Kinetic Analysis of the Thermal Degradation of Recycled Acrylonitrile-Butadiene-Styrene by non-Isothermal Thermogravimetry
Abstract
:1. Introduction
2. Theoretical Background
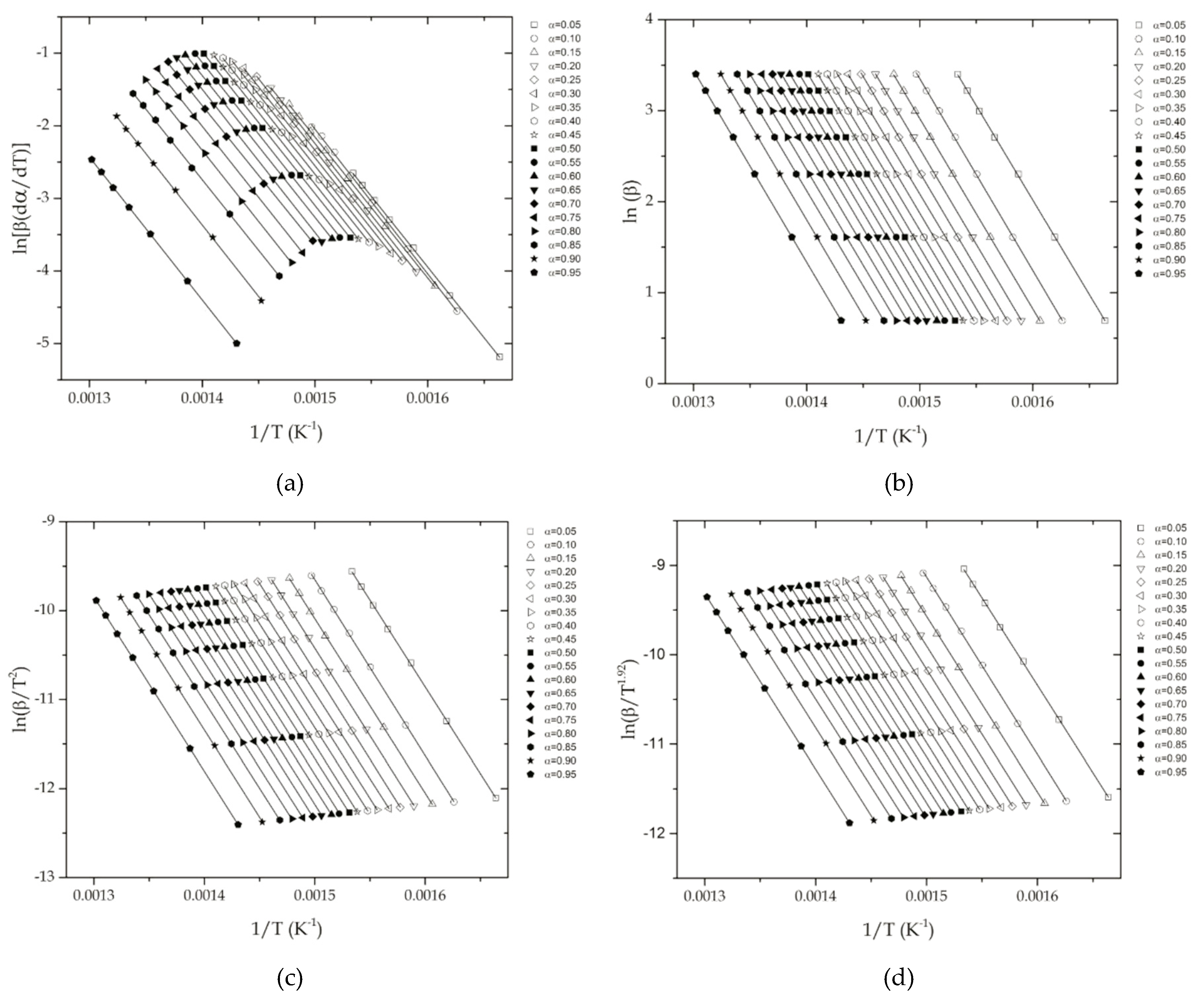
2.1. Isoconversional Methods
2.1.1. Differential Isoconversional Methods
2.1.2. Integral Isoconversional Methods
2.2. Combined Kinetic Analysis (CKA)
2.3. Criado and Maqueda Method
3. Materials and Methods
3.1. Materials
3.2. Thermal Degradation Analysis
4. Results and Discussion
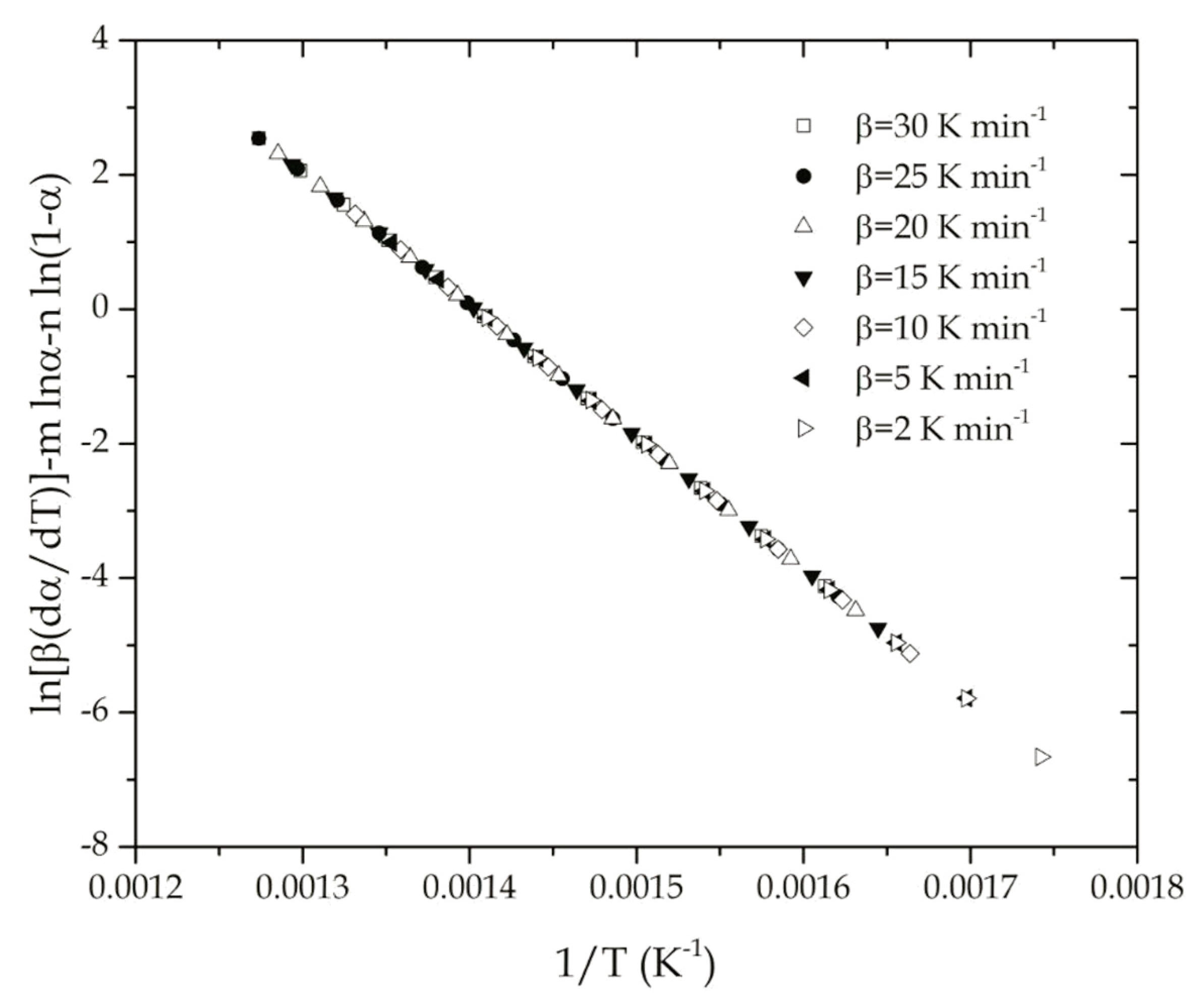
4.1. Determination of the Apparent Activation Energy of the Thermal Degradation of ABS
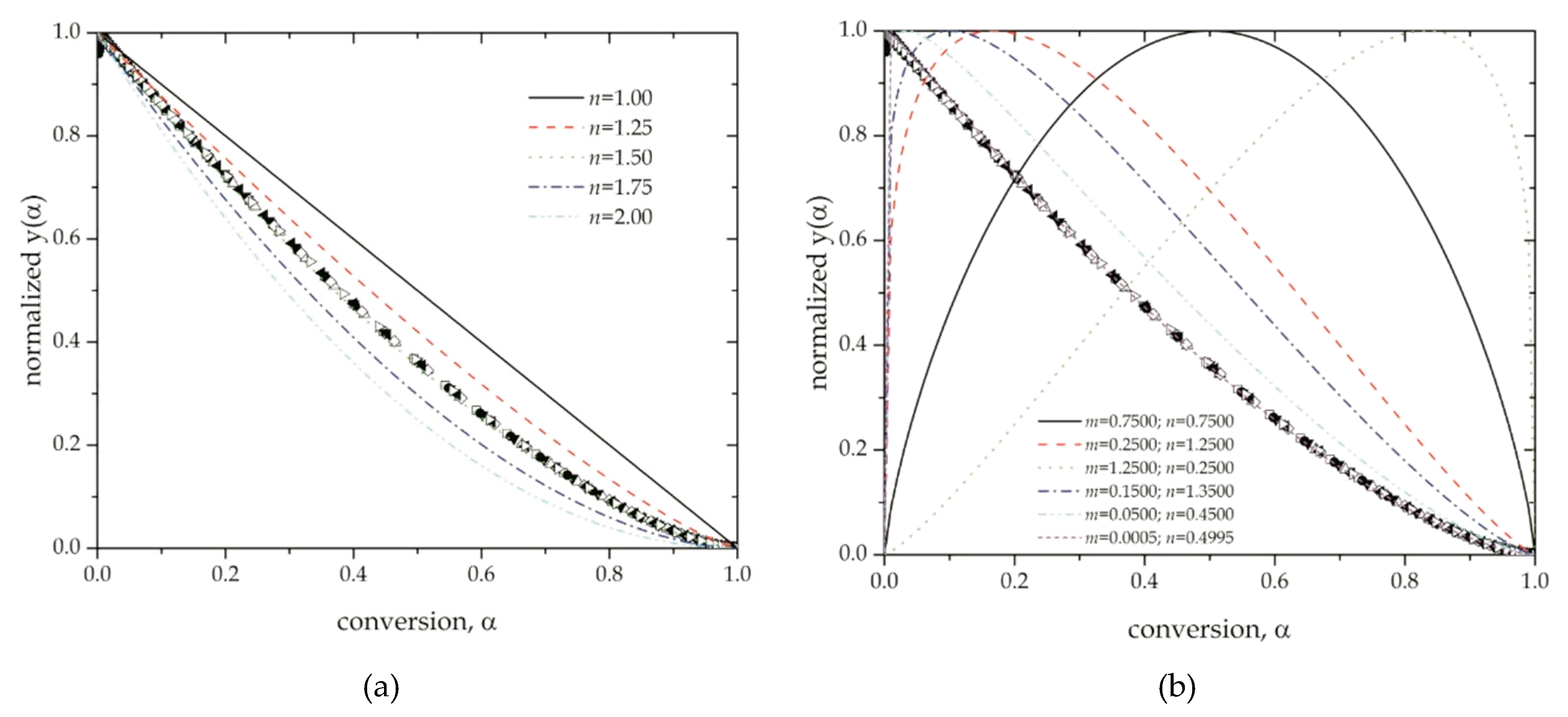
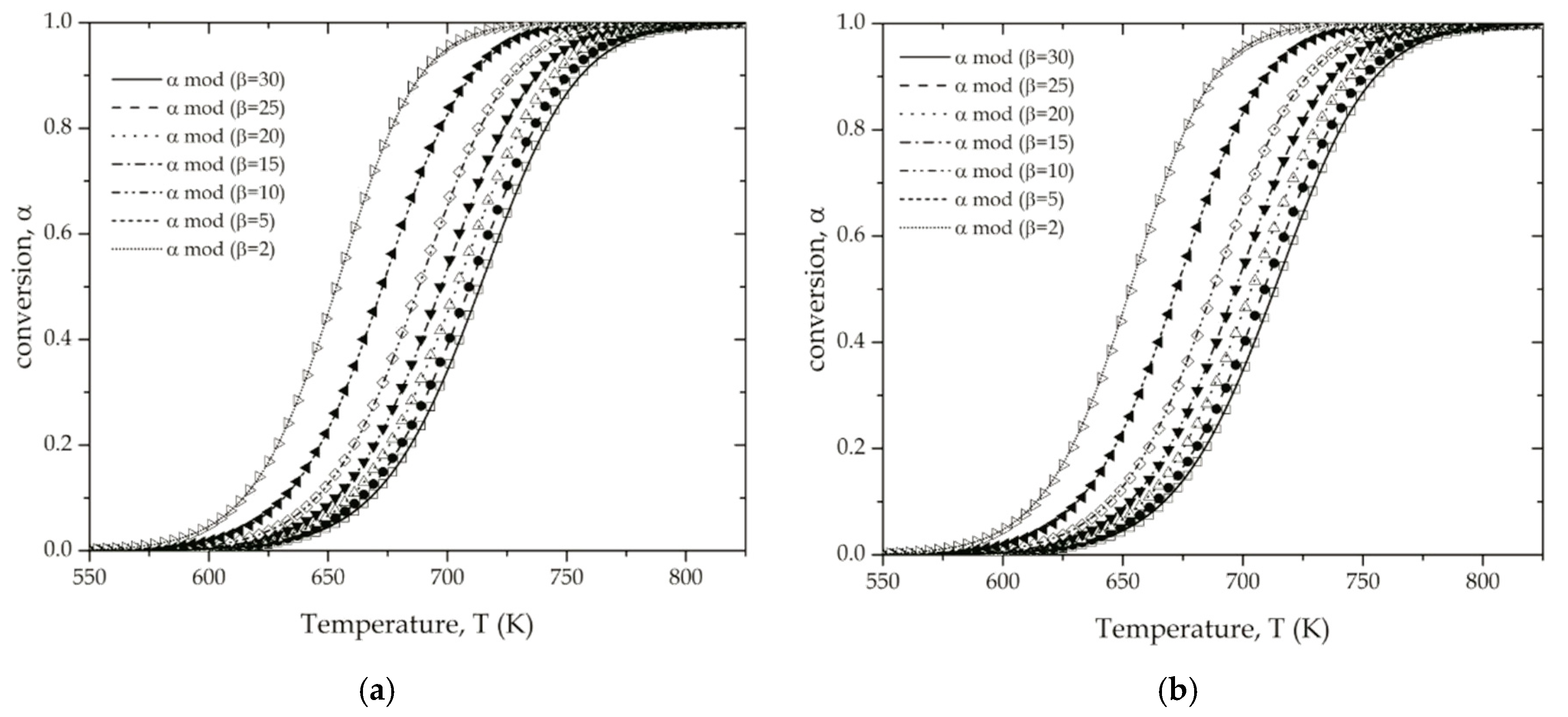
4.2. Determination of the Reaction Model of the Thermal Degradation of ABS
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Scaffaro, R.; Botta, L.; Di Benedetto, G. Physical properties of virgin-recycled abs blends: Effect of post-consumer content and of reprocessing cycles. Eur. Polym. J. 2012, 48, 637–648. [Google Scholar] [CrossRef]
- Tiganis, B.E.; Burn, L.S.; Davis, P.; Hill, A.J. Thermal degradation of acrylonitrile-butadiene-styrene (abs) blends. Polymer Degrad. Stab. 2002, 76, 425–434. [Google Scholar] [CrossRef]
- Niemczyk, A.; Dziubek, K.; Sacher-Majewska, B.; Czaja, K.; Czech-Polak, J.; Oliwa, R.; Lenza, J.; Szolyga, M. Thermal stability and flame retardancy of polypropylene composites containing siloxane-silsesquioxane resins. Polymers 2018, 10. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hussein, M.Z. Poly(lactic acid)/poly(ethylene glycol) polymer nanocomposites: Effects of graphene nanoplatelets. Polymers 2014, 6, 93–104. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Chen, X.; Wen, H.; Xiao, S. Theoretical study on decomposition mechanism of insulating epoxy resin cured by anhydride. Polymers 2017, 9. [Google Scholar] [CrossRef]
- Ramesh, V.; Biswal, M.; Mohanty, S.; Nayak, S.K. Compatibilization effect of eva-g-mah on mechanical, morphological and rheological properties of recycled pc/abs blend. Mater. Express 2014, 4, 499–507. [Google Scholar] [CrossRef]
