Characterization of Polyurethane Foam Waste for Reuse in Eco-Efficient Building Materials
Abstract
:1. Introduction
2. Materials and Methods
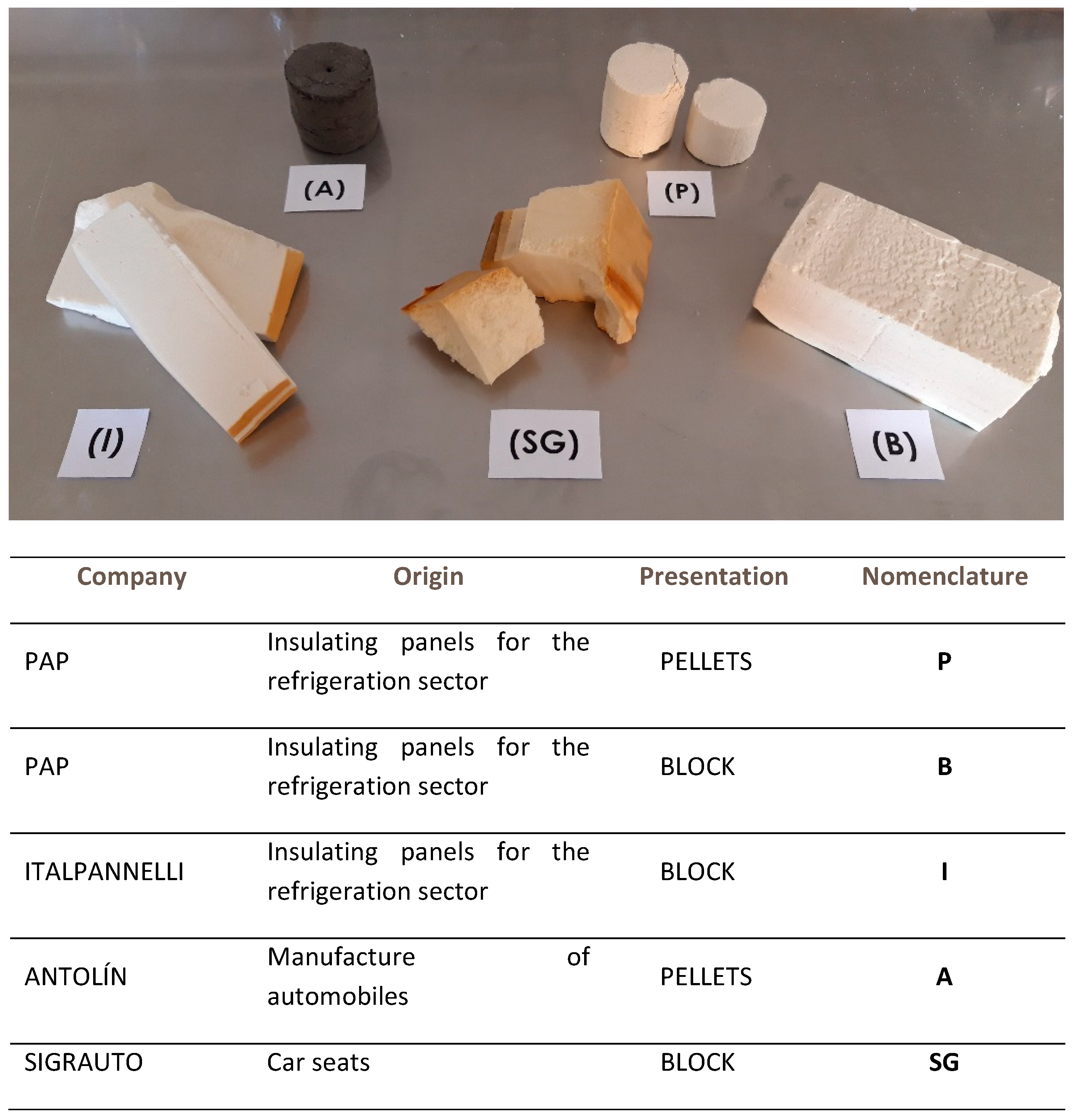
2.1. Materials
- (P): Rigid yellow polyurethane foam waste, in powder, compressed into pellet form (pellets). The waste is generated in the manufacture of insulation panels for the refrigeration sector, at Paneles Aislantes Peninsulares (PAP) factory in Cuenca, Spain. The waste is produced by trimming edges during the production stage.
