Maleinized Linseed Oil as Epoxy Resin Hardener for Composites with High Bio Content Obtained from Linen Byproducts
Abstract
:1. Introduction
2. Materials and Methods
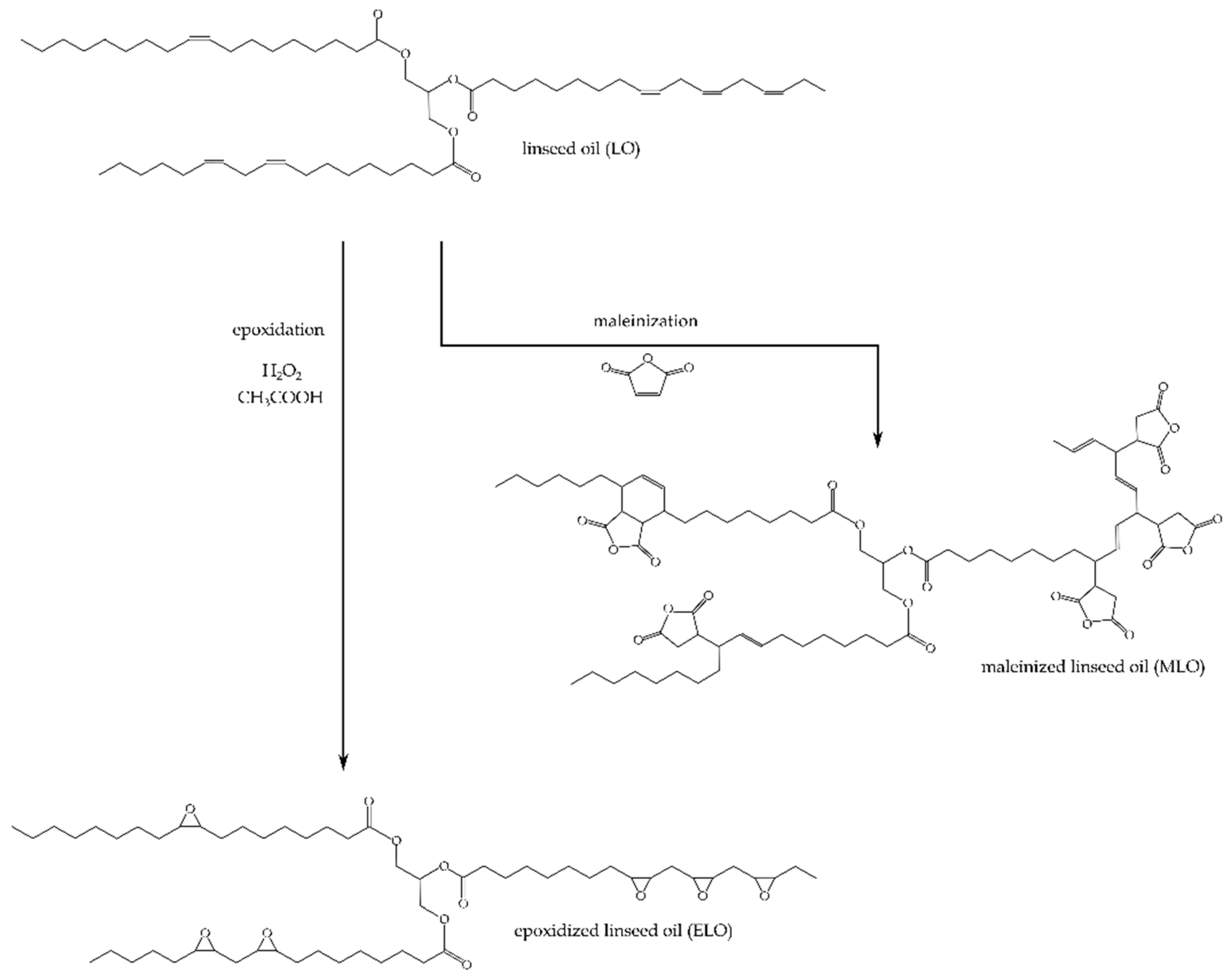
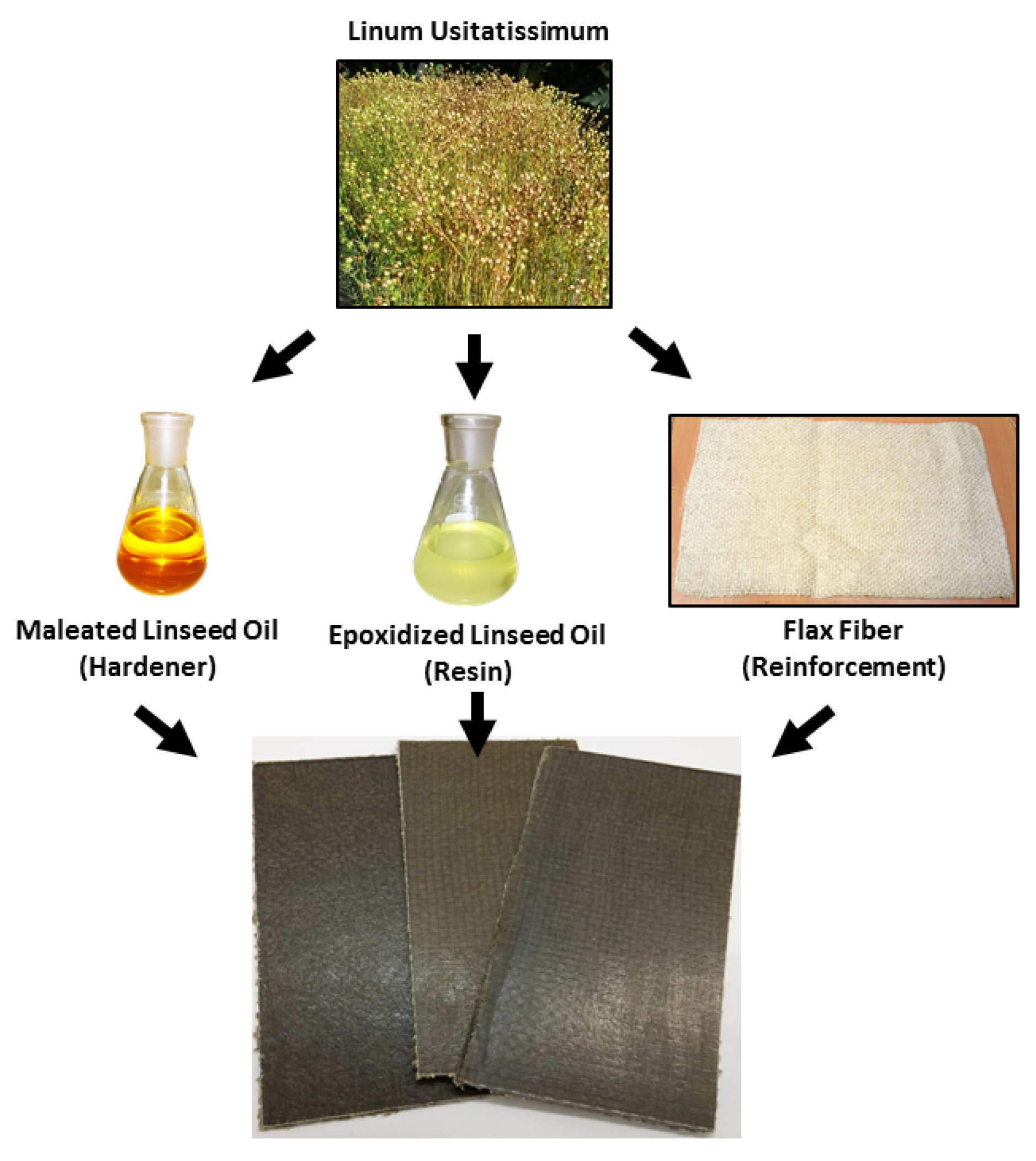