- Kuram, E.; Ozcelik, B.; Yilmaz, F.; Timur, G.; Sahin, Z.M. The effect of recycling number on the mechanical, chemical, thermal, and rheological properties of pbt/pc/abs ternary blends: With and without glass-fiber. Polym. Compos. 2014, 35, 2074–2084. [Google Scholar] [CrossRef]
- Balart, R.; Lopez, J.; Garcia, D.; Salvador, M.D. Recycling of abs and pc from electrical and electronic waste. Effect of miscibility and previous degradation on final performance of industrial blends. Eur. Polym. J. 2005, 41, 2150–2160. [Google Scholar] [CrossRef]
- Khatri, B.; Lappe, K.; Habedank, M.; Mueller, T.; Megnin, C.; Hanemann, T. Fused deposition modeling of abs-barium titanate composites: A simple route towards tailored dielectric devices. Polymers 2018, 10. [Google Scholar] [CrossRef]
- Hart, K.R.; Wetzel, E.D. Fracture behavior of additively manufactured acrylonitrile butadiene styrene (abs) materials. Eng. Fract. Mech. 2017, 177, 1–13. [Google Scholar] [CrossRef]
- Vila Ramirez, N.; Sanchez-Soto, M. Effects of poss nanoparticles on abs-g-ma thermo oxidation resistance. Polym. Compos. 2012, 33, 1707–1718. [Google Scholar] [CrossRef]
- Duh, Y.-S.; Ho, T.-C.; Chen, J.-R.; Kao, C.-S. Study on exothermic oxidation of acrylonitrile-butadiene-styrene (abs) resin powder with application to abs processing safety. Polymers 2010, 2, 174–187. [Google Scholar] [CrossRef]
- Catelli de Souza, A.M.; Cucchiara, M.G.; Ereio, A.V. Abs/hips blends obtained from weee: Influence of processing conditions and composition. J. Appl. Polym. Sci. 2016, 133. [Google Scholar]
- Polli, H.; Pontes, L.A.M.; Araujo, A.S.; Barros, J.M.F.; Fernandes, V.J., Jr. Degradation behavior and kinetic study of abs polymer. J. Therm. Anal. Calorim. 2009, 95, 131–134. [Google Scholar] [CrossRef]
- Suzuki, M.; Wilkie, C.A. The thermal-degradation of acrylonitrile-butadiene-styrene terpolymer as studied by tga/ftir. Polym. Degrad. Stab. 1995, 47, 217–221. [Google Scholar] [CrossRef]
- Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A.; Perejon, A.; Criado, J.M. A new model for the kinetic analysis of thermal degradation of polymers driven by random scission. Polym. Degrad. Stab. 2010, 95, 733–739. [Google Scholar] [CrossRef]
