrossRef]
- Li, R.; Yu, J.C.; Jiang, Z.T.; Zhou, R.H.; Liu, H.Y. A solid-phase fluorescent, quenching method for the determination of trace amounts of nitrite in foods with neutral red. J. Food Drug Anal. 2003, 11, 251–257. [Google Scholar]
- Johansson, L.S.; Tammelin, T.; Campbell, J.M.; Setälä, H.; Österberg, M. Experimental evidence on medium driven cellulose surface adaptation demonstrated using nanofibrillated cellulose. Soft Matter 2011, 7, 10917–10924. [Google Scholar] [CrossRef]
- Kasemo, B.; Fant, C.; Sott, K.; Elwing, H.; Qcm-d, T. Variations in Coupled Water, Viscoelastic Properties, and Film Thickness of a Mefp-1 Protein Film during Adsorption and Cross-Linking: A Quartz Crystal Microbalance with Dissipation Monitoring, Ellipsometry, and Surface Plasmon. Anal. Chem. 2001, 73, 5796–5804. [Google Scholar]
- Fält, S.; Wågberg, L.; Vesterlind, E.L. Swelling of model films of cellulose having different charge densities and comparison to the swelling behavior of corresponding fibers. Langmuir 2003, 19, 7895–7903. [Google Scholar] [CrossRef]
- Tran, C.D.; Duri, S.; Delneri, A.; Franko, M. Chitosan-cellulose composite materials: Preparation, Characterization and application for removal of microcystin. J. Hazard. Mater. 2013, 253, 355–366. [Google Scholar] [CrossRef] [PubMed]












| System | Average Roughness (Ra) [nm] | Root Mean Square Roughness (Rq) [nm] | Average Maximum Height of the Roughness (Rz ISO) [nm] |
|---|---|---|---|
| Spin coated | |||
| CNF | 1.43 | 1.80 | 14.7 |
| CNF-CD | 0.84 | 1.25 | 10.9 |
| CNF-PCD | 1.95 | 2.46 | 15.0 |
| Drop Casted | |||
| CNF | 2.00 | 3.00 | 13.0 |
| CNF-CD | 2.80 | 3.90 | 14.7 |
| CNF-PCD | 4.20 | 4.90 | 16.8 |
| In situ on QCM-D | |||
| CNF/MC | 0.30 | 0.53 | 1.99 |
| CNF-CD/MC | 1.18 | 1.45 | 6.00 |
| CNF-PCD/MC | 2.06 | 2.44 | 9.23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez-Maldonado, D.; Vega Erramuspe, I.B.; Filpponen, I.; Johansson, L.-S.; Lombardo, S.; Zhu, J.; Thielemans, W.; Peresin, M.S. Cellulose-Cyclodextrin Co-Polymer for the Removal of Cyanotoxins on Water Sources. Polymers 2019, 11, 2075. https://doi.org/10.3390/polym11122075
Gomez-Maldonado D, Vega Erramuspe IB, Filpponen I, Johansson L-S, Lombardo S, Zhu J, Thielemans W, Peresin MS. Cellulose-Cyclodextrin Co-Polymer for the Removal of Cyanotoxins on Water Sources. Polymers. 2019; 11(12):2075. https://doi.org/10.3390/polym11122075
Chicago/Turabian StyleGomez-Maldonado, Diego, Iris Beatriz Vega Erramuspe, Ilari Filpponen, Leena-Sisko Johansson, Salvatore Lombardo, Junyong Zhu, Wim Thielemans, and Maria S. Peresin. 2019. "Cellulose-Cyclodextrin Co-Polymer for the Removal of Cyanotoxins on Water Sources" Polymers 11, no. 12: 2075. https://doi.org/10.3390/polym11122075
APA StyleGomez-Maldonado, D., Vega Erramuspe, I. B., Filpponen, I., Johansson, L.-S., Lombardo, S., Zhu, J., Thielemans, W., & Peresin, M. S. (2019). Cellulose-Cyclodextrin Co-Polymer for the Removal of Cyanotoxins on Water Sources. Polymers, 11(12), 2075. https://doi.org/10.3390/polym11122075






