Synthesis of Silver Nanoparticles Loaded onto Polymer-Inorganic Composite Materials and Their Regulated Catalytic Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Polymer-TiO2 Organic-Inorganic Composite Microgels
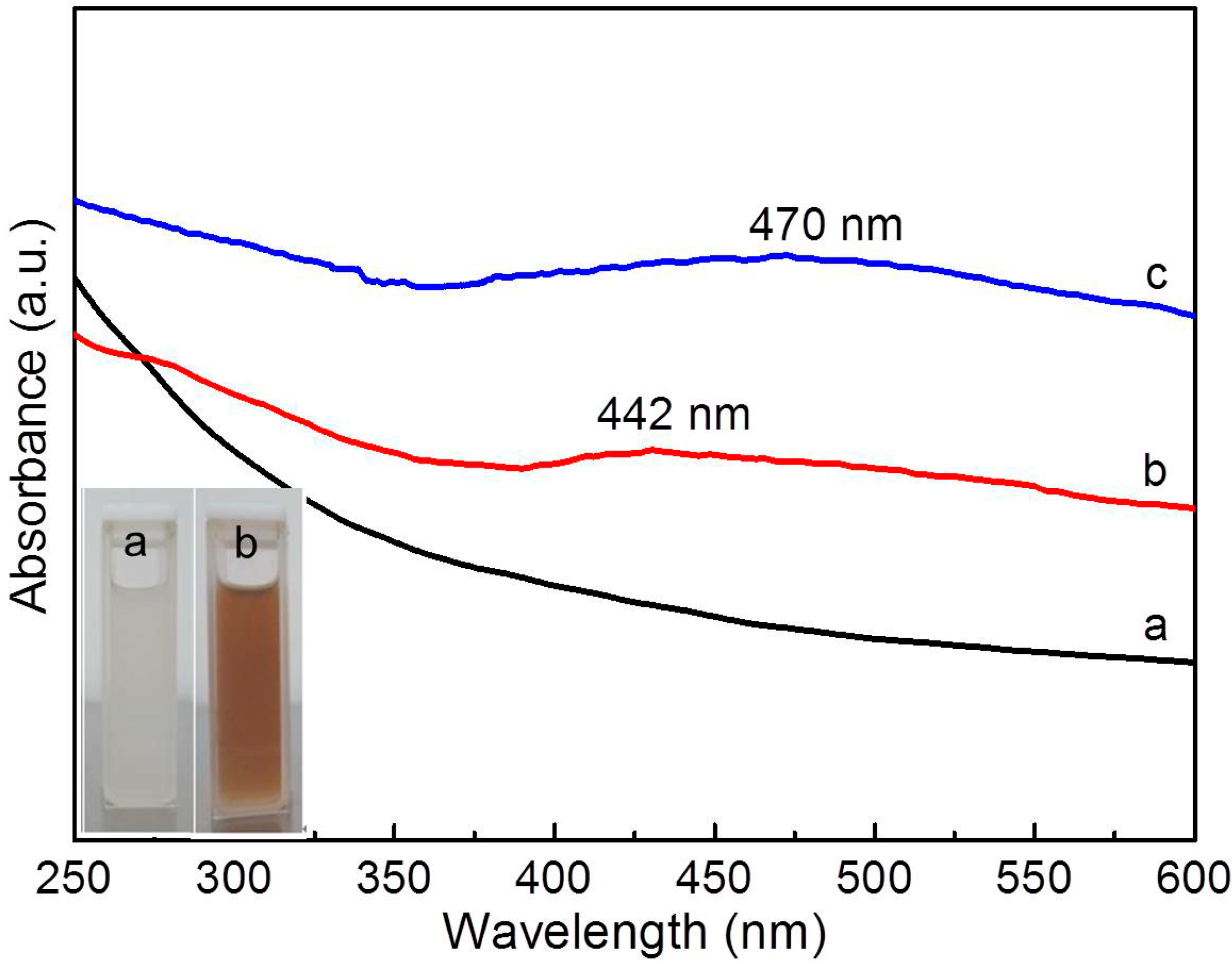
2.3. Syntheses of the Polymer-TiO2/Ag Composite Microgels
2.4. Catalytic Reduction of 4-NP
2.5. Characterization
3. Results and Discussion
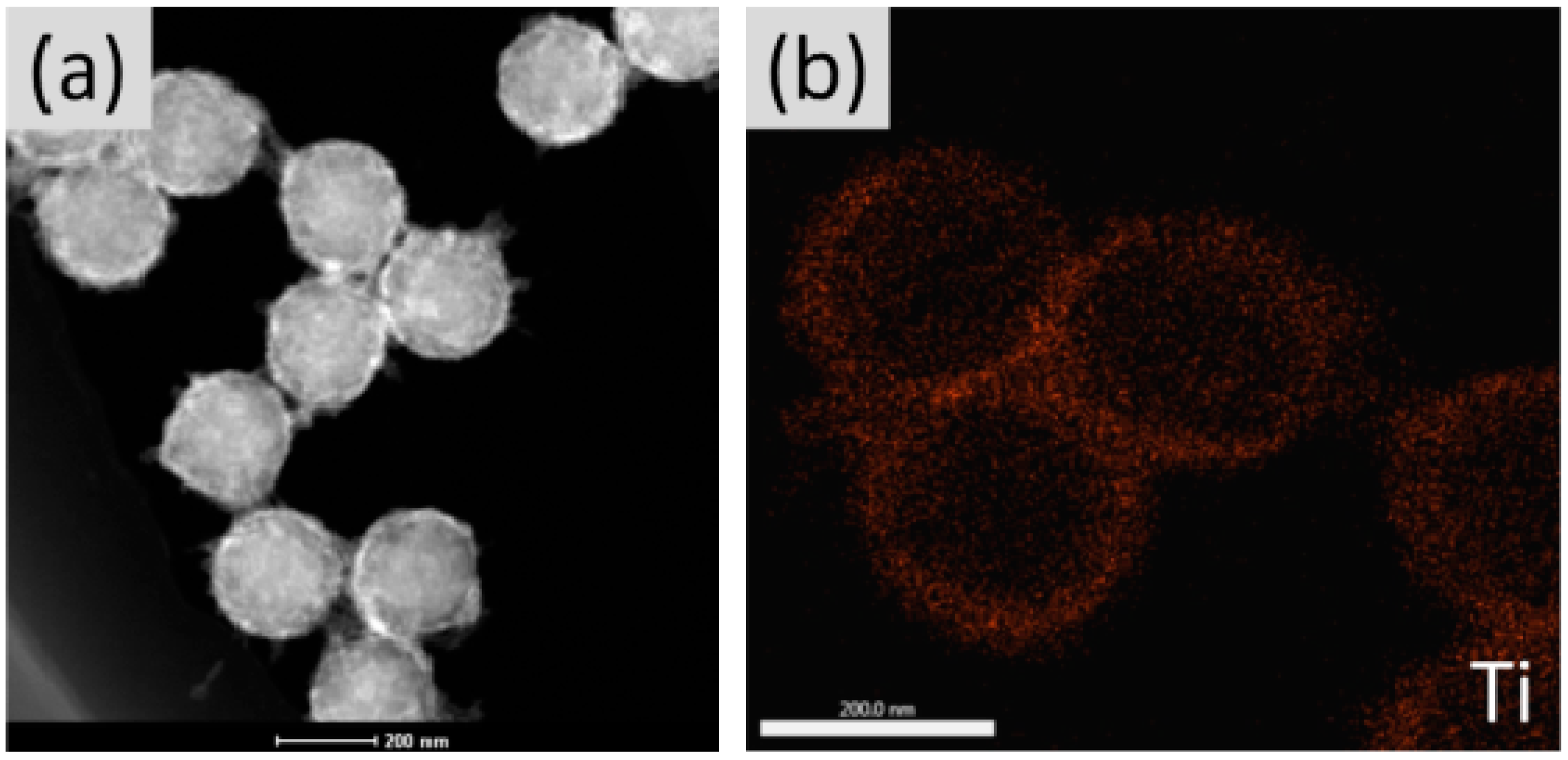
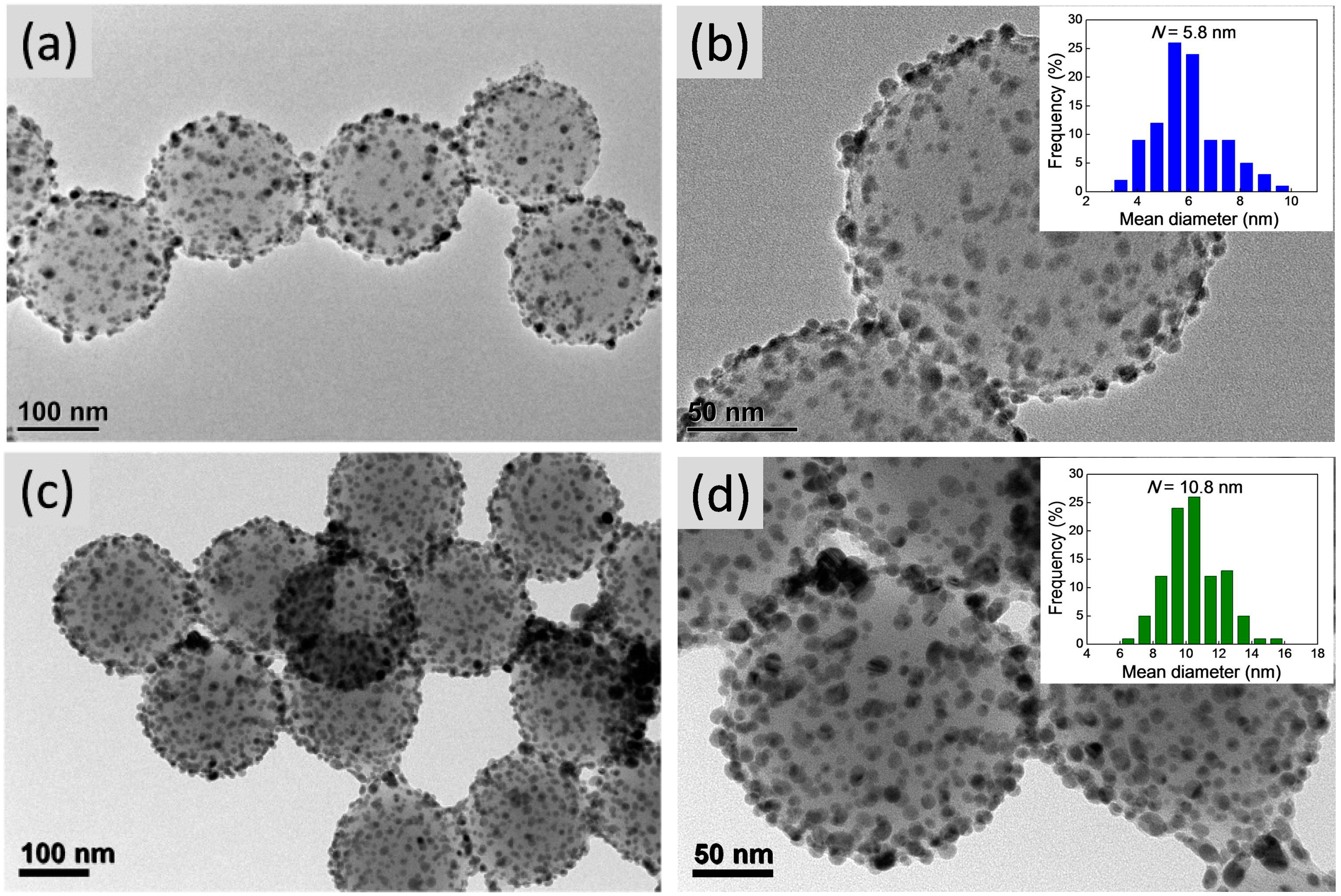
3.1. Morphologies of the Composite Microgels
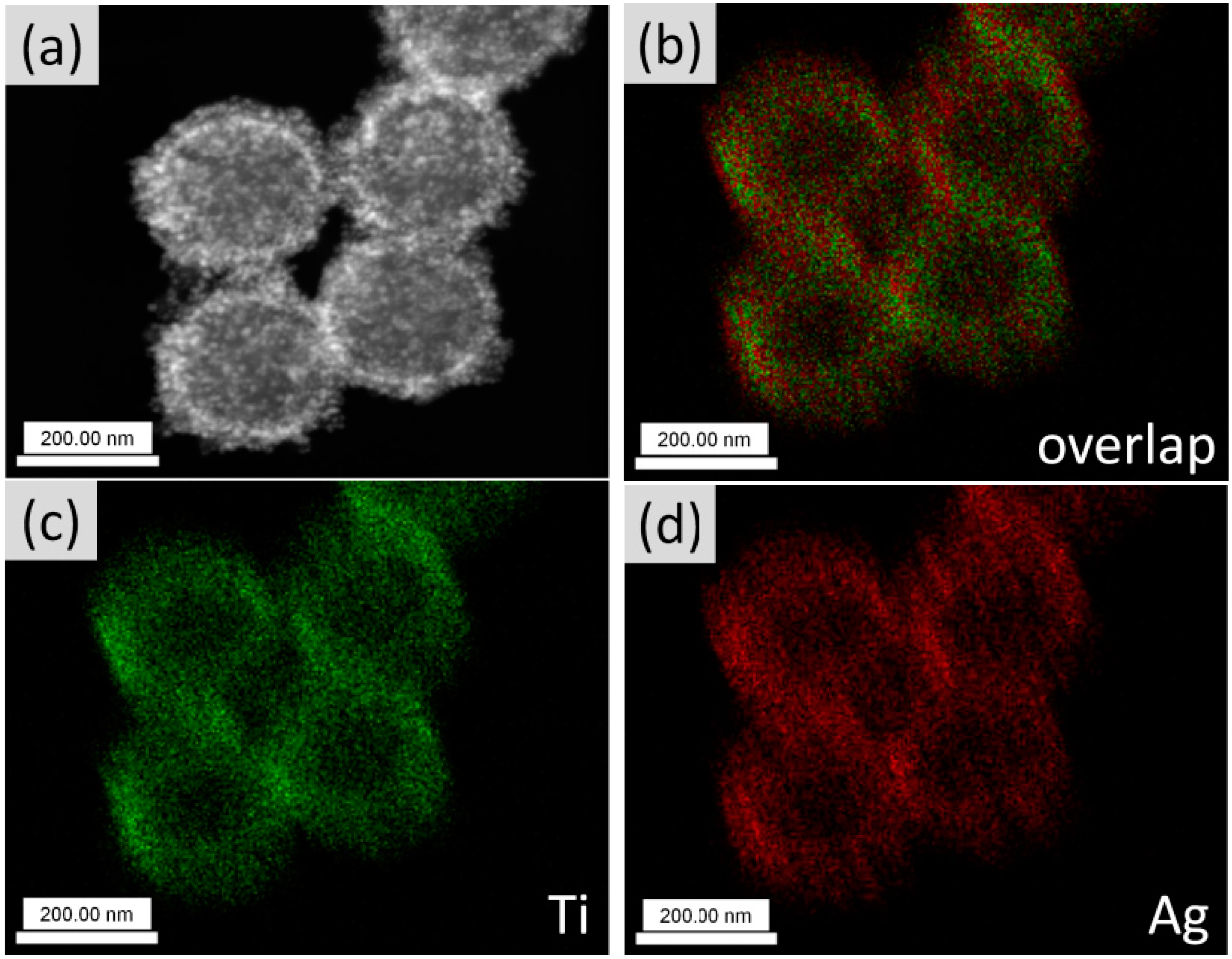
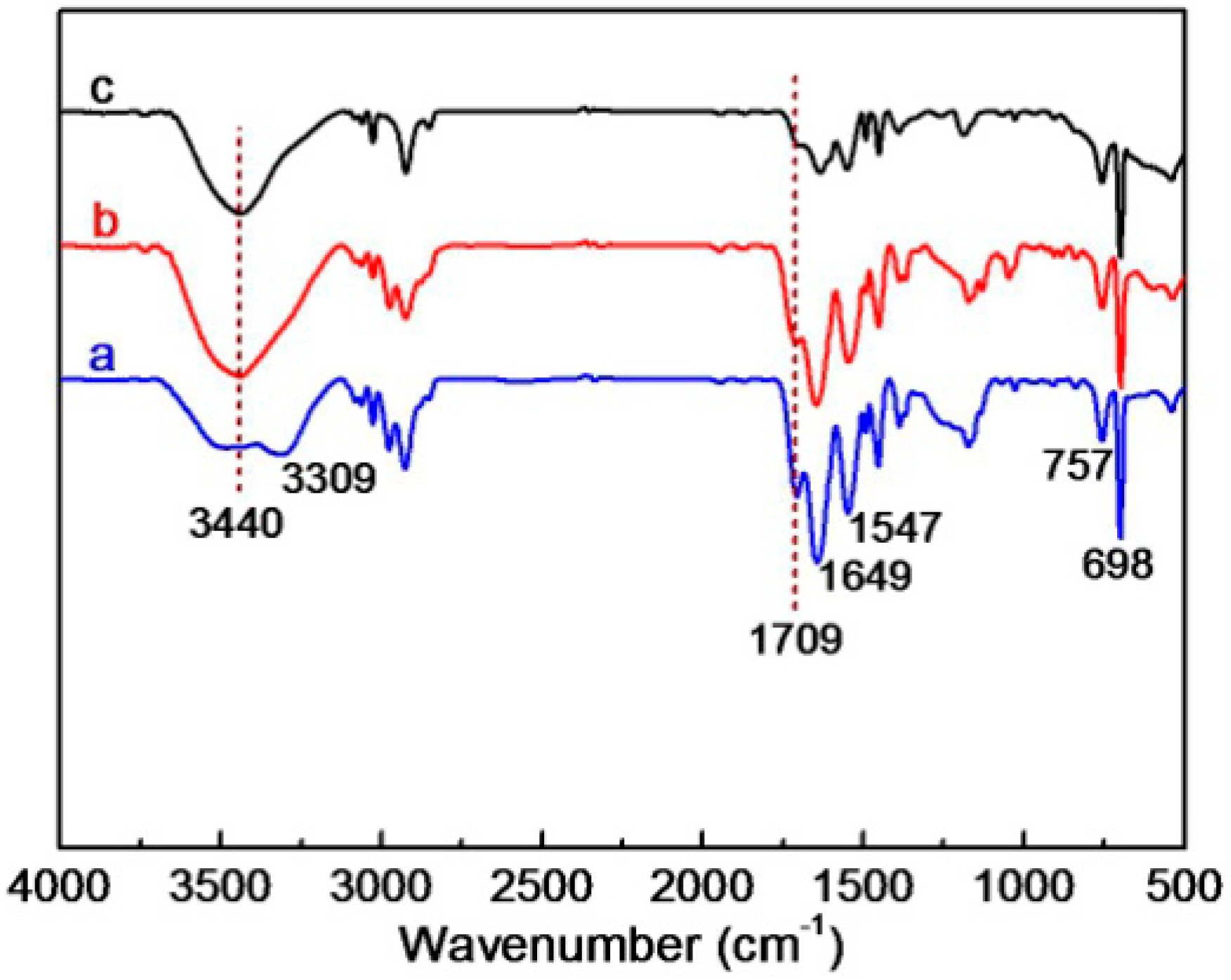
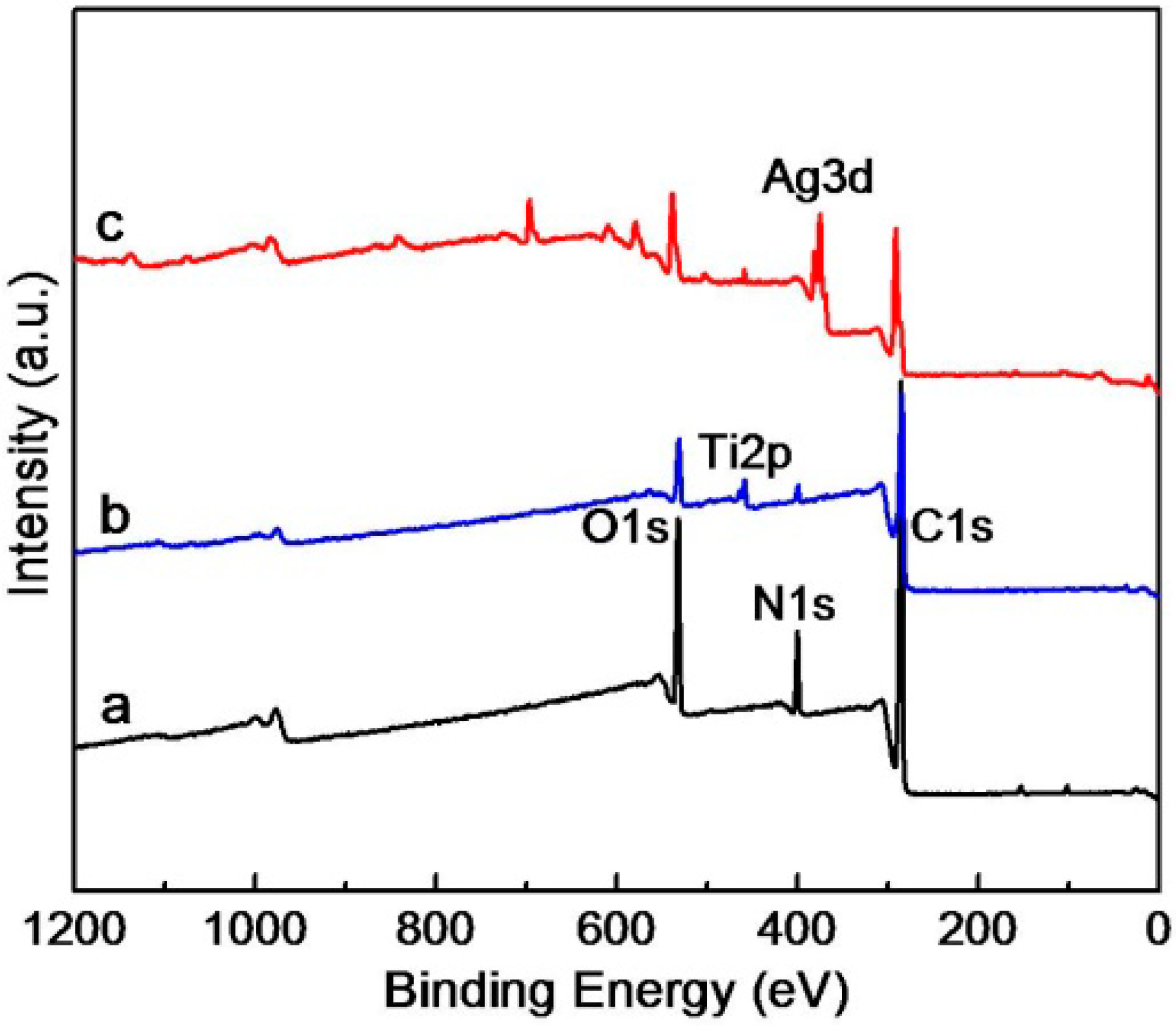
3.2. Composition Analyses of the Composite Microgels
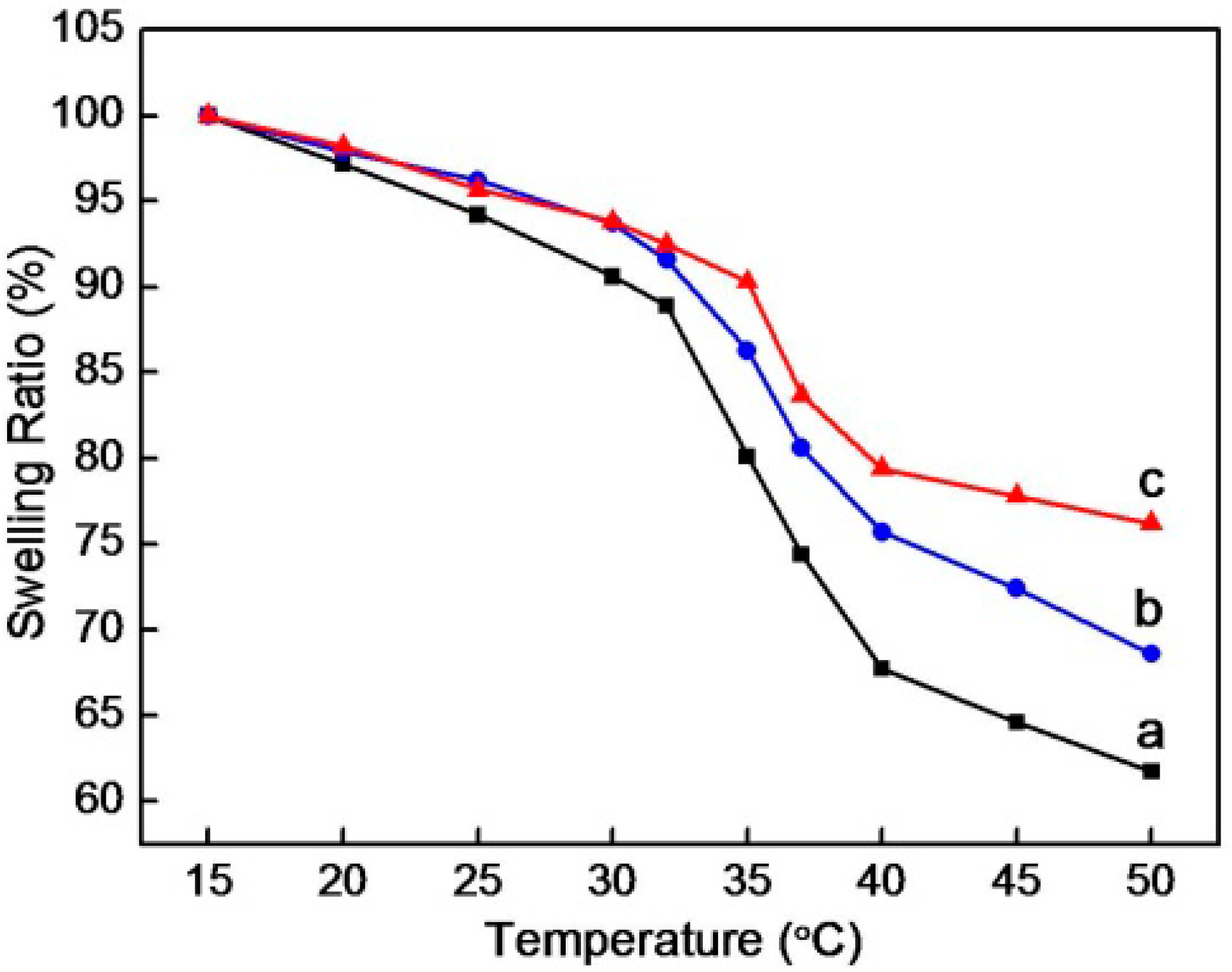
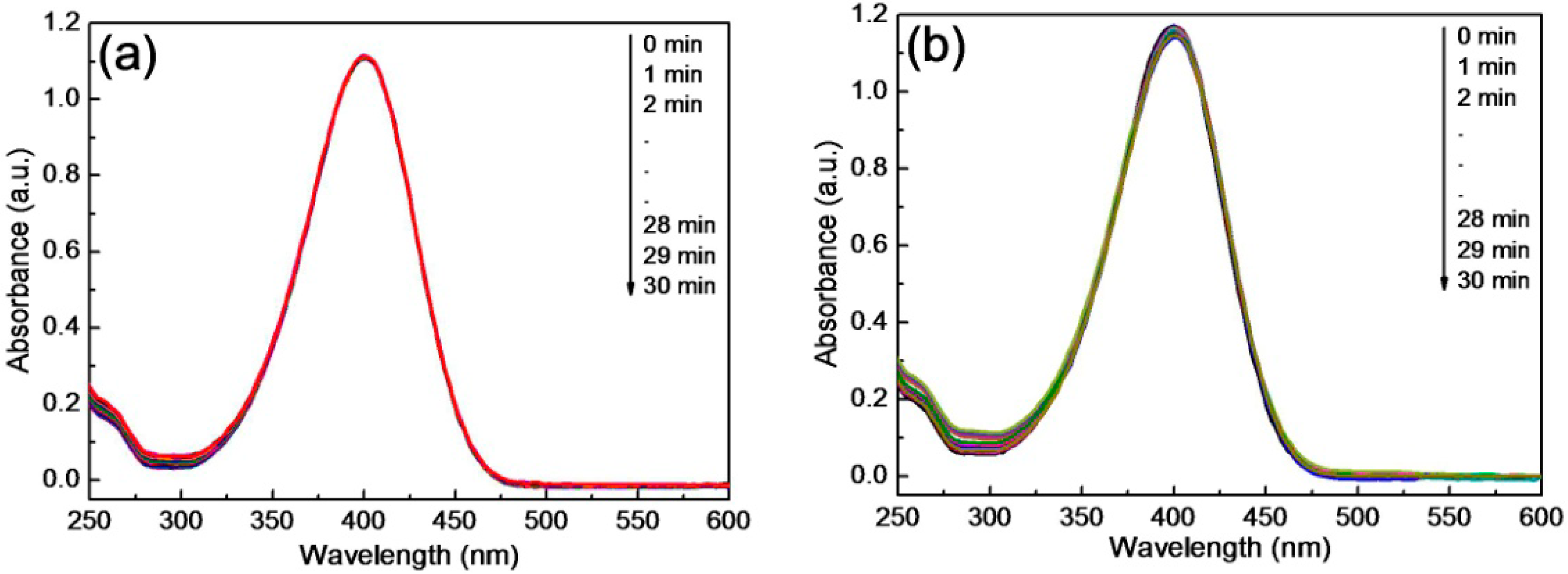
3.3. Thermosensitivity of the Composite Microgels
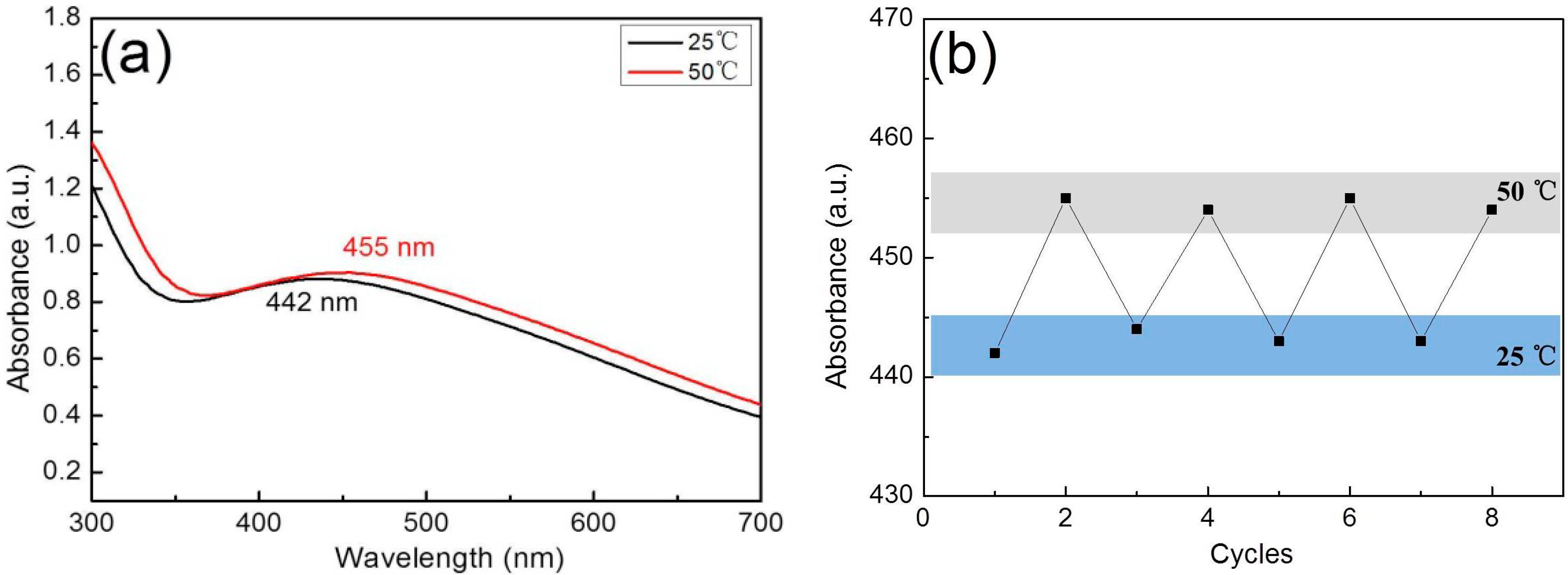
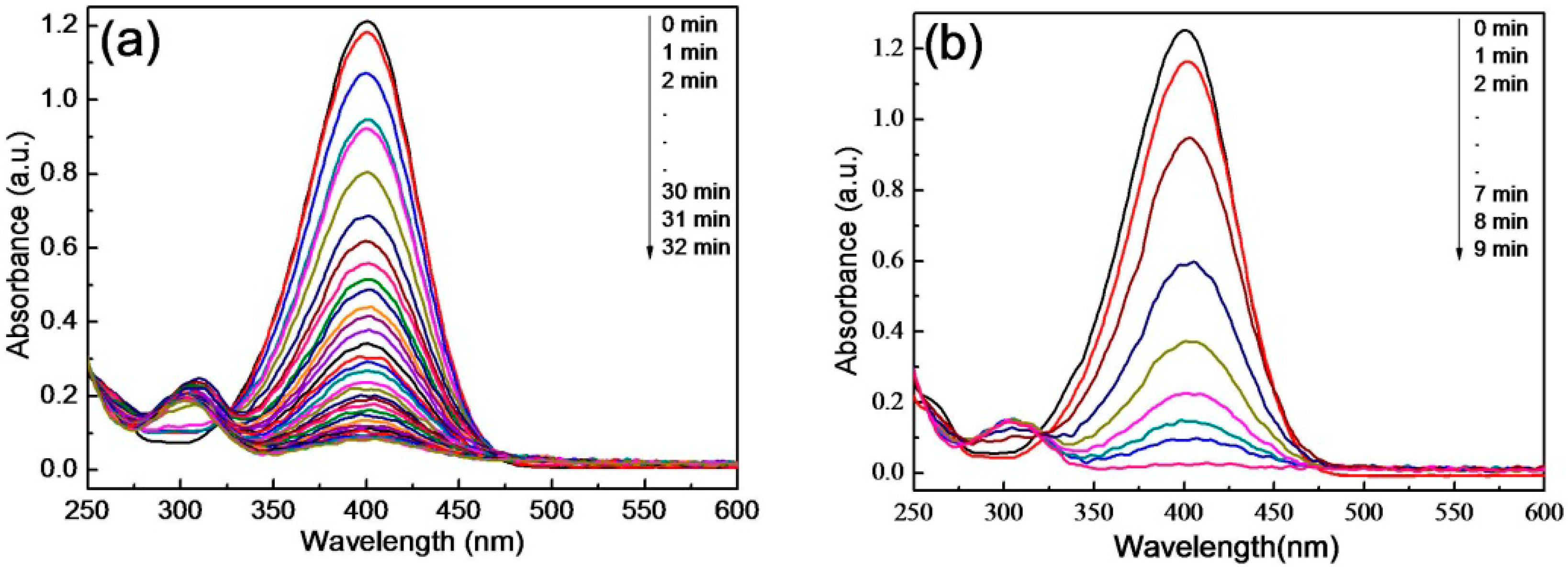
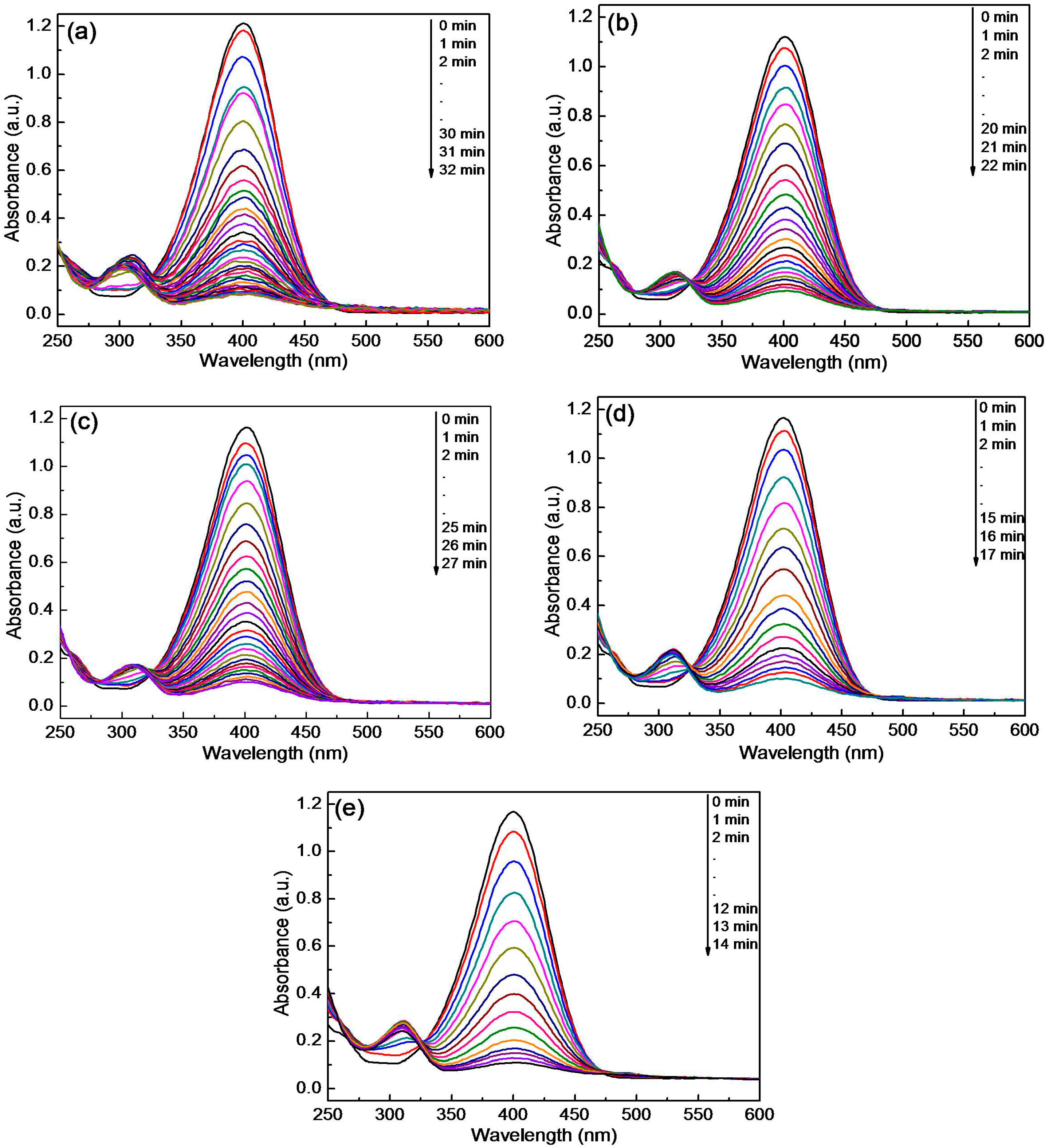
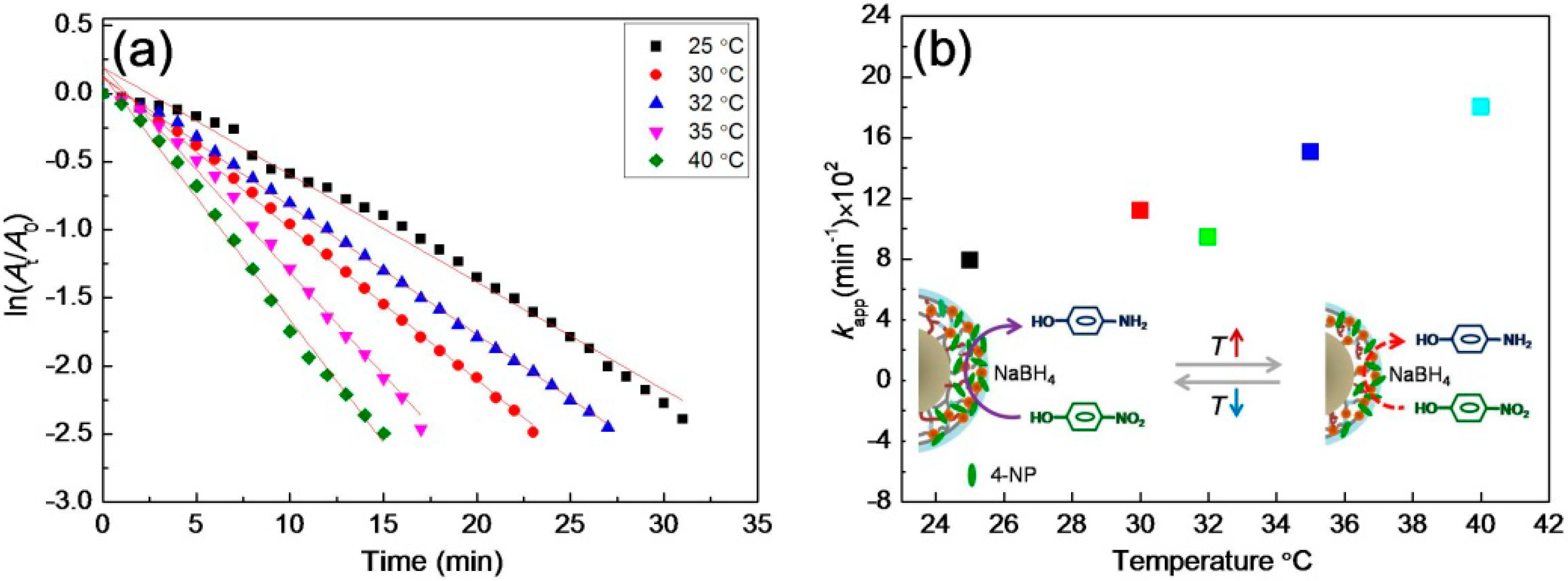
3.4. Catalytic Activity for the Reduction of 4-NP
3.5. Catalytic Cycling of the Composite Materials
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mahmudin, L.; Suharyadi, E.; Utomo, A.B.S.; Abraha, K. Optical properties of silver nanoparticles for surface plasmon resonance (SPR)-based biosensor applications. J. Mod. Phys. 2015, 6, 1071–1076. [Google Scholar] [CrossRef]
- Sharma, R.; Roopak, S.; Pathak, N.K.; Ji, A.; Sharma, R.P. Study of surface plasmon resonances of core-shell nanosphere: A comparison between numerical and analytical approach. Plasmonics 2017, 12, 977–986. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Salim, M.R.; Kueh, A.B.; Hadibarata, T.; Nur, H. A review of silver nanoparticles: Research trends, global consumption, synthesis, properties, and future challenges. J. Chin. Chem. Soc. 2017, 64, 732–756. