Development of Flame-Retarded Nanocomposites from Recycled PET Bottles for the Electronics Industry
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Composition Optimisation
3.1.1. Characterization of Nanoclays and Nanocomposites
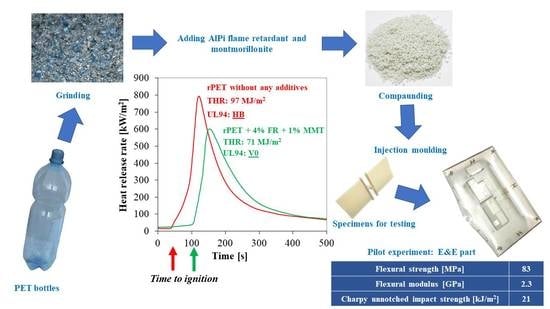
3.1.2. Flammability of rPET Nanocomposites
3.1.3. Mechanical Properties of rPET Nanocomposites
3.2. Pilot Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, N.; Hui, D.; Singh, R.; Ahuja, I.P.S.; Feo, L.; Fraternali, F. Recycling of plastic solid waste: A state of art review and future applications. Compos. Part B Eng. 2017, 115, 409–422. [Google Scholar] [CrossRef]
- Khoonkari, M.; Haghighi, A.H.; Sefidbakht, Y.; Shekoohi, K.; Ghaderian, A. Chemical Recycling of PET Wastes with Different Catalysts. Int. J. Polym. Sci. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Del Mar Castro López, M.; Ares Pernas, A.I.; Abad López, M.J.; Latorre, A.L.; López Vilariño, J.M.; González Rodríguez, M.V. Assessing changes on poly(ethylene terephthalate) properties after recycling: Mechanical recycling in laboratory versus postconsumer recycled material. Mater. Chem. Phys. 2014, 147, 884–894. [Google Scholar] [CrossRef]
- Itim, B.; Philip, M. Effect of multiple extrusions and influence of PP contamination on the thermal characteristics of bottle grade recycled PET. Polym. Degrad. Stab. 2015, 117, 84–89. [Google Scholar] [CrossRef]
- Molnár, B.; Ronkay, F. Effect of solid-state polycondensation on crystalline structure and mechanical properties of recycled polyethylene-terephthalate. Polym. Bull. 2018, 28. [Google Scholar] [CrossRef]
- Alsewailem, F.D.; Alrefaie, J.K. Effect of contaminants and processing regime on the mechanical properties and moldability of postconsumer polyethylene terephthalate bottles. Waste Manag. 2018, 81, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Ronkay, F.; Czigány, T. Development of composites with recycled PET matrix. Polym. Adv. Technol. 2006, 17, 830–834. [Google Scholar] [CrossRef]
- Dairi, B.; Djidjelli, H.; Boukerrou, A.; Migneault, S.; Koubaa, A. Morphological, mechanical, and physical properties of composites made with wood flour-reinforced polypropylene/recycled poly(ethylene terephthalate) blends. Polym. Compos. 2017, 38, 1749–1755. [Google Scholar] [CrossRef]
- Ronkay, F. Influence of short glass fiber reinforcement on the morphology development and mechanical properties of PET/HDPE blends. Polym. Compos. 2011, 32, 586–595. [Google Scholar] [CrossRef]
- Vo, P.P.; Doan, H.N.; Kinashi, K.; Sakai, W.; Tsutsumi, N.; Huynh, D.P. Centrifugally spun recycled PET: Processing and characterization. Polymers (Basel) 2018, 10, 680. [Google Scholar] [CrossRef]
- Romhány, G.; Wu, C.M.; Lai, W.Y.; Karger-Kocsis, J. Fracture behavior and damage development in self-reinforced PET composites assessed by located acoustic emission and thermography: Effects of flame retardant and recycled PET. Compos. Sci. Technol. 2016, 132, 76–83. [Google Scholar] [CrossRef]
- Supaphorn, T.; Takanori, N.; Wiranphat, T.; Hiroyuki, I.; Masayuki, O.; Hiroyuki, H. Effect of ammonium polyphosphate and fillers on flame retardant and mechanical properties of recycled PET injection molded. Polym. Adv. Technol. 2015, 28, 979–985. [Google Scholar] [CrossRef]