- Perejon, A.; Sanchez-Jimenez, P.E.; Gil-Gonzalez, E.; Perez-Maqueda, L.A.; Criado, J.M. Pyrolysis kinetics of ethylene-propylene (epm) and ethylene-propylene-diene (epdm). Polym. Degrad. Stab. 2013, 98, 1571–1577. [Google Scholar] [CrossRef]
- Carrasco, F.; Perez-Maqueda, L.A.; Sanchez-Jimenez, P.E.; Perejon, A.; Santana, O.O.; Maspoch, M.L. Enhanced general analytical equation for the kinetics of the thermal degradation of poly(lactic acid) driven by random scission. Polym. Test. 2013, 32, 937–945. [Google Scholar] [CrossRef]
- Perez-Maqueda, L.A.; Sanchez-Jimenez, P.E.; Perejon, A.; Garcia-Garrido, C.; Criado, J.M.; Benitez-Guerrero, M. Scission kinetic model for the prediction of polymer pyrolysis curves from chain structure. Polym. Test. 2014, 37, 1–5. [Google Scholar] [CrossRef]
- Luda di Cortemiglia, M.P.; Camino, G.; Costa, L.; Guaita, M. Thermal degradation of abs. Thermochim. Acta 1985, 93, 187–190. [Google Scholar] [CrossRef]
- Day, M.; Cooney, J.D.; Mackinnon, M. Degradation of contaminated plastics—A kinetic-study. Polym. Degrad. Stab. 1995, 48, 341–349. [Google Scholar] [CrossRef]
- Balart, R.; Sanchez, L.; Lopez, J.; Jimenez, A. Kinetic analysis of thermal degradation of recycled polycarbonate/acrylonitrile-butadiene-styrene mixtures from waste electric and electronic equipment. Polym. Degrad. Stab. 2006, 91, 527–534. [Google Scholar] [CrossRef]
- Stanko, M.; Stommel, M. Kinetic prediction of fast curing polyurethane resins by model-free isoconversional methods. Polymers 2018, 10. [Google Scholar] [CrossRef]
- Starink, M.J. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
- Lyon, R.E. An integral method of nonisothermal kinetic analysis. Thermochim. Acta 1997, 297, 117–124. [Google Scholar] [CrossRef]
- Shao, J.; Wang, J.; Long, M.; Li, J.; Ma, Y. 5000 h multi-factor accelerated aging test of frp made transmission tower: Characterization, thermal decomposition and reaction kinetics study. Polymers 2017, 9. [Google Scholar] [CrossRef]
