Comparisons of Functional Properties of Polysaccharides from Nostoc flagelliforme under Three Culture Conditions
Abstract
:1. Introduction
2. Materials and Methods
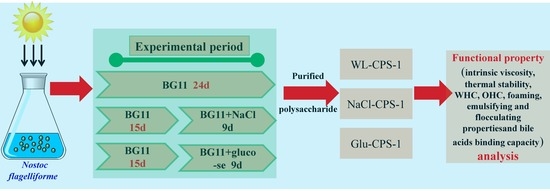
2.1. Strains and Culture Conditions
2.2. The Measurement of Cell Growth and CPS Production
2.3. Isolation and Purification of Polysaccharides
2.4. Determination of Functional Properties
2.4.1. Intrinsic Viscosity Analysis
2.4.2. Thermogravimetric Analysis (TGA)
2.4.3. Water-holding Capacity (WHC) and Oil-holding Capacity (OHC)
2.4.4. Foaming Properties
2.4.5. Emulsifying and Flocculating Properties
2.4.6. Determination of Bile Acids Binding Capacity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Biomass and CPS Production
3.2. Intrinsic Viscosity
3.3. Thermal Characteristic Analysis
3.4. WHC and OHC of Polysaccharides
3.5. Foaming Properties of Polysaccharides
3.6. Emulsifying Properties of Polysaccharides
3.7. Flocculating Activity of Polysaccharides
3.8. Bile Acids Binding Capacity
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bender, J.; Phillips, P. Microbial mats for multiple applications in aquaculture and bioremediation. Bioresource Technol. 2004, 94, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Freire-Nordi, C.S.; Vieira, A.A.H.; Nascimento, O.R. The metal binding capacity of Anabaena spiroides extracellular polysaccharide: An EPR study. Process Biochem. 2005, 40, 2215–2224. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Reis, M.A.M. Advances in bacterial exopolysaccharides: From production to biotechnological applications. Trends Biotechnol. 2011, 29, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.J.; Huang, N.K.; Lur, H.S.; Kuo, C.I.; Lu, M.K. Characterization and biological functions of sulfated polysaccharides from sulfated-salt treatment of Antrodia cinnamomea. Process Biochem. 2009, 44, 453–459. [Google Scholar] [CrossRef]
- Ben Slima, S.; Ktari, N.; Trabelsi, I.; Moussa, H.; Makni, I.; Ben Salah, R. Purification, characterization and antioxidant properties of a novel polysaccharide extracted from Sorghum bicolor (L.) seeds in sausage. Int. J. Biol. Macromol. 2018, 106, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gao, X.D.; Chen, Z.Q.; Chen, Y.; Chen, H.X. Preparation, Characterization and Application of Polysaccharide-Based Metallic Nanoparticles: A Review. Polymers 2017, 9, 689. [Google Scholar] [CrossRef]
- Ktari, N.; Feki, A.; Trabelsi, I.; Triki, M.; Maalej, H.; Slima, S.B.; Nasri, M.; Ben Amara, I.; Ben Salah, R. Structure, functional and antioxidant properties in Tunisian beef sausage of a novel polysaccharide from Trigonella foenum-graecum seeds. Int. J. Biol. Macromol. 2017, 98, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Diana, F.O.; Elizabeth, C.M.; Jose, A.L.E.; Karla, G.M.R.; Anselmo, M.B.; Luis, R.M.C.; Fernando, E.O.; Jose, E.V.H. Chemical characterization and antioxidant activity of sulfated polysaccharides from Navicula sp. Food Hydrocolloids 2018, 75, 229–236. [Google Scholar]
- Lo, T.C.T.; Chang, C.A.; Chiu, K.H.; Tsay, P.K.; Jen, J.F. Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohyd. Polym. 2011, 86, 320–327. [Google Scholar] [CrossRef]
- Yan, J.K.; Wang, Y.Y.; Qiu, W.Y.; Wu, L.X.; Ding, Z.C.; Cai, W.D. Purification, structural characterization and bioactivity evaluation of a novel proteoglycan produced by Corbicula fluminea. Carbohyd. Polym. 2017, 176, 11–18. [Google Scholar] [CrossRef]