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Yang, X.; Wang, S.; Shen, J.; Lin, B.; Li, C. Enhanced visible light photocatalytic activity of interlayer-isolated triplex Ag@SiO2@TiO2 core-shell nanoparticles. Nanoscale 2013, 5, 3359–3366. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Yu, Y.; He, H. Adsorption states of typical intermediates on Ag/Al2O3 catalyst employed in the selective catalytic reduction of NOx by ethanol. Chin. J. Catal. 2015, 36, 1312–1320. [Google Scholar] [CrossRef]
- Ferfera-Harrar, H.; Berdous, D.; Benhalima, T. Hydrogel nanocomposites based on chitosan-g-polyacrylamide and silver nanoparticles synthesized using Curcuma longa for antibacterial applications. Polym. Bull. 2018, 75, 2819–2846. [Google Scholar] [CrossRef]
- Ouay, B.L.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Deng, A.; Zhu, Y. Synthesis of TiO2/SiO2/Ag/Ag2O and TiO2/Ag/Ag2O nanocomposite spheres with photocatalytic performance. Res. Chem. Intermed. 2018, 44, 4227–4243. [Google Scholar] [CrossRef]
- He, H.; Gao, C. Graphene nanosheets decorated with Pd, Pt, Au, and Ag nanoparticles: Synthesis, characterization, and catalysis applications. Sci. China Chem. 2011, 54, 397–404. [Google Scholar] [CrossRef]
- Begum, R.; Naseem, K.; Farooqi, Z.H. A review of responsive hybrid microgels fabricated with silver nanoparticles: Synthesis, classification, characterization and applications. J. Sol-Gel Sci. Technol. 2016, 77, 497–515. [Google Scholar] [CrossRef]
- Wang, Z.; Zong, S.; Li, W.; Wang, C.; Xu, S.; Chen, H.; Cui, Y. SERS-fluorescence joint spectral encoding using organic-metal-QD hybrid nanoparticles with a huge encoding capacity for high-throughput biodetection: Putting theory into practice. J. Am. Chem. Soc. 2012, 134, 2993–3000. [Google Scholar] [CrossRef] [PubMed]
- Lago, V.; De Oliveira, L.F.; Goncalves, K.D.; Kobarg, J.; Cardoso, M.B. Size-selective silver nanoparticles: Future of biomedical devices with enhanced bactericidal properties. J. Mater. Chem. 2011, 21, 12267–12273. [Google Scholar] [CrossRef]