- Doyle, C.D. Kinetic analysis of thermogravimetric data. J. Appl. Polym. Sci. 1961, 5, 285–292. [Google Scholar] [CrossRef]
- Doyle, C.D. Estimating isothermal life from thermogravimetric data. J. Appl. Polym. Sci. 1962, 6, 639–642. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A quick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. B: Polym. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Japan 1965, 38, 1881. [Google Scholar] [CrossRef]
- Zhao, S.F.; Zhang, G.P.; Sun, R.; Wong, C.P. Curing kinetics, mechanism and chemorheological behavior of methanol etherified amino/novolac epoxy systems. Express Polym. Lett. 2014, 8, 95–106. [Google Scholar] [CrossRef]
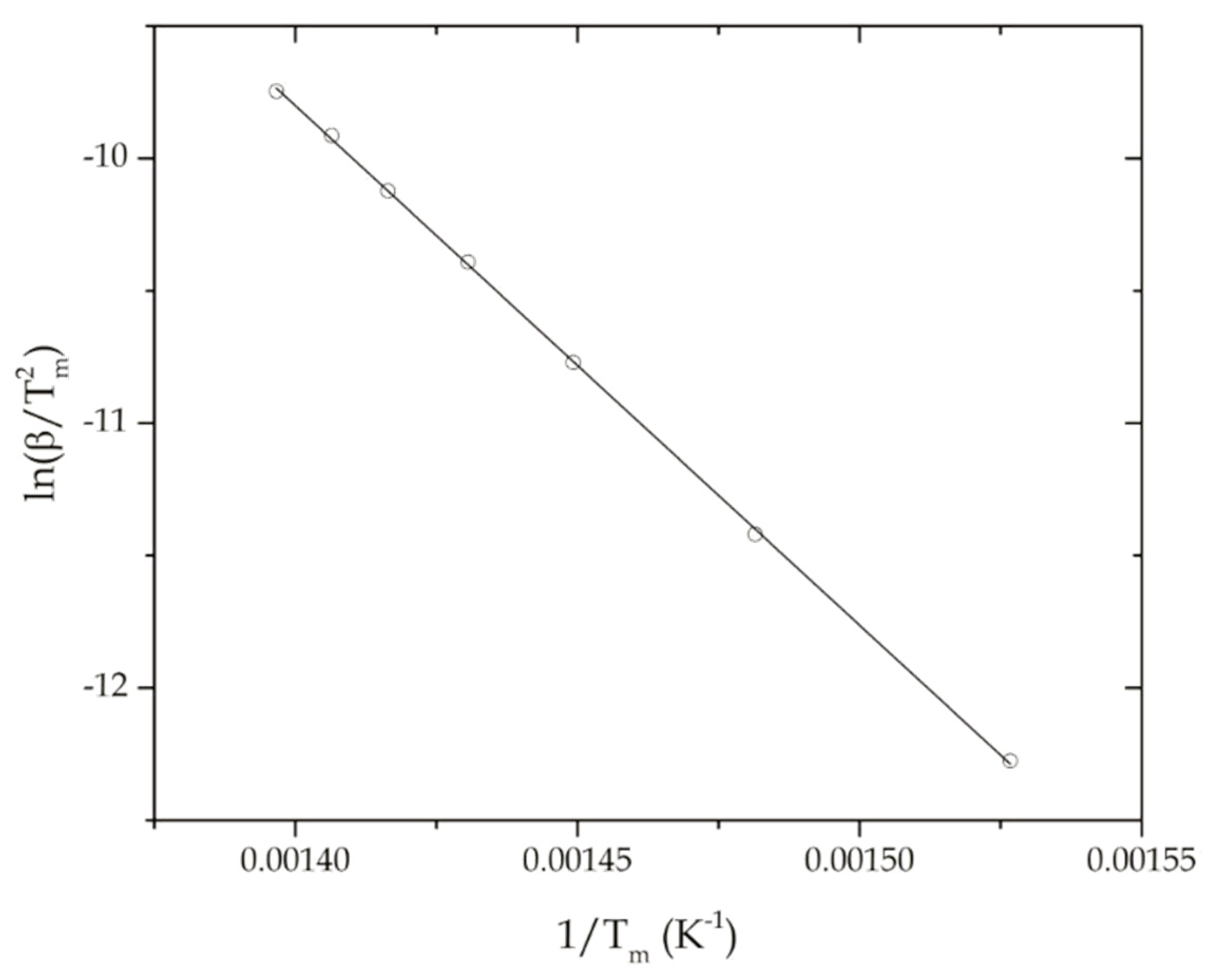
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Perez-Maqueda, L.A.; Criado, J.M.; Sanchez-Jimenez, P.E. Combined kinetic analysis of solid-state reactions: A powerful tool for the simultaneous determination of kinetic parameters and the kinetic model without previous assumptions on the reaction mechanism. J. Phys. Chem. A 2006, 110, 12456–12462. [Google Scholar] [CrossRef] [PubMed]
- Sestak, J.; Berggren, G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim. Acta 1971, 3, 1–12. [Google Scholar] [CrossRef]
- Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A.; Perejon, A.; Criado, J.M. Combined kinetic analysis of thermal degradation of polymeric materials under any thermal pathway. Polym. Degrad. Stab. 2009, 94, 2079–2085. [Google Scholar] [CrossRef]
- Senum, G.I.; Yang, R.T. Rational approximations of integral of arrhenius function. J. Therm. Anal. 1977, 11, 445–449. [Google Scholar] [CrossRef]
- Perez-Maqueda, L.A.; Criado, J.M. The accuracy of senum and yang’s approximations to the arrhenius integral. J. Therm. Anal. Calorim. 2000, 60, 909–915. [Google Scholar] [CrossRef]
- Flynn, J.H. The ‘temperature integral’ - its use and abuse. Thermochim. Acta 1997, 300, 83–92. [Google Scholar] [CrossRef]