- Shi, J.J.; Zhang, J.G.; Sun, Y.H.; Qu, J.; Li, L.; Prasad, C.; Wei, Z.J. Physicochemical properties and antioxidant activities of polysaccharides sequentially extracted from peony seed dreg. Int. J. Biol. Macromol. 2016, 91, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.Z.; Li, Y.M.; Wang, T.X.; Sun, Y.W.; Xu, X.Y.; Zhang, Z.S. Isolation, fine structure and morphology studies of galactomannan from endosperm of Gleditsia japonica var. delavayi. Carbohyd. Polym. 2018, 184, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Han, P.P.; Shen, S.G.; Wang, H.Y.; Sun, Y.; Dai, Y.J.; Jia, S.R. Comparative metabolomic analysis of the effects of light quality on polysaccharide production of cyanobacterium Nostoc flagelliforme. Algal Res. 2015, 9, 143–150. [Google Scholar] [CrossRef]
- Hayashi, K.; Kanekiyo, K.; Ohta, Y.; Lee, J.B.; Takenaka, H.; Hayashi, T. Anti-influenza a virus activity of an acidic polysaccharide from a blue-green alga Nostoc flagelliforme. Planta Med. 2008, 74, PA34. [Google Scholar] [CrossRef]
- Kanekiyo, K.; Lee, J.B.; Hayashi, K.; Takenaka, H.; Hayakawa, Y.; Endo, S.; Hayashi, T. Isolation of an antiviral polysaccharide, nostoflan, from a terrestrial cyanobacterium, Nostoc flagelliforme. J. Nat. Prod. 2005, 68, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, K.; Hayashi, K.; Takenaka, H.; Lee, J.B.; Hayashi, T. Anti-herpes simplex virus target of an acidic polysaccharide, nostoflan, from the edible blue-green alga Nostoc flagelliforme. Biol. Pharm. Bull. 2007, 30, 1573–1575. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.G.; Jia, S.R.; Wu, Y.K.; Yan, R.R.; Lin, Y.H.; Zhao, D.X.; Han, P.P. Effect of culture conditions on the physicochemical properties and antioxidant activities of polysaccharides from Nostoc flagelliforme. Carbohyd. Polym. 2018, 198, 426–433. [Google Scholar] [CrossRef]
- Ding, Z.; Jia, S.R.; Han, P.P.; Yuan, N.N.; Tan, N. Effects of carbon sources on growth and extracellular polysaccharide production of Nostoc flagelliforme under heterotrophic high-cell-density fed-batch cultures. J. Appl. Phycol. 2013, 25, 1017–1021. [Google Scholar] [CrossRef]
- Han, P.P.; Sun, Y.; Wu, X.Y.; Yuan, Y.J.; Dai, Y.J.; Jia, S.R. Emulsifying, Flocculating, and Physicochemical Properties of Exopolysaccharide Produced by Cyanobacterium Nostoc flagelliforme. Appl. Biochem. Biotechnol. 2014, 172, 36–49. [Google Scholar] [CrossRef]
- Liu, X.J.; Jiang, Y.; Chen, F. Fatty acid profile of the edible filamentous cyanobacterium Nostoc flagelliforme at different temperatures and developmental stages in liquid suspension culture. Process Biochem. 2005, 40, 371–377. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Dong, F.Y.; Liu, X.C.; Lv, Q.; Yang, Y.; Liu, F.; Chen, L.; Wang, T.T.; Wang, Z.; Zhang, Y.M. Effects of extraction methods on the yield, chemical structure and anti-tumor activity of polysaccharides from Cordyceps gunnii mycelia. Carbohyd. Polym. 2016, 140, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.A.; de Monredon, F.D.; Dysseler, P.; Guillon, F.; Amado, R.; Thibault, J.F. Hydration properties of dietary fibre and resistant starch: A European collaborative study. LWT-Food Sci. Technol. 2000, 33, 72–79. [Google Scholar] [CrossRef]