- Alaqad, K.; Saleh, T.A. Gold and silver nanoparticles: Synthesis methods, characterization routes and applications towards drugs. J. Environ. Anal. Toxicol. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Siripattanakul-Ratpukdi, S.; Fürhacker, M. Issues of silver nanoparticles in engineered environmental treatment systems. Water Air Soil Pollut. 2014, 225, 1939. [Google Scholar] [CrossRef]
- Li, H.; Qiang, W.; Vuki, M.; Xu, D.; Chen, H.Y. Fluorescence enhancement of silver nanoparticle hybrid probes and ultrasensitive detection of IgE. Anal. Chem. 2011, 83, 8945–8952. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Luo, L.B.; Nie, B.; Yu, S.H. p-Type beta-silver vanadate nanoribbons for nanoelectronic devices with tunable electrical properties. Adv. Funct. Mater. 2013, 23, 5116–5122. [Google Scholar] [CrossRef]
- Begum, R.; Farooqi, Z.H.; Butt, Z.; Wu, Q.S.; Wu, W.T.; Irfan, A. Engineering of responsive polymer based nano-reactors for facile mass transport and enhanced catalytic degradation of 4-nitrophenol. J. Environ. Sci. 2018, 72, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Richtering, W. Responsive emulsions stabilized by stimuli-sensitive microgels: Emulsions with special non-Pickering properties. Langmuir 2012, 28, 17218–17229. [Google Scholar] [CrossRef] [PubMed]
- Sasmal, A.K.; Pal, J.; Sahoo, R.; Kartikeya, P.; Dutta, S.; Pal, T. Superb Dye Adsorption and Dye-Sensitized Change in Cu2O-Ag Crystal Faces in the Dark. J. Phys. Chem. C 2016, 120, 21580–21588. [Google Scholar] [CrossRef]
- Naghibi, S.; Gharagozlou, M. Solvothermal synthesis of M-doped TiO2 nanoparticles for sonocatalysis of methylene blue and methyl orange (M = Cd, Ag, Fe, Ce, and Cu). J. Chin. Chem. Soc. 2017, 64, 640–650. [Google Scholar] [CrossRef]
- Matteis, V.D.; Rizzello, L.; Bello, M.P.; Rinaldi, R. One-step synthesis, toxicity assessment and degradation in tumoral pH environment of SiO2@Ag core/shell nanoparticles. J. Nanopart. Res. 2017, 19, 196. [Google Scholar] [CrossRef]