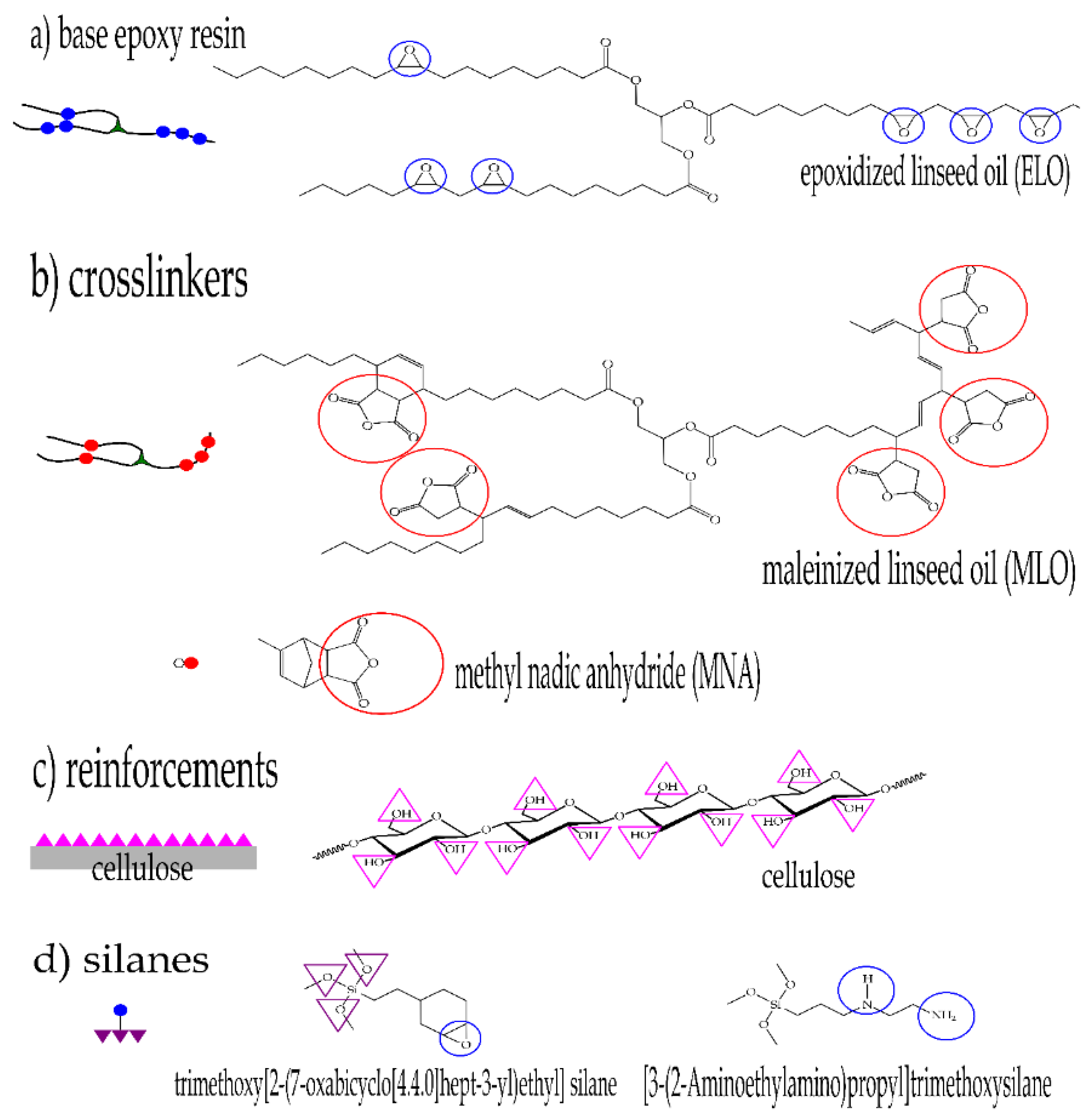
2.1. Materials
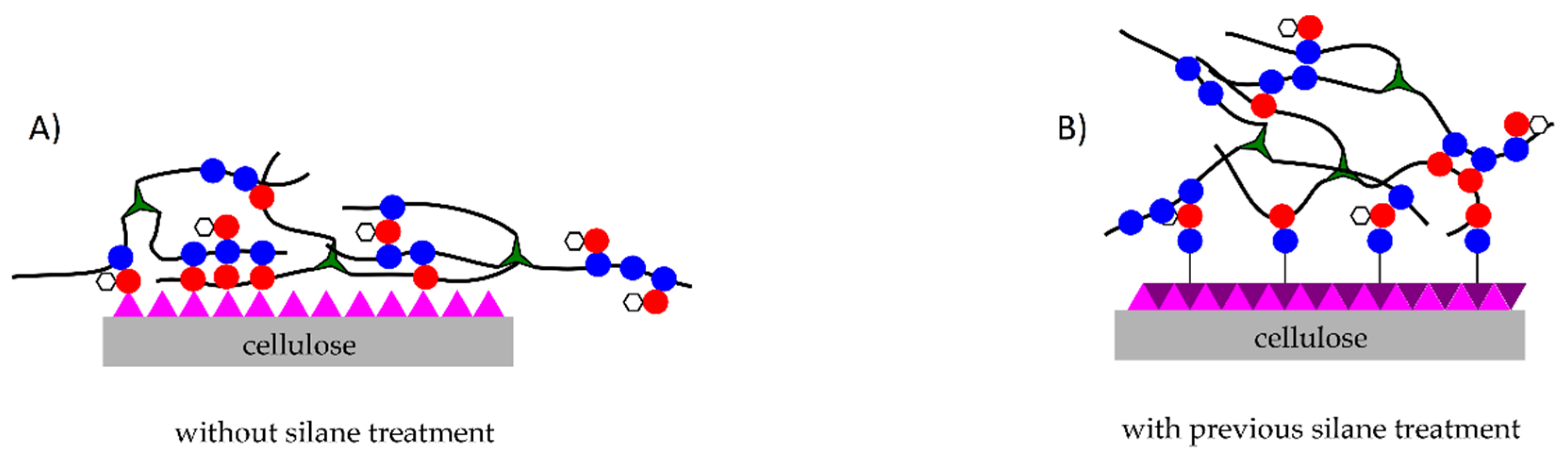
2.2. Surface Treatment
2.3. Composite Manufacturing
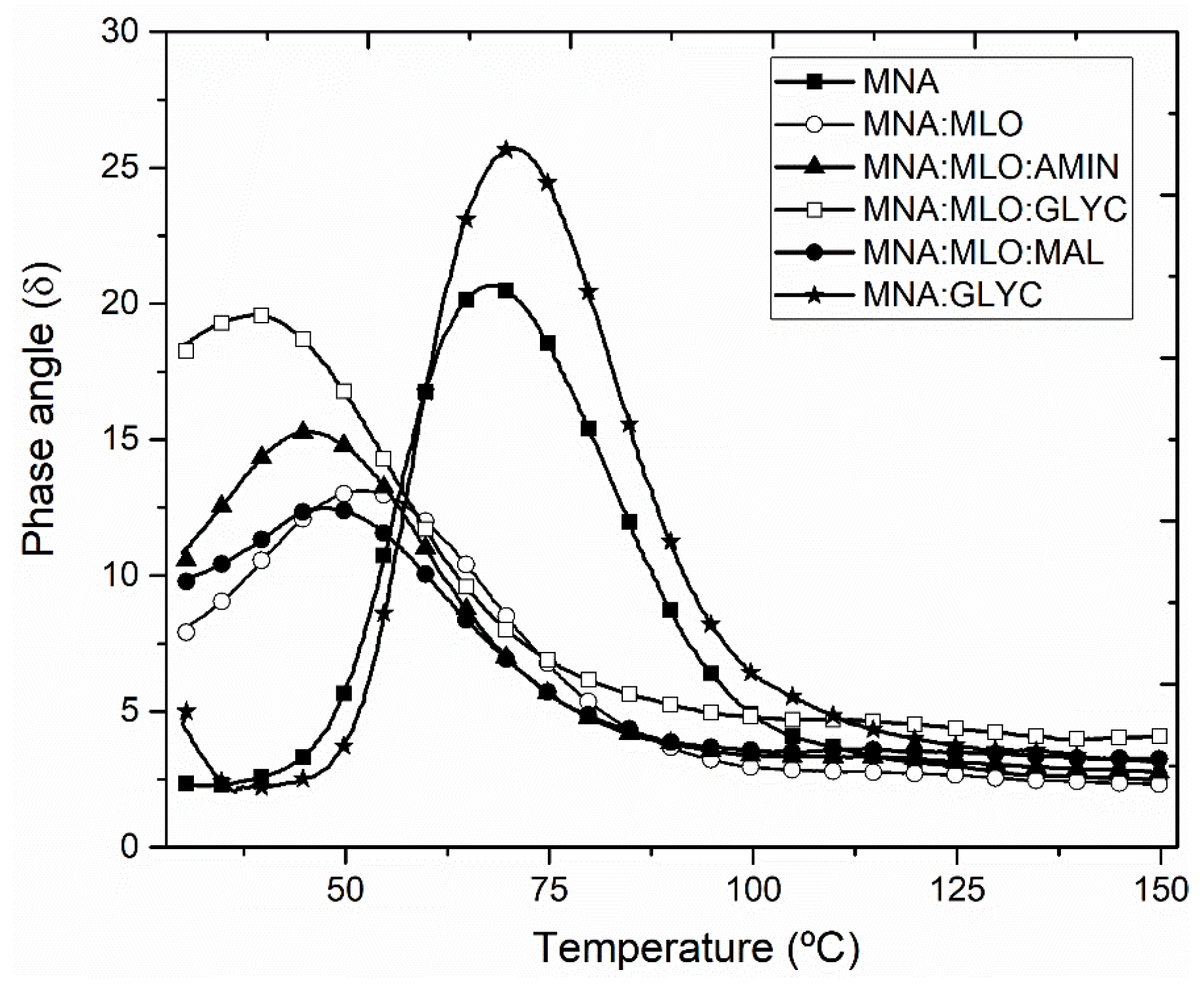
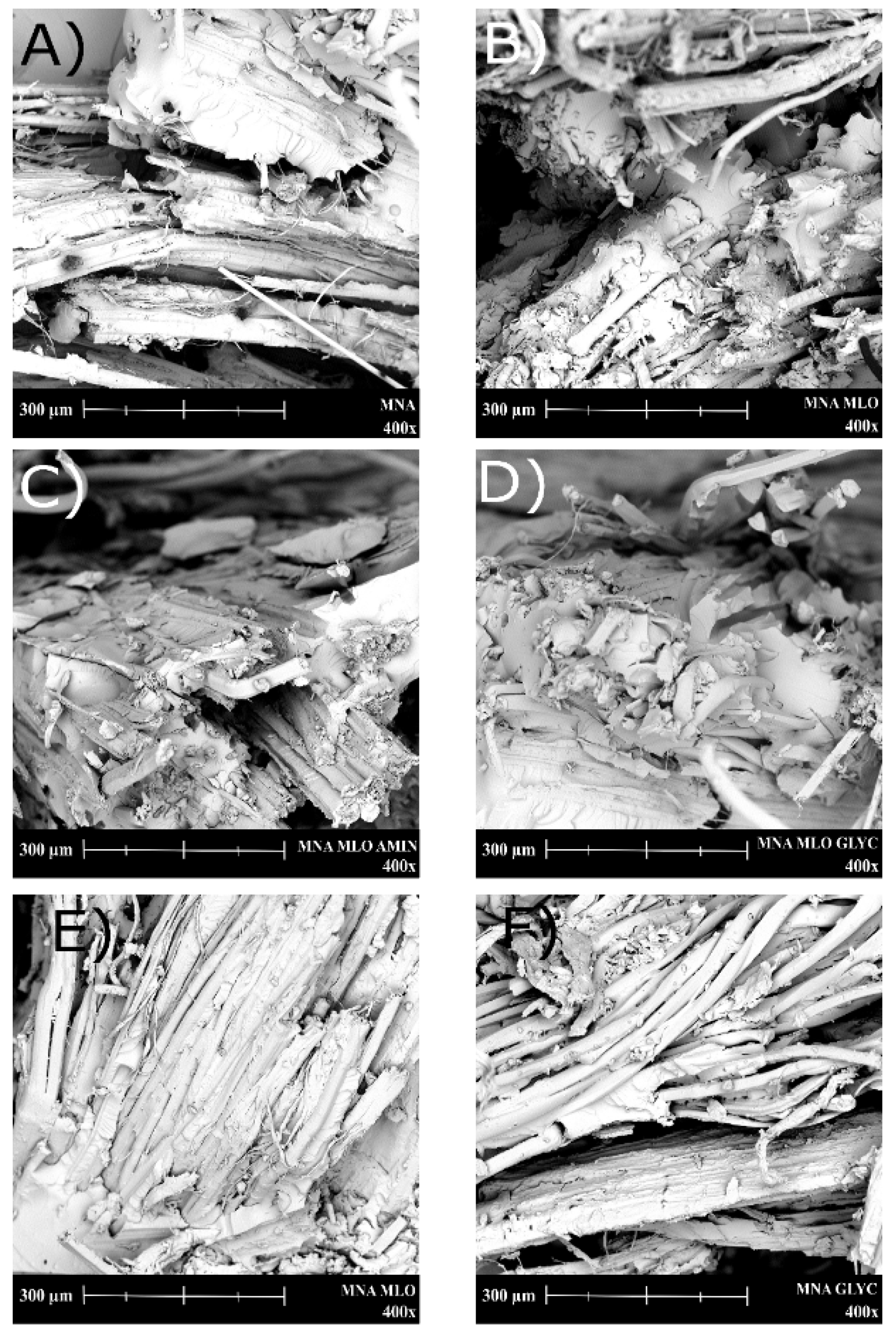
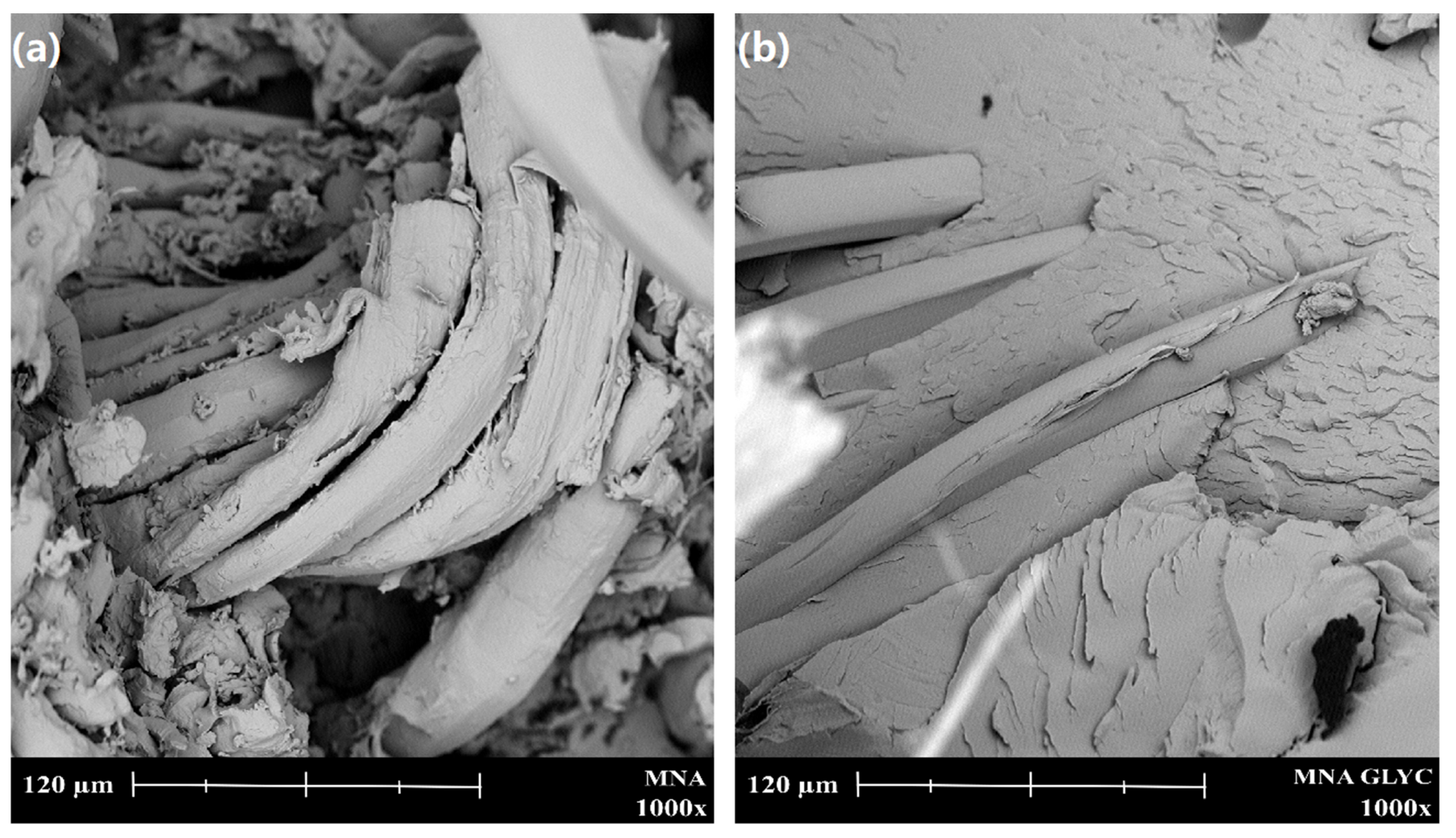