- Zare, Y. Recent progress on preparation and properties of nanocomposites from recycled polymers: A review. Waste Manag. 2013, 33, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, R.; Xu, X. Thermal stability and flame retardancy of PET/magnesium salt composites. Polym. Degrad. Stab. 2010, 95, 1466–1470. [Google Scholar] [CrossRef]
- Chen, L.; Bian, X.C.; Yang, R.; Wang, Y.Z. PET in situ composites improved both flame retardancy and mechanical properties by phosphorus-containing thermotropic liquid crystalline copolyester with aromatic ether moiety. Compos. Sci. Technol. 2012, 72, 649–655. [Google Scholar] [CrossRef]
- Brehme, S.; Schartel, B.; Goebbels, J.; Fischer, O.; Pospiech, D.; Bykovc, Y.; Döring, M. Phosphorus polyester versus aluminum phosphinate in poly(butylene terephthalate) (PBT): Flame retardancy performance and mechanisms. Polym. Degrad. Stab. 2011, 96, 875–884. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Y.Z.; Ban, D.M.; Liu, X.H.; Zhou, Q. Burning behavior and pyrolysis products of flame-retardant PET containing sulfur-containing aryl polyphosphonate. J. Anal. Appl. Pyrolysis 2006, 76, 198–202. [Google Scholar] [CrossRef]
- Swoboda, B.; Buonomo, S.; Leroy, E.; Lopez Cuesta, J.M. Fire retardant poly(ethylene terephthalate)/polycarbonate/triphenyl phosphite blends. Polym. Degrad. Stab. 2008, 93, 910–917. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Xue, B.; Niu, M.; Yang, Y.; Bai, J.; Song, Y.; Peng, Y. Applied Surface Science Multi-functional carbon microspheres with double shell layers for flame retardant poly(ethylene terephthalate). Appl. Surf. Sci. 2018, 435, 656–665. [Google Scholar] [CrossRef]
- Ding, Y.; Stoliarov, S.; Kraemer, R. Development of a Semiglobal Reaction Mechanism for the Thermal Decomposition of a Polymer Containing Reactive Flame Retardants: Application to Glass-Fiber-Reinforced Polybutylene Terephthalate Blended with Aluminum Diethyl Phosphinate and Melamine Polyphosphate. Polymers (Basel) 2018, 10, 1137. [Google Scholar] [CrossRef]
- Braun, U.; Bahr, H.; Schartel, B. Fire retardancy effect of aluminium phosphinate and melamine polyphosphate in glass fibre reinforced polyamide 6. E-Polymers 2010, 10, 1–14. [Google Scholar] [CrossRef]
- Ramani, A.; Dahoe, A.E. On the performance and mechanism of brominated and halogen free flame retardants in formulations of glass fibre reinforced poly(butylene terephthalate). Polym. Degrad. Stab. 2014, 104, 71–86. [Google Scholar] [CrossRef]
- Wu, Z.; Xue, M.; Wang, H.; Tian, X.; Ding, X.; Zheng, K.; Cui, P. Electrical and flame-retardant properties of carbon nanotube/poly(ethylene terephthalate) composites containing bisphenol A bis(diphenyl phosphate). Polymer (Guildf) 2013, 54, 3334–3340. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V.; Milone, C.; Mastronardo, E.; Piperopoulos, E.; Iemmo, L.; Di Bartolomeo, A. Effect of temperature and morphology on the electrical properties of PET/conductive nano fillers composites. Compos. Part B Eng. 2018, 135, 149–154. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C.D. Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. Polym. Sci. 2010, 35, 902–958. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, Q.; Zhang, P.; Xia, Y.; Kong, Q. Thermal stability and flame-retardancy mechanism of poly(ethylene terephthalate)/boehmite nanocomposites. Polym. Degrad. Stab. 2010, 95, 1211–1218. [Google Scholar] [CrossRef]