- (B): Rigid yellow polyurethane foam waste, in the form of plates (Block). Waste generated in the manufacture of insulation panels for the refrigeration sector at Paneles Aislantes Peninsulares (PAP) factory in Cuenca, Spain. The waste comes from rejected panels and remnants of panels used in factory tests.
- (I): Rigid yellow polyurethane foam waste in the form of plates (block). The waste is generated in the manufacture of insulation panels for the refrigeration sector and comes from factory waste, from Italpannelli factory in Zaragoza, Spain.
- (A): Semi-rigid grey polyurethane foam waste, which comes in pieces and powder form; it is compressed into a pellet shape. The waste is generated in the manufacture of automobiles at Grupo Antolín IGA factory in Beaumont, France.
- (SG): Semi-rigid polyurethane foam waste; they are remains of car seats from scrapped cars obtained from the company Sigrauto in Madrid, Spain.
2.2. Methodology
2.2.1. Elemental Analysis (CHNS)
2.2.2. Waste Leaching Test
2.2.3. Thermogravimetric Analysis (TGA)
2.2.4. Density
2.2.5. Water Absorption Capacity
2.2.6. Laser Granulometry
2.2.7. Processing Parameters
2.2.8. Scanning Electron Microscopy (SEM)
2.2.9. Mechanical Properties of the Gypsum/PU Mixtures
2.2.10. Thermal Properties of the Gypsum-PU Mixtures