2.4. Characterization of Composites
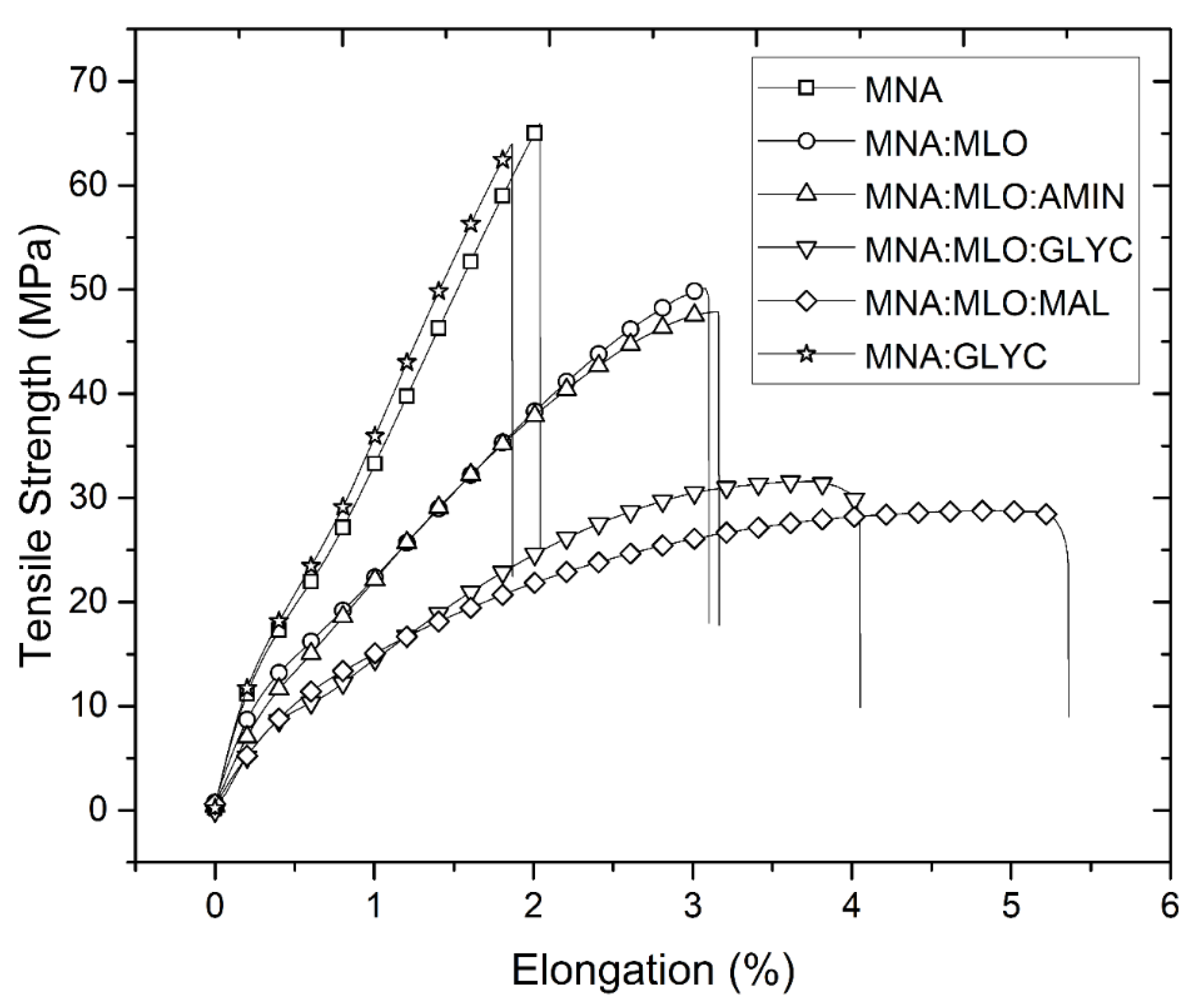
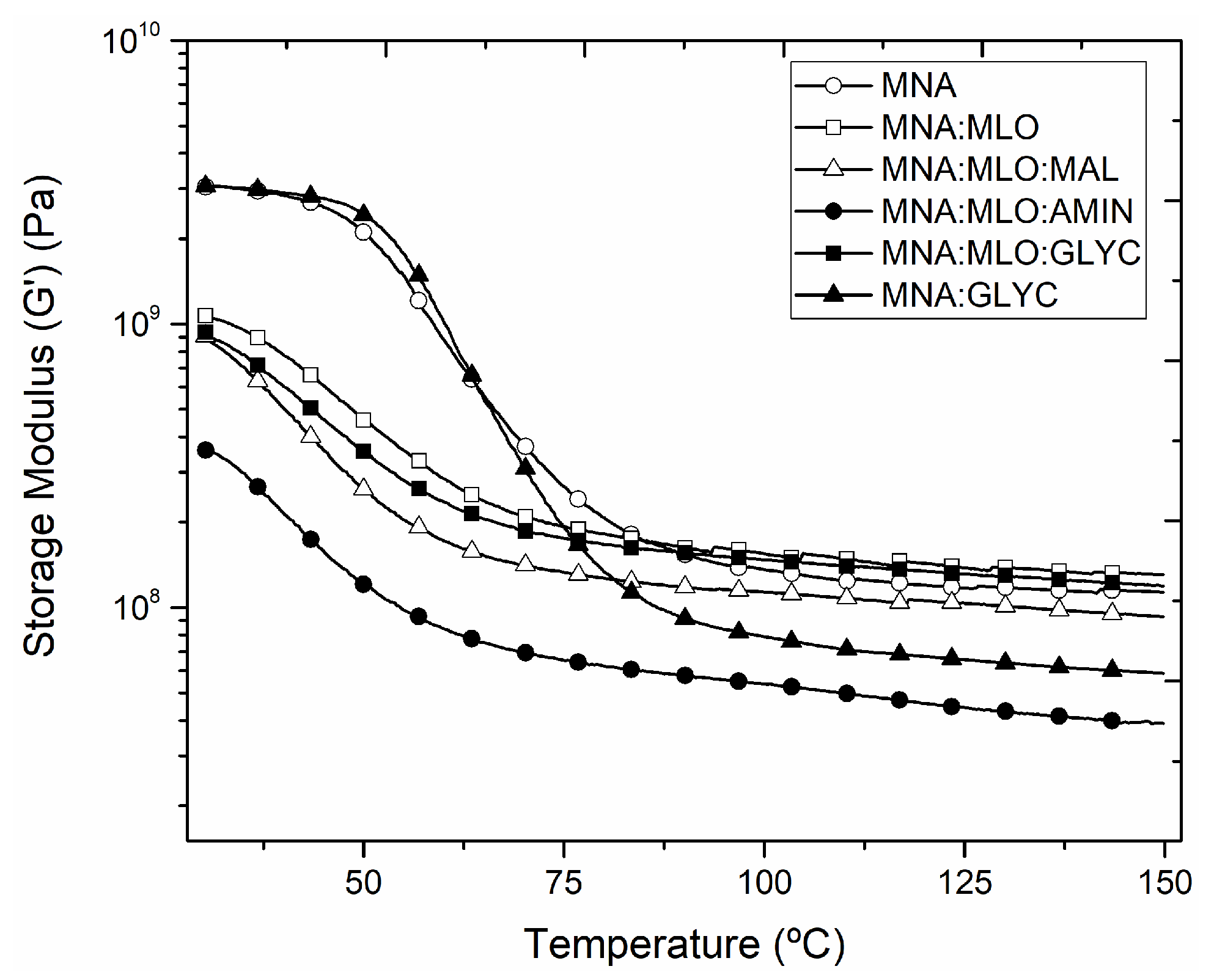
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Susheel, K.; Balbir, S.K.; Inderjeet, K.; Njuguna, J. Biofiber-Reinforced Thermoplastic Composites. Polym. Compos. 2013, 3, 46. [Google Scholar]
- Charlet, K.; Jernot, J.P.; Eve, S.; Gomina, M.; Breard, J. Multi-scale morphological characterisation of flax: From the stem to the fibrils. Carbohydr. Polym. 2010, 82, 54–61. [Google Scholar] [CrossRef]
- Sekhri, S. Textbook of Fabric Science: Fundamentals to Finishing; PHI Learning Pvt. Ltd.: New Delhi, India, 2011; p. 76. [Google Scholar]
- United States Departmente of Agriculture. Flax Profile; United States Departmente of Agriculture, Agricultural Marketing Resource: Washington, DC, USA, 2018.