- Bakirtzis, D.; Ramani, A.; Delichatsios, M.A.; Zhang, J. Structure of the condensed phase and char of fire-retarded PBT nanocomposites by TGA/ATR in N2. Fire Saf. J. 2009, 44, 1023–1029. [Google Scholar] [CrossRef]
- Ye, L.; Ren, J.; Cai, S.Y.; Wang, Z.G.; Li, J.B. Poly(lactic acid) nanocomposites with improved flame retardancy and impact strength by combining of phosphinates and organoclay. Chin. J. Polym. Sci. (Engl. Ed.) 2016, 34, 785–796. [Google Scholar] [CrossRef]
- Kim, J.-C.; Chang, J.-H. Comparison of the properties of poly(butylene terephthalate) nanocomposite fibers with different organoclays. Macromol. Res. 2007, 15, 449–458. [Google Scholar] [CrossRef]
- Louisy, J.; Bourbigot, S.; Duquesne, S.; Desbois, P.; König, A.; Klatt, M. Novel synergists for flame retarded glass-fiber reinforced poly(1,4-butylene terephthalate). Polimery/Polymers 2013, 58, 403–412. [Google Scholar] [CrossRef]
- Ge, X.G.; Wang, D.Y.; Wang, C.; Qu, M.-H.; Wang, J.-S.; Zhao, C.-S.; Jing, X.-K.; Wang, Y.-Z. A novel phosphorus-containing copolyester/montmorillonite nanocomposites with improved flame retardancy. Eur. Polym. J. 2007, 43, 2882–2890. [Google Scholar] [CrossRef]
- Habibi, S.; Rashidi, A.; Bazgir, S.; Katbab, A.A.; Montazer, M. Preparation and flame retardancy of poly(ethylene terephthalate)/ montmorillonite nanocomposites. Asian J. Chem. 2009, 21, 4881–4888. [Google Scholar]
- Bikiaris, D. Thermochimica Acta Can nanoparticles really enhance thermal stability of polymers? Part II: An overview on thermal decomposition of polycondensation polymers. Thermochim. Acta 2011, 523, 25–45. [Google Scholar] [CrossRef]
- Merijs Meri, R.; Zicans, J.; Maksimovs, R.; Ivanova, T.; Kalnins, M.; Berzina, R.; Japins, G. Elasticity and long-term behavior of recycled polyethylene terephthalate (rPET)/montmorillonite (MMT) composites. Compos. Struct. 2014, 111, 453–458. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Karandrea, E.; Giliopoulos, D.; Papageorgiou, D.G.; Ladavos, A.; Katerinopoulou, A.; Achilias, D.S.; Triantafyllidis, K.S.; Bikiaris, D.N. Effect of clay structure and type of organomodifier on the thermal properties of poly(ethylene terephthalate) based nanocomposites. Thermochim. Acta 2014, 576, 84–96. [Google Scholar] [CrossRef]
- Litchfield, D.W.; Baird, D.G. The role of nanoclay in the generation of poly(ethylene terephthalate) fibers with improved modulus and tenacity. Polymer (Guildf) 2008, 49, 5027–5036. [Google Scholar] [CrossRef]
- Lai, M.C.; Chang, K.C.; Huang, W.C.; Hsu, S.C.; Yeh, J.M. Effect of swelling agent on the physical properties of PET-clay nanocomposite materials prepared from melt intercalation approach. J. Phys. Chem. Solids 2008, 69, 1371–1374. [Google Scholar] [CrossRef]
- Pegoretti, A.; Kolarik, J.; Peroni, C.; Migliaresi, C. Recycled poly(ethylene terephthalate)/layered silicate nanocomposites: Morphology and tensile mechanical properties. Polymer (Guildf) 2004, 45, 2751–2759. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, J.; Ma, Y.; Agarwal, U.S. Study on mechanical properties, thermal stability and crystallization behavior of PET/MMT nanocomposites. Compos. Part B Eng. 2006, 37, 399–407. [Google Scholar] [CrossRef]