- Feng, Z.; Yu, J.; Kong, J.; Wang, T. A novel porous Al2O3 layer/AgNPs-Hemin composite for degradation of azo dyes under visible and UV irradiation. Chem. Eng. J. 2016, 294, 236–245. [Google Scholar] [CrossRef]
- Park, J.T.; Lee, C.S.; Park, C.H.; Kim, J.H. Preparation of TiO2/Ag binary nanocomposite as high-activity visible-light-driven photocatalyst via graft polymerization. Chem. Phys. Lett. 2017, 685, 119–126. [Google Scholar] [CrossRef]
- Zheng, B.; Liu, G.; Yao, A.; Xiao, Y.; Du, J.; Guo, Y.; Choi, M.M. A sensitive AgNPs/CuO nanofibers non-enzymatic glucose sensor based on electrospinning technology. Sens. Actuators B 2014, 195, 431–438. [Google Scholar] [CrossRef]
- Kim, D.H.; Vitol, E.A.; Liu, J.; Balasubramanian, S.; Gosztola, D.J.; Cohen, E.E.; Rozhkova, E.A. Stimuli-responsive magnetic nanomicelles as multifunctional heat and cargo delivery vehicles. Langmuir 2013, 29, 7425–7432. [Google Scholar] [CrossRef] [PubMed]
- Kanie, K.; Matsubara, M.; Zeng, X.; Liu, F.; Ungar, G.; Nakamura, H.; Muramatsu, A. Simple cubic packing of gold nanoparticles through rational design of their dendrimeric corona. J. Am. Chem. Soc. 2011, 134, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Tjong, S.C.; Zhao, Q.; Li, R.K. Facile method to prepare monodispersed Ag/polystyrene composite microspheres and their properties. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 4547–4554. [Google Scholar] [CrossRef]
- Godiya, C.B.; Cheng, X.; Li, D.; Chen, Z.; Lu, X.L. Carboxymethyl cellulose/polyacrylamide composite hydrogel for cascaded treatment/reuse of heavy metal ions in wastewater. J. Hazard. Mater. 2019, 364, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Liu, X.Y.; Yang, J.M.; Lin, D.L.; Chen, X.; Zha, L.S. Investigation of Ag nanoparticles loading temperature responsive hybrid microgels and their temperature controlled catalytic activity. Colloids Surf. A 2012, 393, 105–110. [Google Scholar] [CrossRef]