- Mianowski, A. The kissinger law and isokinetic effect - part i. Most common solutions of thermokinetic equations. J. Therm. Anal. Calorim. 2003, 74, 953–973. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Perez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. Ictac kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Criado, J.M.; Ortega, A. Nonisothermal crystallization kinetics of metal glasses—Simultaneous determination of both the activation-energy and the exponent-n of the jma kinetic law. Acta Metall. 1987, 35, 1715–1721. [Google Scholar] [CrossRef]
- Criado, J.M.; Ortega, A. Nonisothermal transformation kinetics—Remarks on the kissinger method. J. Non-Cryst. Solids 1986, 87, 302–311. [Google Scholar] [CrossRef]
- Farjas, J.; Butchosa, N.; Roura, P. A simple kinetic method for the determination of the reaction model from non-isothermal experiments. J. Therm. Anal. Calorim. 2010, 102, 615–625. [Google Scholar] [CrossRef]
- Mamleev, V.; Bourbigot, S.; Le Bras, M.; Lefebvre, J. Three model-free methods for calculation of activation energy in tg. J. Therm. Anal. Calorim. 2004, 78, 1009–1027. [Google Scholar] [CrossRef]
- Nikolaidis, A.K.; Achilias, D.S. Thermal degradation kinetics and viscoelastic behavior of poly(methyl methacrylate)/organomodified montmorillonite nanocomposites prepared via in situ bulk radical polymerization. Polymers 2018, 10. [Google Scholar] [CrossRef]
- Flynn, J.H. A general differential technique for the determination of parameters for d(alpha)/dt = f(alpha)a exp(-e/rt)—energy of activation, preexponential factor and order of reaction (when applicable). J. Therm. Anal. 1991, 37, 293–305. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N.; Vincent, L.; Mija, A.; Guigo, N. Integral, differential and advanced isoconversional methods complex mechanisms and isothermal predicted conversion-time curves. Chemom. Intell. Lab. Syst. 2009, 96, 219–226. [Google Scholar] [CrossRef]
- Flynn, J.H. The isoconversional method for determination of energy of activation at constant heating rates—Corrections for the doyle approximation. J. Therm. Anal. 1983, 27, 95–102. [Google Scholar] [CrossRef]
- Venkatesh, M.; Ravi, P.; Tewari, S.P. Isoconversional kinetic analysis of decomposition of nitroimidazoles: Friedman method vs. flynn-wall-ozawa method. J. Phys. Chem. A 2013, 117, 10162–10169. [Google Scholar] [CrossRef]