- Kráčalík, M.; Mikešová, J.; Puffr, R.; Baldrian, J.; Thomann, R.; Friedrich, C. Effect of 3D structures on recycled PET/organoclay nanocomposites. Polym. Bull. 2007, 58, 313–319. [Google Scholar] [CrossRef]
- Vassiliou, A.A.; Chrissafis, K.; Bikiaris, D.N. In situ prepared PET nanocomposites: Effect of organically modified montmorillonite and fumed silica nanoparticles on PET physical properties and thermal degradation kinetics. Thermochim. Acta 2010, 500, 21–29. [Google Scholar] [CrossRef]
- Hirschler, M.M. How to Measure Smoke Obscuration in a Manner Relevant to Fire Hazard Assessment: Use of Heat Release Calorimetry Test Equipment. J. Fire Sci. 1991, 9, 183–222. [Google Scholar] [CrossRef]
- Hanna, A.A.; Nour, M.A.; Souaya, E.R.; Sherief, M.A.; Abdelmoaty, A.S. Studies on the flammability of polypropylene/ammonium polyphosphate and montmorillonite by using the cone calorimeter test. Open Chem. 2018, 16, 108–115. [Google Scholar] [CrossRef]
- Badia, J.D.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. The role of crystalline, mobile amorphous rigid amorphous fractions in the performance of recycled poly (ethylene terephthalate) (PET). Polym. Degrad. Stabil. 2012, 1, 98–107. [Google Scholar] [CrossRef]
- Koo, J.H. Fundamentals, Properties, and Applications of Polymer Nanocomposites; Cambridge University Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Pomogailo, A.D.; Dzhardimalieva, G.I. Nanostructured Materials Preparation via Condensation Ways; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA, 2014. [Google Scholar]
- Xia, Y.; Ghasemlou, M.; Rubino, M.; Auras, R.; Baghdachi, J. Novel Active Surface Prepared by Embedded Functionalized Clays in an Acrylate Coating. ACS Appl. Mater. Interfaces 2015, 7, 24944–24949. [Google Scholar] [CrossRef] [PubMed]
- Bocz, K.; Molnár, B.; Marosi, G.; Ronkay, F. Preparation of Low-Density Microcellular Foams from Recycled PET Modified by Solid State Polymerization and Chain Extension. J. Polym. Environ 2018, 8. [Google Scholar] [CrossRef]














| Paper | Type of Polyester | Mechanical Properties | Nature of Nanoclay/Dispersion |
|---|---|---|---|
| Ye et al. [30] | PLA | Little increase in impact strength and tensile strength | oMMT: Mixed intercalated/exfoliated structures. |
| Kim et al. [31] | PBT | Increase in tensile strength | oMMT: Intercalation and clusters |
| Ramani et al. [23] | PBT | Not tested | oMMT: Not tested |
| Louisy et al. [32] | PBT | Not tested | oMMT: Not tested |
| Ge et al. [33] | PET–2-carboxyethyl (phenylphosphinic) acid (PET-co-HPPPA) copolymer | Not tested | oMMT: Strong intercalation |
| Habibi et al. [34] | PET | Not tested | oMMT: Intercalated morphology |
| Pegoretti et al. [40] | recycled PET | MMT and oMMT had no significant effect on tensile strength, elongation at break decreased, and modulus increased in both case | MMT: Weak intercalation oMMT: Strong intercalation |
| Wang et al. [41] | PET | Impact strength and elongation at break decrease in the function of oMMT | oMMT: Intercalation |
| Kracalik et al. [42] | recycled PET | Not tested | oMMT: Partial or no exfoliation |
| Vassiliou et al. [43] | PET | Increase in tensile strength | oMMT: Exfoliation |
| rPET [%] | FR [%] | MMT [%] | oMMT [%] | |
|---|---|---|---|---|
| 0 FR | 100 | |||
| 0 FR + 1 oMMT | 99 | 1 | ||