- Naseem, K.; Farooqi, Z.H.; Begum, R.; Wu, W.T.; Irfan, A.; Al-Sehemi, A.G. Silver nanoparticles engineered polystyrene-poly(N-isopropylmethacrylamide-acrylic acid) core shell hybrid polymer microgels for catalytic reduction of Congo Red. Macromol. Chem. Phys. 2018, 219, 1800211. [Google Scholar] [CrossRef]
- Khan, S.R.; Farooqi, Z.H.; Ali, A.; Begum, R.; Kanwal, F.; Siddiq, M. Kinetics and mechanism of reduction of nitrobenzene catalyzed by silver-poly (N-isopropylacryl amide-co-allylacetic acid) hybrid microgels. Mater. Chem. Phys. 2016, 171, 318–327. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.I.; Uflyand, I.E. Preparation of metal-polymer nanocomposites by chemical reduction of metal ions: Functions of polymer matrices. J. Polym. Res. 2018, 25, 255. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Q.; Wang, J.; Ma, Y.; Wang, D.; Wu, Z.; Zhang, M. Temperature responsive copolymer as support for metal nanoparticle catalyst: A recyclable catalytic system. React. Funct. Polym. 2017, 112, 60–67. [Google Scholar] [CrossRef]
- Begum, R.; Farooqi, Z.H.; Ahmed, E.; Naseem, K.; Ashraf, S.; Sharif, A.; Rehan, R. Catalytic reduction of 4-nitrophenol using silver nanoparticles-engineered poly (N-isopropylacrylamide-co-acrylamide) hybrid microgels. Appl. Organomet. Chem. 2017, 31, 3563–3571. [Google Scholar] [CrossRef]
- Agrawal, G.; Agrawal, R. Stimuli-Responsive Microgels and Microgel-Based Systems: Advances in the Exploitation of Microgel Colloidal Properties and Their Interfacial Activity. Polymers 2018, 10, 418. [Google Scholar] [CrossRef]
- Shah, L.A.; Haleem, A.; Sayed, M.; Siddiq, M. Synthesis of sensitive hybrid polymer microgels for catalytic reduction of organic pollutants. J. Environ. Chem. Eng. 2016, 4, 3492–3497. [Google Scholar] [CrossRef]