- McHughen, A. Flax (Linum usitatissimum L.): In Vitro Studies. Biotechnol. Agric. For. 1990, 10, 12. [Google Scholar]
- Yan, L.B.; Chouw, N.; Yuan, X.W. Improving the mechanical properties of natural fibre fabric reinforced epoxy composites by alkali treatment. J. Reinf. Plast. Compos. 2012, 31, 425–437. [Google Scholar] [CrossRef]
- Charlet, K.; Eve, S.; Jernot, J.P.; Gomina, M.; Breard, J. Tensile deformation of a flax fiber. In Mesomechanics 2009; Korsunsky, A.M., Dini, D., Sih, G.C., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2009; Volume 1, pp. 233–236. [Google Scholar]
- Carus, M.; Scholz, L. Targets for bio-based composites and natural fibres. JEC Compos. 2011, 63, 31–32. [Google Scholar]
- Balsam, M.; Barghoorn, P.; Stebani, U. Trends in applied macromolecular chemistry. Angew. Makromol. Chem. 1999, 267, 1–9. [Google Scholar] [CrossRef]
- Khalil, H.; Rozman, H.D.; Ahmad, M.N.; Ismail, H. Acetylated plant-fiber-reinforced polyester composites: A study of mechanical, hygrothermal, and aging characteristics. Polym. Plast. Technol. Eng. 2000, 39, 757–781. [Google Scholar] [CrossRef]
- Lilholt, H.; Toftegaard, H.; Thomsen, A.B.; Schmidt, A.S. Natural composites based on cellulosic fibres and polypropylene matrix. In Proceedings of the ICCM-12, Paris, France, 5–9 July 1999. [Google Scholar]
- Baley, C. Analysis of the flax fibres tensile behaviour and analysis of the tensile stiffness increase. Compos. A Appl. Sci. Manuf. 2002, 33, 939–948. [Google Scholar] [CrossRef]
- Bos, H.L. The Potential of Flax Fiber as Reinforcement for Composite Materials; Eindhoven University: Eindhoven, The Netherlands, 2004. [Google Scholar]
- Sparnins, E. Mechanical Properties of Flax Fibers and Their Composites; Luleå University of Technology: Luleå, Sweden, 2009. [Google Scholar]
- Symington, M.C.; Banks, W.M.; West, O.D.; Pethrick, R.A. Tensile Testing of Cellulose Based Natural Fibers for Structural Composite Applications. J. Compos. Mater. 2009, 43, 1083–1108. [Google Scholar] [CrossRef]
- Baroncini, E.A.; Yadav, S.K.; Palmese, G.R.; Stanzione, J.F. Recent advances in bio-based epoxy resins and bio-based epoxy curing agents. J. Appl. Polym. Sci. 2016, 133, 19. [Google Scholar] [CrossRef]
- Karak, N. Vegetable Oil-Based Polymers: Properties, Processing and Applications; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Meier, M.A.; Metzger, J.O.; Schubert, U.S. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, A.R.; Wuzella, G.; Kandelbauer, A.; Aust, N. Thermal cure kinetics of epoxidized linseed oil with anhydride hardener. J. Therm. Anal. Calorim. 2012, 107, 989–998. [Google Scholar] [CrossRef]
- Ngo, T.T.; Lambert, C.A.; Kohl, J.G. Characterization of Compostability and Mechanical Properties for Linseed Oil Resin Composites Reinforced with Natural Fibers. Polym. Plast. Technol. Eng. 2014, 53, 1215–1222. [Google Scholar] [CrossRef]
- Samper, M.D.; Fombuena, V.; Boronat, T.; Garcia-Sanoguera, D.; Balart, R. Thermal and Mechanical Characterization of Epoxy Resins (ELO and ESO) Cured with Anhydrides. J. Am. Oil Chem. Soc. 2012, 89, 1521–1528. [Google Scholar] [CrossRef]
- Samper, M.D.; Petrucci, R.; Sanchez-Nacher, L.; Balart, R.; Kenny, J.M. Properties of composite laminates based on basalt fibers with epoxidized vegetable oils. Mater. Des. 2015, 72, 9–15. [Google Scholar] [CrossRef]
- Paul, A.; Joseph, K.; Thomas, S. Effect of surface treatments on the electrical properties of low-density polyethylene composites reinforced with short sisal fibers. Compos. Sci. Technol. 1997, 57, 67–79. [Google Scholar] [CrossRef]
- De Espinosa, L.M.; Ronda, J.C.; Galia, M.; Cadiz, V. A New Enone-Containing Triglyceride Derivative as Precursor of Thermosets from Renewable Resources. J. Polym. Sci. A Polym. Chem. 2008, 46, 6843–6850. [Google Scholar] [CrossRef]
- Gerbase, A.E.; Petzhold, C.L.; Costa, A.P.O. Dynamic mechanical and thermal behavior of epoxy resins based on soybean oil. J. Am. Oil Chem. Soc. 2002, 79, 797–802. [Google Scholar] [CrossRef]
- Brydson, J.A. Plastic Materials; Butterworth-Heinemann: Boston, MA, USA, 2000; p. 760. [Google Scholar]
- Fombuena, V.; Sanchez-Nacher, L.; Samper, M.D.; Juarez, D.; Balart, R. Study of the Properties of Thermoset Materials Derived from Epoxidized Soybean Oil and Protein Fillers. J. Am. Oil Chem. Soc. 2013, 90, 449–457. [Google Scholar] [CrossRef]
- Wang, H.J.; Rong, M.Z.; Zhang, M.Q.; Hu, J.; Chen, H.W.; Czigany, T. Biodegradable foam plastics based on castor oil. Biomacromolecules 2008, 9, 615–623. [Google Scholar] [CrossRef]
- Mistri, E.; Routh, S.; Ray, D.; Sahoo, S.; Misra, M. Green composites from maleated castor oil and jute fibres. Indust. Crops Prod. 2011, 34, 900–906. [Google Scholar] [CrossRef]
- Campanella, A.; La Scala, J.J.; Wool, R.P. Fatty Acid-Based Comonomers as Styrene Replacements in Soybean and Castor Oil-Based Thermosetting Polymers. J. Appl. Polym. Sci. 2011, 119, 1000–1010. [Google Scholar] [CrossRef]