| 0 FR + 3 oMMT | 97 | 3 | ||
| 0 FR + 1 MMT | 99 | 1 | ||
| 0 FR + 3 MMT | 97 | 3 | ||
| 4 FR | 96 | 4 | ||
| 4 FR + 1 oMMT | 95 | 4 | 1 | |
| 4 FR + 3 oMMT | 93 | 4 | 3 | |
| 4 FR + 1 MMT | 95 | 4 | 1 | |
| 4 FR + 3 MMT | 93 | 4 | 3 | |
| 8 FR | 92 | 8 | ||
| 8 FR + 1 oMMT | 91 | 8 | 1 | |
| 8 FR + 3 oMMT | 89 | 8 | 3 | |
| 8 FR + 1 MMT | 91 | 8 | 1 | |
| 8 FR + 3 MMT | 89 | 8 | 3 |
| Diffraction Angle (2θ) [°] | Interlayer Spacing [nm] | |
|---|---|---|
| MMT | 7.07 | 1.25 |
| rPET + 1% MMT | 7.01 | 1.26 |
| rPET + 3% MMT | 7.01 | 1.26 |
| oMMT | 2.70 | 3.27 |
| rPET + 1% oMMT | 2.70 | 3.27 |
| rPET + 3% oMMT | 2.72 | 3.25 |
| Char Amount [%] | |
|---|---|
| rPET | 11.3 |
| rPET + 1% MMT | 14.5 |
| rPET + 3% MMT | 16.2 |
| rPET + 1% oMMT | 11.9 |
| rPET + 3% oMMT | 14.5 |
| TTI [s] | HRRmax Time [s] | HRRmax [kW/m2] | THR [MJ/m2] | AEHC [MJ/kg] | FPI [sm2/kW] | Residual Mass [%] | UL-94 Rating | |
|---|---|---|---|---|---|---|---|---|
| 0 FR | 39 | 119 | 773 | 97 | 17.7 | 0.05 | 0 | HB |
| 0 FR + 1 oMMT | 38 | 135 | 706 | 129 | 23.5 | 0.054 | 0 | HB |
| 0 FR + 3 oMMT | 37 | 143 | 679 | 140 | 25.6 | 0.054 | 0 | HB |
| 0 FR + 1 MMT | 61 | 123 | 649 | 80 | 14.6 | 0.094 | 2.7 | V2 |
| 0 FR + 3 MMT | 63 | 134 | 674 | 89 | 17.3 | 0.093 | 5.7 | V2 |
| 4 FR | 58 | 145 | 401 | 73 | 13.6 | 0.145 | 0.6 | V2 |
| 4 FR + 1 oMMT | 69 | 148 | 433 | 84 | 15.7 | 0.159 | 2 | V2 |
| 4 FR + 3 oMMT | 38 | 150 | 506 | 117 | 21.2 | 0.075 | 0.2 | V2 |
| 4 FR + 1 MMT | 104 | 151 | 579 | 71 | 15.2 | 0.18 | 14.8 | V0 |
| 4 FR + 3 MMT | 102 | 166 | 543 | 87 | 17.4 | 0.188 | 8.9 | V0 |
| 8 FR | 109 | 167 | 418 | 60 | 11.3 | 0.261 | 4.6 | V2 |
| 8 FR + 1 oMMT | 60 | 144 | 434 | 84 | 15.6 | 0.138 | 2.5 | V2 |
| 8 FR + 3 oMMT | 22 | 172 | 420 | 100 | 18.1 | 0.052 | 0 | V2 |
| 8 FR + 1 MMT | 93 | 164 | 367 | 71 | 14.9 | 0.253 | 15.9 | V0 |
| 8 FR + 3 MMT | 91 | 172 | 295 | 58 | 12.7 | 0.308 | 15.8 | V0 |
| rPET+ 4 FR + 1 MMT | PC/ABS NH-1237* | HIPS VE-1801* | |
|---|---|---|---|
| UL 94 rating [2 mm] | V0 | V0 | V0 |
| Flexural strength[MPa] | 83 | 85 | 32 |
| Flexural modulus[GPa] | 2.25 | 4.2 | 1.80 |
| Charpy unnotched impact strength [kJ/m2] | 20.6 | no data | no data |
| Charpy notched impact strength[kJ/m2] | 2.1 | 5.0 | 10.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronkay, F.; Molnár, B.; Szalay, F.; Nagy, D.; Bodzay, B.; Sajó, I.E.; Bocz, K. Development of Flame-Retarded Nanocomposites from Recycled PET Bottles for the Electronics Industry. Polymers 2019, 11, 233. https://doi.org/10.3390/polym11020233
Ronkay F, Molnár B, Szalay F, Nagy D, Bodzay B, Sajó IE, Bocz K. Development of Flame-Retarded Nanocomposites from Recycled PET Bottles for the Electronics Industry. Polymers. 2019; 11(2):233. https://doi.org/10.3390/polym11020233
Chicago/Turabian StyleRonkay, Ferenc, Béla Molnár, Ferenc Szalay, Dóra Nagy, Brigitta Bodzay, István E. Sajó, and Katalin Bocz. 2019. "Development of Flame-Retarded Nanocomposites from Recycled PET Bottles for the Electronics Industry" Polymers 11, no. 2: 233. https://doi.org/10.3390/polym11020233
APA StyleRonkay, F., Molnár, B., Szalay, F., Nagy, D., Bodzay, B., Sajó, I. E., & Bocz, K. (2019). Development of Flame-Retarded Nanocomposites from Recycled PET Bottles for the Electronics Industry. Polymers, 11(2), 233. https://doi.org/10.3390/polym11020233