- Begum, R.; Najeeb, J.; Ahmad, G.; Wu, W.T.; Irfan, A.; Al-Sehemi, A.G.; Farooqi, Z.H. Synthesis and characterization of poly(N-isopropylmethacrylamide-co-acrylic acid) microgels for in situ fabrication and stabilization of silver nanoparticles for catalytic reduction of o-nitroaniline in aqueous medium. React. Funct. Polym. 2018, 132, 89–97. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, T.; Hu, B.; Yang, Q.; Liu, L.; Yu, B.; Ye, S. Synthesis of thermo-and pH-responsive Ag nanoparticle-embedded hybrid microgels and their catalytic activity in methylene blue reduction. Mater. Chem. Phys. 2015, 149, 460–466. [Google Scholar] [CrossRef]
- Li, K.; Chen, X.; Wang, Z.; Xu, L.; Fu, W.; Zhao, L.; Chen, L. Temperature-responsive catalytic performance of Ag nanoparticles endowed by poly (N-isopropylacrylamide-co-acrylic acid) microgels. Polym. Compos. 2017, 38, 708–718. [Google Scholar] [CrossRef]
- Scherzinger, C.; Schwarz, A.; Bardow, A.; Leonhard, K.; Richtering, W. Cononsolvency of poly-N-isopropyl acrylamide (PNIPAM): Microgels versus linear chains and macrogels. Curr. Opin. Colloid Interface Sci. 2014, 19, 84–94. [Google Scholar] [CrossRef]
- Lu, Y.; Mei, Y.; Drechsler, M.; Ballauff, M. Thermosensitive core-shell particles as carriers for Ag nanoparticles: Modulating the catalytic activity by a phase transition in networks. Angew. Chem. Int. Ed. 2006, 45, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Proch, S.; Schrinner, M.; Drechsler, M.; Kempe, R.; Ballauff, M. Thermosensitive core-shell microgel as a “nanoreactor” for catalytic active metal nanoparticles. J. Mater. Chem. 2009, 19, 3955–3961. [Google Scholar] [CrossRef]
- Yang, L.Q.; Hao, M.M.; Wang, H.Y.; Zhang, Y. Amphiphilic polymer-Ag composite microgels with tunable catalytic activity and selectivity. Colloid Polym. Sci. 2015, 293, 2405–2417. [Google Scholar] [CrossRef]
- Wang, M.Y.; Niu, R.; Huang, M.; Zhang, Y. The surface structural changes of self-assembly monolayer Au nanoparticles and their regulated catalytic activity. Sci. Sin. Chim. 2015, 45, 76–89. [Google Scholar]