- Sawpan, M.A.; Pickering, K.L.; Fernyhough, A. Effect of various chemical treatments on the fibre structure and tensile properties of industrial hemp fibres. Compos. A Appl. Sci. Manuf. 2011, 42, 888–895. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Mamun, A.A.; Lucka-Gabor, M.; Gutowski, V.S. The effects of acetylation on properties of flax fibre and its polypropylene composites. Express Polym. Lett. 2008, 2, 413–422. [Google Scholar] [CrossRef]
- Cruz, J.; Fangueiro, R. Surface modification of natural fibers: A review. In Tensinet—Cost Tu1303 International Symposium 2016—Novel Structural Skins—Improving Sustainability and Efficiency through New Structural Textile Materials and Designs; Chilton, J., Gosling, P., Mollaert, M., Stimpfle, B., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2016; Volume 155, pp. 285–288. [Google Scholar]
- Kalia, S.; Kaith, B.S.; Kaur, I. Pretreatments of Natural Fibers and their Application as Reinforcing Material in Polymer Composites—A Review. Polym. Eng. Sci. 2009, 49, 1253–1272. [Google Scholar] [CrossRef]
- Li, X.; Tabil, L.G.; Panigrahi, S. Chemical treatments of natural fiber for use in natural fiber-reinforced composites: A review. J. Polym. Environ. 2007, 15, 25–33. [Google Scholar] [CrossRef]
- Seki, Y. Innovative multifunctional siloxane treatment of jute fiber surface and its effect on the mechanical properties of jute/thermoset composites. Mater. Sci. Eng. A Struct. Mater. Prop. Microstructur. Process. 2009, 508, 247–252. [Google Scholar] [CrossRef]
- Valadez-Gonzalez, A.; Cervantes-Uc, J.M.; Olayo, R.; Herrera-Franco, P.J. Chemical modification of henequen fibers with an organosilane coupling agent. Compos. Part B Eng. 1999, 30, 321–331. [Google Scholar] [CrossRef]
- Luo, H.L.; Zhang, C.Y.; Xiong, G.Y.; Wan, Y.Z. Effects of alkali and alkali/silane treatments of corn fibers on mechanical and thermal properties of its composites with polylactic acid. Polym. Compos. 2016, 37, 3499–3507. [Google Scholar] [CrossRef]
- Zhu, J.C.; Zhu, H.J.; Njuguna, J.; Abhyankar, H. Recent Development of Flax Fibres and Their Reinforced Composites Based on Different Polymeric Matrices. Materials 2013, 6, 5171–5198. [Google Scholar] [CrossRef]
- Apolinario, G.; Ienny, P.; Corn, S.; Leger, R.; Bergeret, A.; Haudin, J.M. Effects of Water Ageing on the Mechanical Properties of Flax and Glass Fibre Composites: Degradation and Reversibility. In Natural Fibres: Advances in Science and Technology Towards Industrial Applications: From Science to Market; Fangueiro, R., Rana, S., Eds.; Springer: Dordrecht, The Netherlands, 2016; Volume 12, pp. 183–196. [Google Scholar]
- Kodal, M.; Topuk, Z.D.; Ozkoc, G. Dual effect of chemical modification and polymer precoating of flax fibers on the properties of short flax fiber/poly(lactic acid) composites. J. Appl. Polym. Sci. 2015, 132, 13. [Google Scholar] [CrossRef]
- Phillips, S.; Kuo, P.Y.; Demaria, C.; Lessard, L.; Sain, M.; Hubert, P. Effect of Common Chemical Treatments on the Process Kinetics and Mechanical Properties of Flax/Epoxy Composites Manufactured by Resin Infusion. J. Polym. Environ. 2015, 23, 143–155. [Google Scholar] [CrossRef]
- Pickering, K.L.; Efendy, M.G.A.; Le, T.M. A review of recent developments in natural fibre composites and their mechanical performance. Compos. A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef]
- Tayfun, U.; Dogan, M.; Bayramli, E. Influence of Surface Modifications of Flax Fiber on Mechanical and Flow Properties of Thermoplastic Polyurethane Based Eco-Composites. J. Nat. Fibers 2016, 13, 309–320. [Google Scholar] [CrossRef]
- Wu, C.M.; Lai, W.Y.; Wang, C.Y. Effects of Surface Modification on the Mechanical Properties of Flax/beta-Polypropylene Composites. Materials 2016, 9, 314. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.L.; Liu, W.T.; Zhou, L.Y.; Hua, Z.X.; Liu, H.; He, S.Q. Modification of flax fiber surface and its compatibilization in polylactic acid/flax composites. Iranian Polym. J. 2016, 25, 25–35. [Google Scholar] [CrossRef]
- Zhu, J.C.; Immonen, K.; Avril, C.; Brighton, J.; Zhu, H.J.; Abhyankar, H. Novel Hybrid Flax Reinforced Supersap Composites in Automotive Applications. Fibers 2015, 3, 76–89. [Google Scholar] [CrossRef]
- Pickering, K.L.; Abdalla, A.; Ji, C.; McDonald, A.G.; Franich, R.A. The effect of silane coupling agents on radiata pine fibre for use in thermoplastic matrix composites. Compos. A Appl. Sci. Manuf. 2003, 34, 915–926. [Google Scholar] [CrossRef]
- Cantero, G.; Arbelaiz, A.; Llano-Ponte, R.; Mondragon, I. Effects of fibre treatment on wettability and mechanical behaviour of flax/polypropylene composites. Compos. Sci. Technol. 2003, 63, 1247–1254. [Google Scholar] [CrossRef]