3. Results and Discussion
3.1. Elementary Analysis (CNHS)
3.2. Thermogravimetric Analysis (TGA)
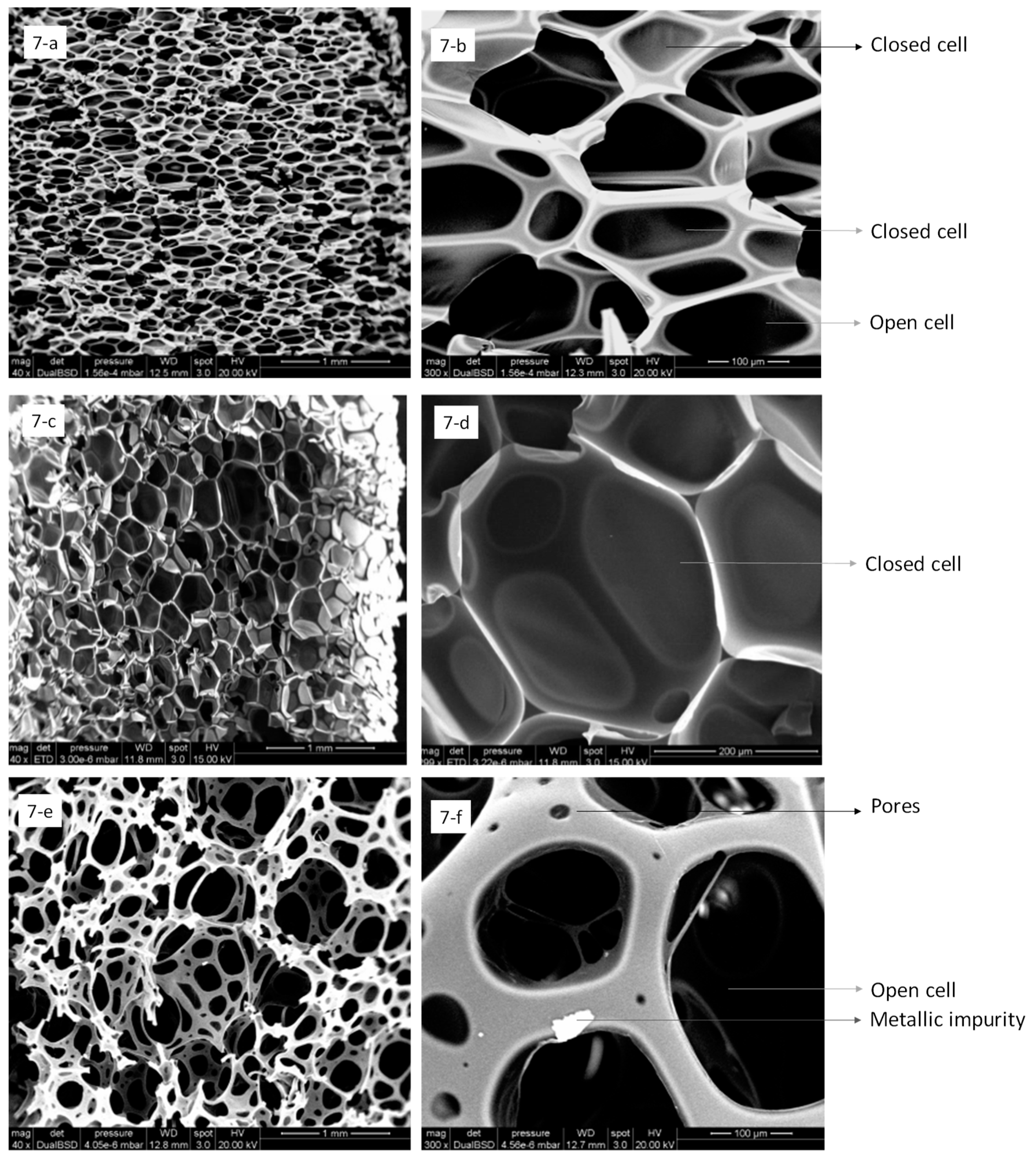
3.3. Scanning Electron Microscopy (SEM)
3.4. Waste Leaching Test
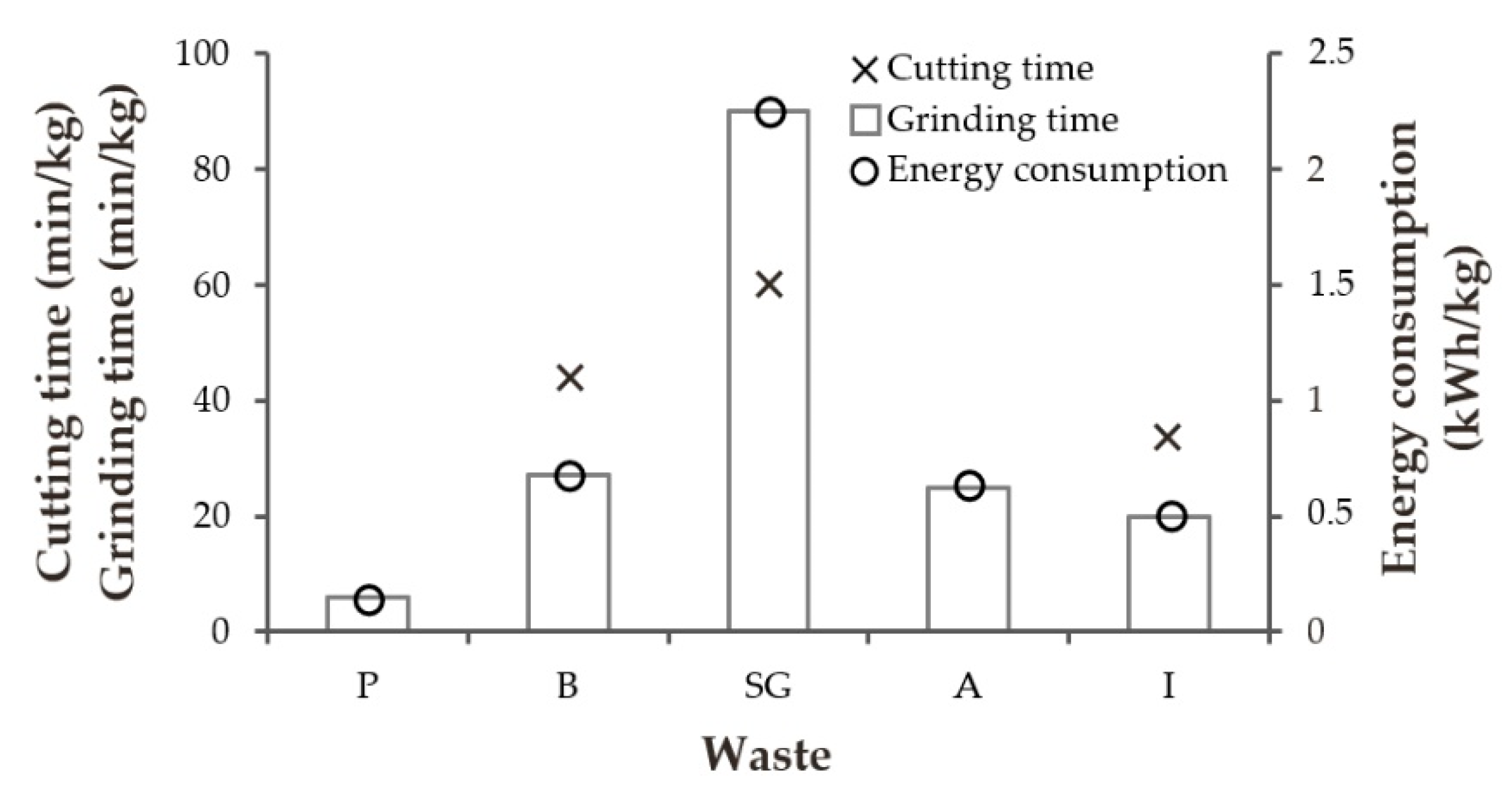
3.5. Processing Parameters
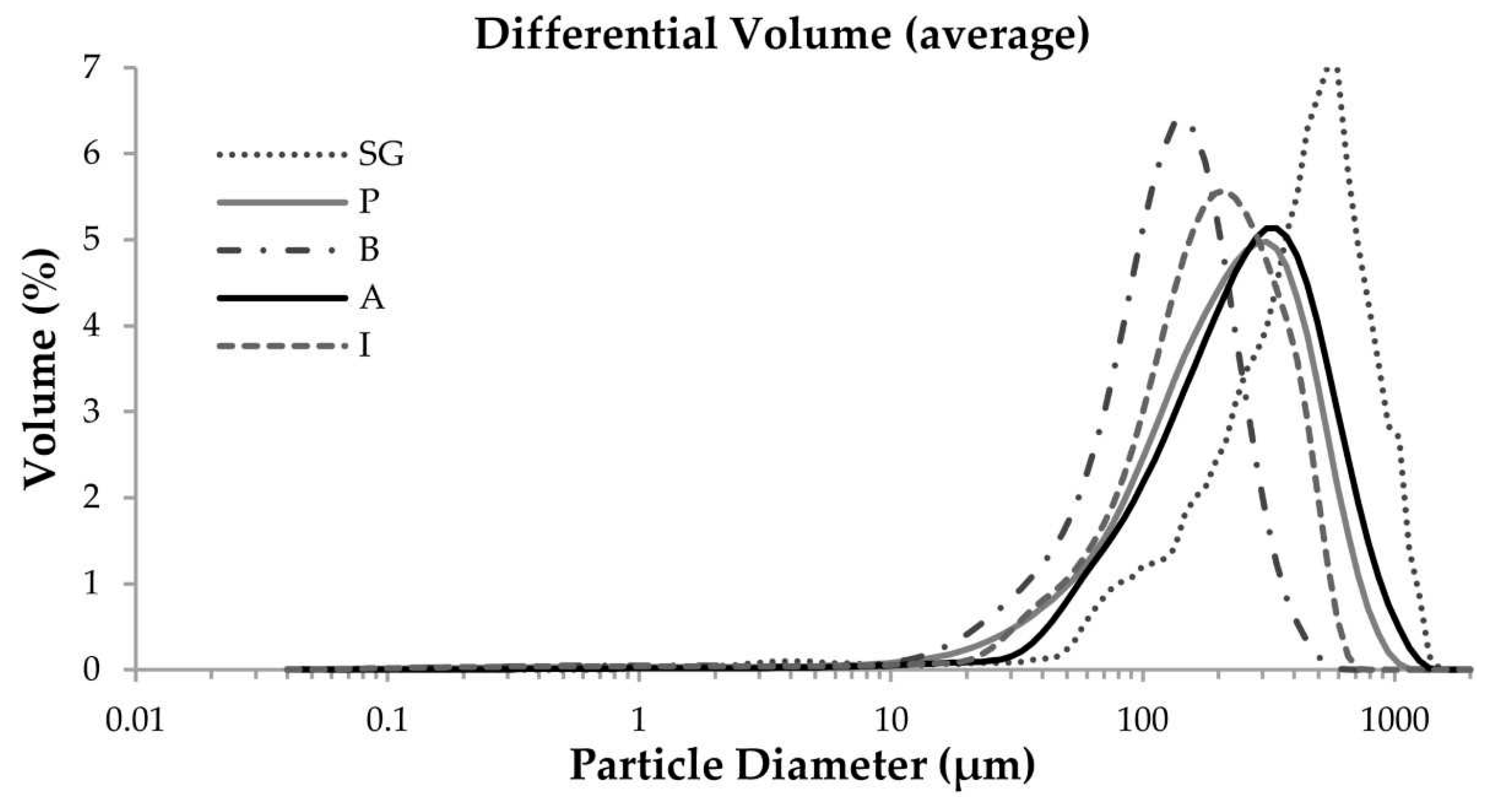
3.6. Laser Diffraction Granulometry
3.7. Determination of Apparent and Real Density, Water Absorption Capacity
3.8. The Possibilities of the Uses of the Wastes Studied
4. Conclusions
- All of the polymers degrade at above 200 °C. In the case of polyurethane SG, degradation occurs at a higher temperature (400 °C).