- Bayar, N.; Bouallegue, T.; Achour, M.; Kriaa, M.; Bougatef, A.; Kammoun, R. Ultrasonic extraction of pectin from Opuntia ficus indica cladodes after mucilage removal: Optimization of experimental conditions and evaluation of chemical and functional properties. Food Chem. 2017, 235, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kahlon, T.S.; Smith, G.E.; Shao, Q. In vitro binding of bile acids by kidney bean (Phaseolus vulgaris), black gram (Vigna mungo), bengal gram (Cicer arietinum) and moth bean (Phaseolus aconitifolins). Food Chem. 2005, 90, 241–246. [Google Scholar] [CrossRef]
- Parikh, A.; Madamwar, D. Partial characterization of extracellular polysaccharides from cyanobacteria. Bioresource. Technol. 2006, 97, 1822–1827. [Google Scholar] [CrossRef] [PubMed]
- Gallaher, C.M.; Munion, J.; Hesslink, R.; Wise, J.; Gallaher, D.D. Cholesterol reduction by glucomannan and chitosan is mediated by changes in cholesterol absorption and bile acid and fat excretion in rats. J. Nutr. 2000, 130, 2753–2759. [Google Scholar] [CrossRef]
- Leroux, J.; Langendorff, V.; Schick, G.; Vaishnav, V.; Mazoyer, J. Emulsion stabilizing properties of pectin. Food Hydrocolloids 2003, 17, 455–462. [Google Scholar] [CrossRef]
- Funami, T.; Nakauma, M.; Ishihara, S.; Tanaka, R.; Inoue, T.; Phillips, G.O. Structural modifications of sugar beet pectin and the relationship of structure to functionality. Food Hydrocolloids 2011, 25, 221–229. [Google Scholar] [CrossRef]
- Ghribi, A.M.; Sila, A.; Gafsi, I.M.; Blecker, C.; Danthine, S.; Attia, H.; Bougatef, A.; Besbes, S. Structural, functional, and ACE inhibitory properties of water-soluble polysaccharides from chickpea flours. Int. J. Biol. Macromol. 2015, 75, 276–282. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Dietary fibre and physicochemical properties of several edible seaweeds from the northwestern Spanish coast. Food Res. Int. 2010, 43, 2289–2294. [Google Scholar] [CrossRef]
- Kolsi, R.B.A.; Fakhfakh, J.; Krichen, F.; Jribi, I.; Chiarore, A.; Patti, F.P.; Blecker, C.; Allouche, N.; Belghith, H.; Belghith, K. Structural characterization and functional properties of antihypertensive Cymodocea nodosa sulfated polysaccharide. Carbohyd. Polym. 2016, 151, 511–522. [Google Scholar] [CrossRef]
- Fleury, N.; Lahaye, M. Chemical and physico-chemical characterisation of fibres from Laminaria digitata (kombu breton): A physiological approach. J. Sci. Food Agric. 1991, 55, 389–400. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, X.; Jing, C.L.; Zou, P.; Zhang, C.S.; Li, Y.Q. Microwave assisted hydrothermal extraction of polysaccharides from Ulva prolifera: Functional properties and bioactivities. Carbohyd. Polym. 2018, 181, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.Q.; Xia, W.S.; Zhang, C.; Yu, L.L. In vitro binding of bile acids and triglycerides by selected chitosan preparations and their physico-chemical properties. LWT-Food Sci. Technol. 2006, 39, 1087–1092. [Google Scholar] [CrossRef]
- Shad, M.A.; Nawaz, H.; Hussain, M.; Yousuf, B. Proximate composition and functional properties of rhizomes of lotus (Nelumbo nucifera) from Punjab, Pakistan. Pak. J. Bot. 2011, 43, 895–904. [Google Scholar]
- Jeddou, K.B.; Chaari, F.; Maktouf, S.; Nouri-Ellouz, O.; Helbert, C.B.; Ghorbel, R.E. Structural, functional, and antioxidant properties of water-soluble polysaccharides from potatoes peels. Food Chem. 2016, 205, 97–105. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Sessa, M.; Chouaibi, M.; Ferrari, G.; Donsì, F.; Hamdi, S. Assessment of emulsifying ability of almond gum in comparison with gum Arabic using response surface methodology. Food Hydrocolloids 2014, 37, 49–59. [Google Scholar] [CrossRef]