- Zhao, J.; Liu, L.F.; Zhang, Y. Synthesis of Silver Nanoparticles Loaded onto a Structural Support and Their Catalytic Activity. Acta Phys. Chim. Sin. 2015, 31, 1549–1558. [Google Scholar]
- Wang, P.; Chen, D.; Tang, F.Q. Preparation of titania-coated polystyrene particles in mixed solvents by ammonia catalysis. Langmuir 2006, 22, 4832–4835. [Google Scholar] [CrossRef] [PubMed]
- Aditya, T.; Pal, A.; Pal, T. Nitroarene reduction: A trusted model reaction to test nanoparticle catalysts. Chem. Commun. 2015, 51, 9410–9431. [Google Scholar] [CrossRef] [PubMed]
- Bastús, N.G.; Merkoçi, F.; Piella, J.; Puntes, V. Synthesis of highly monodisperse citrate-stabilized silver nanoparticles of up to 200 nm: Kinetic control and catalytic properties. Chem. Mater. 2014, 26, 2836–2846. [Google Scholar] [CrossRef]
- Sun, D.; Yang, J.; Wang, X. Bacterial cellulose/TiO2 hybrid nanofibers prepared by the surface hydrolysis method with molecular precision. Nanoscale 2010, 2, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, X.; Fang, J.; Zhou, G.; Liu, Z.; Wu, S.; Zhu, X. Synthesis of BiOI-TiO2 composite nanoparticles by microemulsion method and study on their photocatalytic activities. Sci. World J. 2014, 2014, 647040. [Google Scholar]
- Su, W.; Wei, S.; Hu, Q.; Tang, J.X. Preparation of TiO2/Ag colloids with ultraviolet resistance and antibacterial property using short chain polyethylene glycol. J. Hazard. Mater. 2009, 172, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, Y.; Wang, J.; Guo, Q.; Li, J. Size-controlled synthesis of dispersed equiaxed amorphous TiO2 nanoparticles. Ceram. Int. 2015, 41, 9057–9062. [Google Scholar] [CrossRef]
- Zhang, C.X.; Li, C.; Chen, Y.; Zhang, Y. Synthesis and catalysis of Ag nanoparticles trapped into temperature-sensitive and conductive polymers. J. Mater. Sci. 2014, 49, 6872–6882. [Google Scholar] [CrossRef]