- Huang, F.-Y. Thermal properties and thermal degradation of cellulose tri-stearate (cts). Polymers 2012, 4, 1012–1024. [Google Scholar] [CrossRef]
- Perez-Maqueda, L.A.; Criado, J.M.; Gotor, F.J.; Malek, J. Advantages of combined kinetic analysis of experimental data obtained under any heating profile. J. Phys. Chem. A 2002, 106, 2862–2868. [Google Scholar] [CrossRef]
- Garcia-Garrido, C.; Perez-Maqueda, L.A.; Criado, J.M.; Sanchez-Jimenez, P.E. Combined kinetic analysis of multistep processes of thermal decomposition of polydimethylsiloxane silicone. Polymer 2018, 153, 558–564. [Google Scholar] [CrossRef]
- Yan, Q.-L.; Zeman, S.; Sanchez-Jimenez, P.E.; Zhao, F.-Q.; Perez-Maqueda, L.A.; Malek, J. The effect of polymer matrices on the thermal hazard properties of rdx-based pbxs by using model-free and combined kinetic analysis. J. Hazard. Mater. 2014, 271, 185–195. [Google Scholar] [CrossRef]
- Yahyaoui, R.; Sanchez Jimenez, P.E.; Perez Maqueda, L.A.; Nahdi, K.; Criado Luque, J.M. Synthesis, characterization and combined kinetic analysis of thermal decomposition of hydrotalcite (Mg6Al2(OH)(16)CO3 center dot 4H(2)O). Thermochim. Acta 2018, 667, 177–184. [Google Scholar] [CrossRef]
- Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A.; Perejon, A.; Criado, J.M. Constant rate thermal analysis for thermal stability studies of polymers. Polym. Degrad. Stab. 2011, 96, 974–981. [Google Scholar] [CrossRef]
- Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A.; Perejon, A.; Criado, J.M. Generalized kinetic master plots for the thermal degradation of polymers following a random scission mechanism. J. Phys. Chem. A 2010, 114, 7868–7876. [Google Scholar] [CrossRef] [PubMed]
- Malek, J.; Koga, N.; Perez-Maqueda, L.A.; Criado, J.M. The ozawa’s generalized time concept and yz-master plots as a convenient tool for kinetic analysis of complex processes. J. Therm. Anal. Calorim. 2013, 113, 1437–1446. [Google Scholar] [CrossRef]