- None of the PU wastes have a leaching capacity and they are all considered to be suitable for use in new construction materials.
- The wastes that had been compacted had the best processing times, with the same prior cutting time and low energy use. This characteristic that these types of foams display, along with the fact that they have the greatest apparent density, create an advantage with respect to the other wastes with regard to PU being productively reused in building materials, both in terms of transportation (from the factory where the waste is generated) and in the collection of the waste and the rendering of the mixture.
- It was observed that the polyurethane that underwent a milling process had a high level of fineness with average particle sizes being around 250 µm and had greater levels of apparent density in respect of the rest of the wastes. The flexible foam had a larger average particle size of approximately 400 µm. In this case, the apparent density is lower compared to the rest of the foams.
- The microstructure of the polyurethanes is different depending on the industry from where they came. In the case of board and slab shaped wastes that come from the refrigeration industry, the structure is hexagonal semi-closed celled. Open and closed cells can be observed in the images from the SEM. The waste from the refrigeration industry is in slab form (I) and has a closed-cell structure. In both cases, adequate thermal behaviour was predicted, which could be used in improving thermal insulation when they are included in construction material.
- It is observed that SG waste has a structure suited to be used as a possible acoustic absorber, because of its open pore structure.
- The wastes that result from milling processes have a structure that is in the form of overlapping layers with no defined hexagonal structure. In this case, the wastes had some metal impurities in their structure associated with the actual milling process.
- As a final aim of this research, there is the possibility of including the wastes in plaster matrices in ratio with volume (1/1.5) thus obtaining adequate mechanical resistance to compression of over 2 MPa, a reduction in thermal conductivity by 33%, and a reduction in the weight of the material by 31%. In regards to the non-combustibility test and calorific value test, only the rigid PU foam wastes (B), and (I) met the standards to have an A1 classification, which is ideal for interior cladding materials for buildings. There was worse fire reaction behaviour in samples (P), (A) and (SG) due to the impurities that they contain. Nevertheless, it must be determined whether the classification in these two cases would be that of A2 or worse and alternative ways for the material to be applied in different areas of a building will need to be found.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plastics, The Facts 2017. An Analysis of European Plastics Production, Demand and Waste Data. Plastics Europe. Available online: https://www.plasticseurope.org/application/files/5715/1717/4180/Plastics_the_facts_2017_FINAL_for_website_one_page.pdf (accessed on 5 December 2018).
- Członka, S.; Bertino, M.F.; Strzelec, K.; Strąkowska, A.; Masłowski, M. Rigid polyurethane foams reinforced with solid waste generated in leather industry. Polym. Test. 2018, 69, 225–237. [Google Scholar] [CrossRef]
- Magnin, A.; Pollet, E.; Perrin, R.; Ullmann, C.; Persillon, C.; Phalip, V.; Avérous, L. Enzymatic recycling of thermoplastic polyurethanes: Synergistic effect of an esterase and an amidase and recovery of building blocks. Waste Manag. 2019, 85, 141–150. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, N.; Zuo, J.; Chen, G.; Du, H. Characteristics and trends of research on waste-to-energy incineration: A bibliometric analysis, 1999–2015. Renew. Sustain. Energy Rev. 2016, 66, 95–104. [Google Scholar] [CrossRef]
- Directive 2000/76/EC of the European Parliament and of the Council of 4 December 2000 on the Incineration of Waste. Available online: https://eur-lex.europa.eu/eli/dir/2000/76/oj (accessed on 10 February 2019).
- Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32008L0098 (accessed on 10 February 2019).
- Junco, C.; Rodríguez, A.; Calderón, V.; Muñoz-Rupérez, C.; Gutiérrez-González, S. Fatigue durability test of mortars incorporating polyurethane foam wastes. Constr. Build. Mater. 2018, 190, 373–381. [Google Scholar] [CrossRef]
- Mounanga, P.; Gbongbon, W.; Poullain, P.; Turcry, P. Proportioning and characterization of lightweight concrete mixtures made with rigid polyurethane foam wastes. Cem. Concr. Compos. 2008, 30, 806–814. [Google Scholar] [CrossRef]
- Kismi, M.; Mounanga, P. Comparison of short and long-term performances of lightweight aggregate mortars made with polyurethane foam waste and expanded polystyrene beads. In Proceedings on 2nd International Seminar on Innovation and Valorization in Civil Engineering and Construction Materials; MATEC Web of Conferences: Paris, France, 2012; Volume 2, p. 02019. [Google Scholar]
- Gadea, J.; Rodríguez, A.; Campos, P.L.; Garabito, J.; Calderón, V. Lightweight mortar made with recycled polyurethane foam. Cem. Concr. Compos. 2010, 32, 672–677. [Google Scholar] [CrossRef]
- Arroyo, R.; Horgnies, M.; Junco, C.; Rodríguez, A.; Calderón, V. Lightweight structural eco-mortars made with polyurethane wastes and non-Ionic surfactants. Constr. Build. Mater. 2019, 197, 157–163. [Google Scholar] [CrossRef]
- Gutiérrez-González, S.; Gadea, J.; Rodríguez, A.; Junco, C.; Calderón, V. Lightweight plaster materials with enhanced thermal properties made with polyurethane foam wastes. Constr. Build. Mater. 2012, 28, 653–658. [Google Scholar] [CrossRef]
- Garabito, J.; Alameda, L.; Gadea, J.; Gutiérrez-González, S. Influence of superplasticizers on the properties of lightweight mortar plaster made with recycled polymers. Adv. Mater. Res. 2015, 1129, 546–553. [Google Scholar] [CrossRef]
- 12457-2:2003. Characterisation of waste–Leaching–Compliance Test for Leaching of Granular Waste Materials and Sludges. Part 1: One Stage Batch Test at a Liquid to Solid Ration of 2 L/kg with Particle Size below 4 mm (without or with Size Reduction). CEN Comité Européen de Normalisation: European Committee for Standardisation. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0029596 (accessed on 10 February 2019).
- EN 1936:2007. Natural Stone Test Methods–Determination of Real Density and Apparent Density, and of Total and Open Porosity. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0038621 (accessed on 10 February 2019).
- EN 13755:2008. Natural Stone Test Methods. Determination of Water Absorption at Atmospheric Pressure. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0042047 (accessed on 10 February 2019).
- EN 13279-2. Gypsum Binders and Gypsum Plasters. Test Methods. 2014. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0052932 (accessed on 10 February 2019).
- EN 12667:2001. Thermal Performance of Building Materials and Products. Determination of Thermal Resistance by Means of Guarded Hot Plate and Heat Flow Meter Methods. High and Medium Thermal Resistance Products. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0027459 (accessed on 10 February 2019).
- EN ISO 1182:2011. Reaction to Fire Tests for Building Products–Non-combustibility Test. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0047993 (accessed on 10 February 2019).
- ISO 1716:2011. Reaction to Fire Tests for Products–Determination of the Gross Heat of Combustion (Calorific Value). Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0047995 (accessed on 10 February 2019).