- Marais, E.; Nyokong, T. Adsorption of 4-nitrophenol onto Amberlite®IRA-900 modified with metallophthalocyanines. J. Hazard. Mater. 2008, 152, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Bo, L.L.; Zhang, Y.B.; Quan, X.; Zhao, B. Microwave assisted catalytic oxidation of p-nitrophenol in aqueous solution using carbon-supported copper catalyst. J. Hazard. Mater. 2008, 153, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Mele, G.; Pio, I.; Li, J.; Palmisano, L.; Vasapollo, G. Degradation of 4-nitrophenol (4-NP) using Fe-TiO2 as a heterogeneous photo-Fenton catalyst. J. Hazard. Mater. 2010, 176, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Davoodnia, A.; Khojastehnezhad, A. Carbon-based solid acid catalyzed synthesis of polyhydroquinoline derivatives via Hantzsch reaction under solvent-free conditions. J. Chil. Chem. Soc. 2012, 57, 1385–1387. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; Jiang, C.; Guo, J.; Fan, Y.; Zhang, Y. Synthesis of Silver Nanoparticles Loaded onto Polymer-Inorganic Composite Materials and Their Regulated Catalytic Activity. Polymers 2019, 11, 401. https://doi.org/10.3390/polym11030401
Yan S, Jiang C, Guo J, Fan Y, Zhang Y. Synthesis of Silver Nanoparticles Loaded onto Polymer-Inorganic Composite Materials and Their Regulated Catalytic Activity. Polymers. 2019; 11(3):401. https://doi.org/10.3390/polym11030401
Chicago/Turabian StyleYan, Sen, Chunge Jiang, Jianwu Guo, Yinglan Fan, and Ying Zhang. 2019. "Synthesis of Silver Nanoparticles Loaded onto Polymer-Inorganic Composite Materials and Their Regulated Catalytic Activity" Polymers 11, no. 3: 401. https://doi.org/10.3390/polym11030401
APA StyleYan, S., Jiang, C., Guo, J., Fan, Y., & Zhang, Y. (2019). Synthesis of Silver Nanoparticles Loaded onto Polymer-Inorganic Composite Materials and Their Regulated Catalytic Activity. Polymers, 11(3), 401. https://doi.org/10.3390/polym11030401