- Perez-Maqueda, L.A.; Ortega, A.; Criado, J.M. The use of master plots for discriminating the kinetic model of solid state reactions from a single constant-rate thermal analysis (crta) experiment. Thermochim. Acta 1996, 277, 165–173. [Google Scholar] [CrossRef]
- Malek, J.; Mitsuhashi, T. Kinetic study of solid-state processes. JAERI Conf. 2003, 1, 149–155. [Google Scholar]



| Code | Reaction Model | f(α) | g(α) |
|---|---|---|---|
| P2 | Power law | 2α1/2 | α1/2 |
| P3 | Power law | 3α2/3 | α1/3 |
| P4 | Power law | 4α3/4 | α1/4 |
| A2 | Nucleation and growth (Avrami Equation 1) | 2(1−α)[−ln(1−α)]1/2 | [−ln(1−α)]1/2 |
| A3 | Nucleation and growth (Avrami Equation 2) | 3(1−α)[−ln(1−α)]2/3 | [−ln(1−α)]1/3 |
| A4 | Nucleation and growth (Avrami Equation 3) | 4(1−α)[−ln(1−α)]3/4 | [−ln(1−α)]1/4 |
| R1 | Controlled reaction on the surface (motion in one dimension) | 1 | |
| R2 | Controlled surface reaction (contracting cylinder) | 2(1−α)1/2 | [−ln(1−α)]1/2 |
| R3 | Controlled reaction on the surface (migration volume; contracting sphere) | 3(1−α)2/3 | [−ln(1−α)]1/3 |
| D1 | Diffusion in one dimension | (1/2)α −1 | 2 |
| D2 | Diffusion in two dimensions (Valensi Equation ) | − [ln(1−α)]−1 | (1−α) ln(1−α) + |
| D3 | Diffusion in three dimensions (Jander Equation ) | (3/2)[1−(1−α)1/3]−1(1−α)2/3 | [1 − (1−α)1/3]2 |
| F1 | Random nucleation with one nucleus of individual particle. Mample first order. | (1−α) | −ln(1−α) |
| F2 | Random nucleation with two nuclei of individual particle | (1−α)2 | (1−α)−1 |
| F3 | Random nucleation with three nuclei of individual particle | (1/2)(1−α)3 | (1−α)−2 |
| Fn | nth order | (1−α)n | [(1−α)1−n−1]/(n−1) |
| ePT Bna | Extended Prout-Tompkins | (1−)n m | No analytical solution |
| Degree | P(x) |
|---|---|
| 1 | |
| 2 | |
| 3 | |
| 4 |
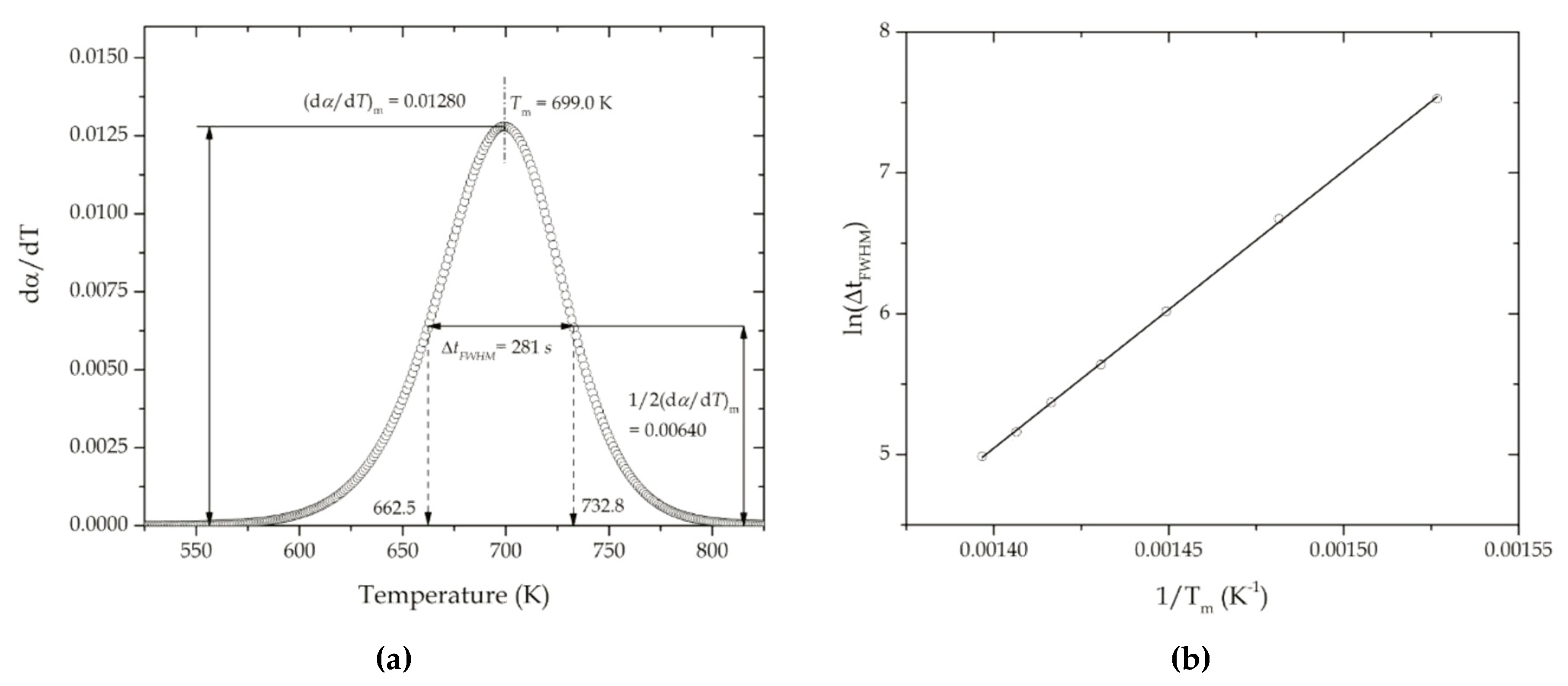
| Heating Rate (K min−1) | Tm (K) | m | |
|---|---|---|---|
| 2 | 655 | 0.526 | 0.01454 |
| 5 | 675 | 0.535 | 0.01373 |
| 10 | 690 | 0.529 | 0.01314 |
| 15 | 699 | 0.525 | 0.01280 |
| 20 | 706 | 0.528 | 0.01256 |
| 25 | 711 | 0.524 | 0.01238 |
| 30 | 716 | 0.531 | 0.01223 |
| Heating Rate (K min−1) | Ea (kJ mol−1) | ln A c | r | n | m |
|---|---|---|---|---|---|
| 2 | 163.9 | 27.6810 | −1.0000 | 1.4920 | 0.0024 |