- Calderón, V.; Gutiérrez-González, S.; Gadea, J.; Rodríguez, A. Construction Applications of Polyurethane Foam Wastes. In Recycling of Polyurethane Foams, 1st ed.; Sabu, T.A., Kanny, K.B., Thomas, M.G.C., Vasudeo Rani, A.D., Abitha, V.K.E., Eds.; Elsevier: Cambridge, UK, 2018; Volume 10, pp. 115–125. [Google Scholar]
- Jiao, L.; Xiao, H.; Wang, Q.; Sun, J. Thermal degradation characteristics of rigid polyurethane foam and the volatile products analysis with TG-FTIR-MS. Polym. Degrad. Stab. 2013, 98, 2687–2696. [Google Scholar] [CrossRef]
- Klempner, D.; Sendijaveri’c, V.; Aseeva, R.M. Handbook of Polymeric Foams and Foam Technology; Hanser Publishers: Munich, Germany, 2004. [Google Scholar]
- Abdollahi, S.; Khorasani, M.; Mohamad, G.M. Acoustic damping flexible polyurethane foams: Effect of isocyanate index and water content on the soundproofing. J. Appl. Polym. Sci. 2019, 136, 47363. [Google Scholar]
- Bandow, N.; Gartiser, S.; Ilvonen, O.; Schoknecht, U. Evaluation of the impact of construction products on the environment by leaching of possibly hazardous substances. Environ. Sci. Eur. 2018, 30, 14. [Google Scholar] [CrossRef] [PubMed]
- Lithner, D.; Damberg, J.; Dave, G.; Larsson, Å. Leachates from plastic consumer products – Screening for toxicity with Daphnia magna. Chemosphere 2009, 74, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Junco, C.; Gadea, J.; Rodríguez, A.; Gutiérrez-González, S.; Calderón, V. Durability of lightweight masonry mortars made with white recycled polyurethane foam. Cem. Concr. Compos. 2012, 34, 1174–1179. [Google Scholar] [CrossRef]
- EN ISO 2440. Flexible and Rigid Cellular Polymeric Materials. Accelerated Ageing Tests. 2014. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0054132 (accessed on 10 February 2019).
- Mostafa, H.E.; El-Dakhakhni, W.W.; Mekky, W.F. Use of reinforced rigid polyurethane foam for blast hazard mitigation. J. Reinf. Plastics Compos. 2010, 29, 3048–3057. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Rodríguez, J.F. Recycling of polyurethanes from laboratory to industry, a journey towards the sustainability. Waste Manag. 2018, 76, 147–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pan, Y.; Shen, C.; Liu, C.; Liu, X. Facile Thermally Impacted Water-Induced Phase Separation Approach for the Fabrication of Skin-Free Thermoplastic Polyurethane Foam and Its Recyclable Counterpart for Oil–Water Separation. Macromol. Rapid Commun. 2018, 39, 1800635. [Google Scholar] [CrossRef] [PubMed]
- Alameda, L.; Calderón, V.; Junco, C.; Rodríguez, A.; Gadea, J.; Gutiérrez-González, S. Characterization of gypsum plasterboard with polyurethane foam waste reinforced with polypropylene fibers. Mater. Constr. 2016, 66, e100. [Google Scholar] [CrossRef]
- Gutiérrez González, S.; Junco, C.; Calderon, V.; Rodríguez, A.; Gadea, J. Design and Manufacture of a Sustainable Lightweight Prefabricated Material Based on Gypsum Mortar with Semi-Rigid Polyurethane Foam Waste. In Proceedings of International Congress on Polymers in Concrete (ICPIC 2018); Taha, M., Ed.; Springer: Cham, Switzerland, 2018; pp. 449–455. [Google Scholar]
- Herrero, S.; Mayor, P.; Hernández-Olivares, F. Influence of proportion and particle size gradation of rubber from end-of-life tires on mechanical, thermal and acoustic properties of plaster–rubber mortars. Mater. Des. 2013, 47, 633–642. [Google Scholar] [CrossRef] [Green Version]
- Commission Decision of 8 February 2000 Implementing Council Directive 89/106/EEC as Regards the classification of the Reaction to Fire Performance of Construction Products. Official Journal of the European Communities No L. 50. 23.2.2000. Available online: http://data.europa.eu/eli/dec/2000/147/corrigendum/2001-03-24/oj (accessed on 10 February 2019).