- Gernaat, C.R. Correlation between Rhological and Mechanical Properties in a Low-Temperature Cure Prepreg Composite; Tenesee Technological University: Cookeville, TN, USA, 2002. [Google Scholar]
- Lackinger, E.; Sartori, J.; Potthast, A.; Rosenau, T. Novel Vegetable Oil Based Paper Sizing Agents as Substitutes for Crude Oil Based ASA. Lenzinger Berichte 2013, 91, 107–111. [Google Scholar]
- Ray, D.; Ghorui, S.; Bandyopadhyay, N.R.; Sengupta, S.; Kar, T. New Materials from Maleated Castor Oil/Epoxy Resin Blend Reinforced with Fly Ash. Ind. Eng. Chem. Res. 2012, 51, 2603–2608. [Google Scholar] [CrossRef]
- Baley, C.; Busnel, F.; Grohens, Y.; Sire, O. Influence of chemical treatments on surface properties and adhesion of flax fibre-polyester resin. Compos. A Appl. Sci. Manuf. 2006, 37, 1626–1637. [Google Scholar] [CrossRef]
- Karnani, R.; Krishnan, M.; Narayan, R. Biofiber-reinforced polypropylene composites. Polym. Eng. Sci. 1997, 37, 476–483. [Google Scholar] [CrossRef]
- Yan, L.; Chouw, N.; Jayaraman, K. Flax fibre and its composites—A review. Compos. B Eng. 2014, 56, 296–317. [Google Scholar] [CrossRef]
- Fombuena, V.; Bernardi, L.; Fenollar, O.; Boronat, T.; Balart, R. Characterization of green composites from biobased epoxy matrices and bio-fillers derived from seashell wastes. Mater. Des. 2014, 57, 168–174. [Google Scholar] [CrossRef]
- Samper, M.D.; Petrucci, R.; Sanchez-Nacher, L.; Balart, R.; Kenny, J.M. New environmentally friendly composite laminates with epoxidized linseed oil (ELO) and slate fiber fabrics. Compos. B Eng. 2015, 71, 203–209. [Google Scholar] [CrossRef]
- Al-Mulla, E.A.J.; Yunus, W.M.Z.W.; Ibrahim, N.A.B.; Ab Rahman, M.Z. Properties of epoxidized palm oil plasticized polytlactic acid. J. Mater. Sci. 2010, 45, 1942–1946. [Google Scholar] [CrossRef]
- Balart, J.F.; Montanes, N.; Fombuena, V.; Boronat, T.; Sanchez-Nacher, L. Disintegration in Compost Conditions and Water Uptake of Green Composites from Poly(Lactic Acid) and Hazelnut Shell Flour. J. Polym. Environ. 2018, 26, 701–715. [Google Scholar] [CrossRef]
- Chern, Y.C.; Tseng, S.M.; Hsieh, K.H. Damping properties of interpenetrating polymer networks of polyurethane-modified epoxy and polyurethanes. J. Appl. Polym. Sci. 1999, 74, 328–335. [Google Scholar] [CrossRef]
- Amiri, A. Silane coupling agents used for natural fiber/polymer composites: A review. In Proceedings of the 65th FLAX INSTITUTES of the United States, Fargo, ND, USA, 27–28 March 2014. [Google Scholar]
- Xie, Y.; Hill, C.A.S.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. A Appl. Sci. Manuf. 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Raza, M.A.; Ashraf, M.A.; Westwood, A.V.K.; Jamil, T.; Ahmad, R.; Inam, A.; Deen, K.M. Maleated High Oleic Sunflower Oil-Treated Cellulose Fiber-Based Styrene Butadiene Rubber Composites. Polym. Compos. 2016, 37, 1113–1121. [Google Scholar] [CrossRef]


| Parameter | E-Glass | Flax Fiber |
|---|---|---|
| Density (g cm−3) | 2.5 | 1.4 |
| E-modulus (GPa) | 76 | 50–70 |
| Specific E-modulus (GPa cm3 g−1) | 30 | 36–50 |
| Tensile strength (GPa) | 1.4–2.5 | 0.5–1.5 |
| Specific tensile strength (GPa cm3 g−1) | 0.5–1 | 0.4–1.1 |
| Renewability | No | Yes |
| Recyclability | No | Yes |
| Disposal | Biodegradable | Not Biodegradable |
| Health risk when inhaled | Yes | No |
| No. Sample | Epoxy Resin | Hardener | Surface Treatment | Flax Fabrics Layers | Code |
|---|---|---|---|---|---|
| 1 | ELO | MNA | - | 3 | MNA |
| 2 | ELO | MNA:MLO | - | 3 | MNA:MLO |
| 3 | ELO | MNA:MLO | Amine silane | 3 | MNA:MLO:AMIN |
| 4 | ELO | MNA:MLO | Glycidyl silane | 3 | MNA:MLO:GLYC |
| 5 | ELO | MNA:MLO | Maleic anydride | 3 | MNA:MLO:MAL |
| 6 | ELO | MNA | Glycidyl Silane | 3 | MNA:GLYC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fombuena, V.; Petrucci, R.; Dominici, F.; Jordá-Vilaplana, A.; Montanes, N.; Torre, L. Maleinized Linseed Oil as Epoxy Resin Hardener for Composites with High Bio Content Obtained from Linen Byproducts. Polymers 2019, 11, 301. https://doi.org/10.3390/polym11020301
Fombuena V, Petrucci R, Dominici F, Jordá-Vilaplana A, Montanes N, Torre L. Maleinized Linseed Oil as Epoxy Resin Hardener for Composites with High Bio Content Obtained from Linen Byproducts. Polymers. 2019; 11(2):301. https://doi.org/10.3390/polym11020301
Chicago/Turabian StyleFombuena, Vicent, Roberto Petrucci, Franco Dominici, Amparo Jordá-Vilaplana, Néstor Montanes, and Luigi Torre. 2019. "Maleinized Linseed Oil as Epoxy Resin Hardener for Composites with High Bio Content Obtained from Linen Byproducts" Polymers 11, no. 2: 301. https://doi.org/10.3390/polym11020301
APA StyleFombuena, V., Petrucci, R., Dominici, F., Jordá-Vilaplana, A., Montanes, N., & Torre, L. (2019). Maleinized Linseed Oil as Epoxy Resin Hardener for Composites with High Bio Content Obtained from Linen Byproducts. Polymers, 11(2), 301. https://doi.org/10.3390/polym11020301