| 5 | 163.9 | 27.6660 | −1.0000 | 1.4930 | 0.0024 |
| 10 | 163.8 | 27.6510 | −1.0000 | 1.4930 | 0.0024 |
| 15 | 163.5 | 27.5890 | −1.0000 | 1.4920 | 0.0044 |
| 20 | 163.7 | 27.6220 | −1.0000 | 1.4930 | 0.0032 |
| 25 | 163.6 | 27.6160 | −1.0000 | 1.4930 | 0.0032 |
| 30 | 163.4 | 27.5810 | −1.0000 | 1.4920 | 0.0044 |
| Heating Rate (K min−1) | ||
|---|---|---|
| 2 | 0.0005 | 0.5547 |
| 5 | 0.0006 | 0.5621 |
| 10 | 0.0006 | 0.5556 |
| 15 | 0.0005 | 0.5635 |
| 20 | 0.0005 | 0.5333 |
| 25 | 0.0005 | 0.5611 |
| 30 | 0.0005 | 0.5557 |
| Heating Rate (K min−1) | nth order: (1−α)n | autocatalytic: αm(1−α)n | |||||
|---|---|---|---|---|---|---|---|
| n | ln A | r | n | m | ln A | R2 | |
| 2 | 1.4958 | 27.5423 | 0.9999 | 1.4929 | 0.0050 | 27.5659 | 1.000 |
| 5 | 1.4957 | 27.5419 | 1.0000 | 1.4935 | 0.0048 | 27.5655 | 1.000 |
| 10 | 1.4951 | 27.5411 | 1.0000 | 1.4938 | 0.0046 | 27.5649 | 1.000 |
| 15 | 1.4949 | 27.5408 | 1.0000 | 1.4939 | 0.0045 | 27.5646 | 1.000 |
| 20 | 1.4947 | 27.5405 | 1.0000 | 1.4941 | 0.0044 | 27.5644 | 1.000 |
| 25 | 1.4945 | 27.5403 | 1.0000 | 1.4942 | 0.0044 | 27.5643 | 1.000 |
| 30 | 1.4943 | 27.5402 | 1.0000 | 1.4952 | 0.0069 | 27.5684 | 1.000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balart, R.; Garcia-Sanoguera, D.; Quiles-Carrillo, L.; Montanes, N.; Torres-Giner, S. Kinetic Analysis of the Thermal Degradation of Recycled Acrylonitrile-Butadiene-Styrene by non-Isothermal Thermogravimetry. Polymers 2019, 11, 281. https://doi.org/10.3390/polym11020281
Balart R, Garcia-Sanoguera D, Quiles-Carrillo L, Montanes N, Torres-Giner S. Kinetic Analysis of the Thermal Degradation of Recycled Acrylonitrile-Butadiene-Styrene by non-Isothermal Thermogravimetry. Polymers. 2019; 11(2):281. https://doi.org/10.3390/polym11020281
Chicago/Turabian StyleBalart, Rafael, David Garcia-Sanoguera, Luis Quiles-Carrillo, Nestor Montanes, and Sergio Torres-Giner. 2019. "Kinetic Analysis of the Thermal Degradation of Recycled Acrylonitrile-Butadiene-Styrene by non-Isothermal Thermogravimetry" Polymers 11, no. 2: 281. https://doi.org/10.3390/polym11020281