- Gutiérrez-González, S.; Gadea, J.; Rodríguez, A.; Blanco-Varela, M.T.; Calderón, V. Compatibility between gypsum and polyamide powder waste to produce lightweight plaster with enhanced thermal properties. Constr. Build. Mater. 2012, 34, 179–185. [Google Scholar] [CrossRef] [Green Version]





| Waste | Chemical Element (%) | Others | |||
|---|---|---|---|---|---|
| C | H | N | S | ||
| P | 64.48 | 5.63 | 6.74 | 0.00 | 23.15 |
| B | 62.06 | 5.07 | 6.58 | 0.00 | 26.29 |
| SG | 64.67 | 7.75 | 4.80 | 0.00 | 22.78 |
| A | 63.74 | 6.15 | 6.04 | 0.00 | 24.07 |
| I | 63.34 | 5.58 | 7.28 | 0.00 | 23.80 |
| Waste | EC (µs/cm) | TDS (mg/L) | Salt (mg/L) | pH |
|---|---|---|---|---|
| Distilled water | 1.8 | 1.24 | Out of scale | 6.6 |
| P | 38.2 | 19.7 | 14.3 | 6.6 |
| B | 63.4 | 40.5 | 28.3 | 7.5 |
| SG | 149.2 | 95.4 | 69.0 | 7.9 |
| A | 27.9 | 21.5 | 15.6 | 7.7 |
| I | 32.8 | 20.7 | 15.3 | 6.8 |
| Waste | Apparent Density (Unprocessed) (kg/m3) | Apparent Density (Processed) (kg/m3) | Real Density (kg/m3) | Total Absorption (%) |
|---|---|---|---|---|
| P | 451.4 | 141.7 | 1052.7 | 2.0 |
| B | 37.6 | 45.5 | 1370.9 | 28.0 |
| SG | 33.1 | 39.8 | 1211.1 | 645.0 |
| A | 212.5 | 86.1 | 1378.6 | 333.5 |
| I | 33.8 | 56.0 | 1105.0 | 49.0 |
| Sample | 1/1.5 (P) | 1/1.5 (SG) | 1/1.5 (A) | 1/1.5 (B) | 1/1.5 (I) |
|---|---|---|---|---|---|
| Compression strength at 28 days | 2.00 | 3.71 | 3.70 | 3.95 | 4.33 |
| Flexion strength at 28 days | 1.15 | 1.71 | 1.97 | 2.23 | 2.20 |
| Sample | 1/1.5 (P) | 1/1.5 (SG) | 1/1.5 (A) | 1/1.5 (B) | 1/1.5 (I) |
|---|---|---|---|---|---|
| Temperature increase (°C) | 71.15 | * | * | 16.6 | 19.5 |
| Flaming time (s) | 339 | * | * | NONE | NONE |
| Loss of mass (%) | 37.89 | * | * | 26.63 | 27.72 |
| Superior Calorific Power (MJ/kg) | - | - | - | 1.048 | 1.596 |
| Parameter | Standard Plaster | Gypsum-PU 1/1.5 (B) | Gypsum-PU 1/1.5 (I) |
|---|---|---|---|
| Thermal conductivity (W/m × k) | 0.30 | 0.20 | 0.18 |
| Weight (kg/m2) | 8.33 | 5.88 | 5.60 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Rojo, R.; Alameda, L.; Rodríguez, Á.; Calderón, V.; Gutiérrez-González, S. Characterization of Polyurethane Foam Waste for Reuse in Eco-Efficient Building Materials. Polymers 2019, 11, 359. https://doi.org/10.3390/polym11020359
Gómez-Rojo R, Alameda L, Rodríguez Á, Calderón V, Gutiérrez-González S. Characterization of Polyurethane Foam Waste for Reuse in Eco-Efficient Building Materials. Polymers. 2019; 11(2):359. https://doi.org/10.3390/polym11020359
Chicago/Turabian StyleGómez-Rojo, Raúl, Lourdes Alameda, Ángel Rodríguez, Verónica Calderón, and Sara Gutiérrez-González. 2019. "Characterization of Polyurethane Foam Waste for Reuse in Eco-Efficient Building Materials" Polymers 11, no. 2: 359. https://doi.org/10.3390/polym11020359
APA StyleGómez-Rojo, R., Alameda, L., Rodríguez, Á., Calderón, V., & Gutiérrez-González, S. (2019). Characterization of Polyurethane Foam Waste for Reuse in Eco-Efficient Building Materials. Polymers, 11(2), 359. https://doi.org/10.3390/polym11020359