- Bach, Q.V.; Chen, W.H. Pyrolysis characteristics and kinetics of microalgae via thermogravimetric analysis (TGA): A state-of-the-art review. Bioresour. Technol. 2017, 246, 88–100. [Google Scholar] [CrossRef]
- Rizzo, A.M.; Prussi, M.; Bettucci, L.; Libelli, I.M.; Chiaramonti, D. Characterization of microalga Chlorella as a fuel and its thermogravimetric behavior. Appl. Energ. 2013, 102, 24–31. [Google Scholar] [CrossRef]
- Gannasin, S.P.; Adzahan, N.M.; Mustafa, S.; Muhammad, K. Techno-functional properties and in vitro bile acid-binding capacities of tamarillo (Solanum betaceum Cav.) hydrocolloids. Food Chem. 2016, 196, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.L.; Roy, S.; Vergara-Jimenez, M. Resistant starch and cholestyramine have distinct effects on hepatic cholesterol metabolism in guinea pigs fed a hypercholesterlemic diet. Nutr. Res. 2000, 20, 837–849. [Google Scholar] [CrossRef]
- Kahlon, T.S.; Woodruff, C.L. In vitro binding of bile acids by soy protein, pinto beans black beans and wheat gluten. Food Chem. 2002, 79, 425–429. [Google Scholar] [CrossRef]
- Yin, J.Y.; Nie, S.P.; Li, J.; Li, C.; Cui, S.W.; Xie, M.Y. Mechanism of Interactions between Calcium and Viscous Polysaccharide from the Seeds of Plantago asiatica L. J. Agric. Food Chem. 2012, 60, 7981–7987. [Google Scholar] [CrossRef] [PubMed]







| Treatment | Bile Acid Bound (μmol) | Relative Bile Acids Binding ability (%) a | |
|---|---|---|---|
| WL-CPS (mg/mL) | 0.2 | 0.253 ± 0.02 * | 68.94 ± 4.72 * |
| 0.5 | 0.267 ± 0.01 * | 72.75 ± 1.78 * | |
| 1.0 | 0.271 ± 0.01 * | 73.84 ± 3.24 * | |
| NaCl-CPS (mg/mL) | 0.2 | 0.252 ± 0.01 * | 68.66 ± 3.83 * |
| 0.5 | 0.262 ± 0.01 * | 71.39 ± 1.77 * | |
| 1.0 | 0.274 ± 0.01 * | 74.66 ± 2.06 * | |
| Glu-CPS (mg/mL) | 0.2 | 0.267 ± 0.01 * | 72.75 ± 3.54 * |
| 0.5 | 0.271 ± 0.01 * | 73.84 ± 2.65 * | |
| 1.0 | 0.291 ± 0.01 * | 79.29 ± 2.36 * | |
| Cholestyramine (mg) | 10 | 0.367 ± 0.02 | 100 ± 1.56 |
| Cellulose (mg) | 10 | 0.030 ± 0.01 | 8.17 ± 0.54 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, S.-G.; Lin, Y.-H.; Zhao, D.-X.; Wu, Y.-K.; Yan, R.-R.; Zhao, H.-B.; Tan, Z.-L.; Jia, S.-R.; Han, P.-P. Comparisons of Functional Properties of Polysaccharides from Nostoc flagelliforme under Three Culture Conditions. Polymers 2019, 11, 263. https://doi.org/10.3390/polym11020263
Shen S-G, Lin Y-H, Zhao D-X, Wu Y-K, Yan R-R, Zhao H-B, Tan Z-L, Jia S-R, Han P-P. Comparisons of Functional Properties of Polysaccharides from Nostoc flagelliforme under Three Culture Conditions. Polymers. 2019; 11(2):263. https://doi.org/10.3390/polym11020263
Chicago/Turabian StyleShen, Shi-Gang, Ya-Hui Lin, Dong-Xue Zhao, Yi-Kai Wu, Rong-Rong Yan, Hua-Bing Zhao, Zhi-Lei Tan, Shi-Ru Jia, and Pei-Pei Han. 2019. "Comparisons of Functional Properties of Polysaccharides from Nostoc flagelliforme under Three Culture Conditions" Polymers 11, no. 2: 263. https://doi.org/10.3390/polym11020263
APA StyleShen, S.-G., Lin, Y.-H., Zhao, D.-X., Wu, Y.-K., Yan, R.-R., Zhao, H.-B., Tan, Z.-L., Jia, S.-R., & Han, P.-P. (2019). Comparisons of Functional Properties of Polysaccharides from Nostoc flagelliforme under Three Culture Conditions. Polymers, 11(2), 263. https://doi.org/10.3390/polym11020263