Enhancing the Mechanical and Thermal Properties of Epoxy Resin via Blending with Thermoplastic Polysulfone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the PSF/Epoxy Composites
2.3. Characterizations
3. Results and Discussion
3.1. The Solubility of PSF in Epoxy Resin
3.2. Curing Kinetics of PSF/Epoxy Resin Blends
3.3. The Chemical Structures of the PSF/Epoxy Resin Blends
3.4. Dynamic Mechanical Thermal Analysis of the PSF/Epoxy Blends
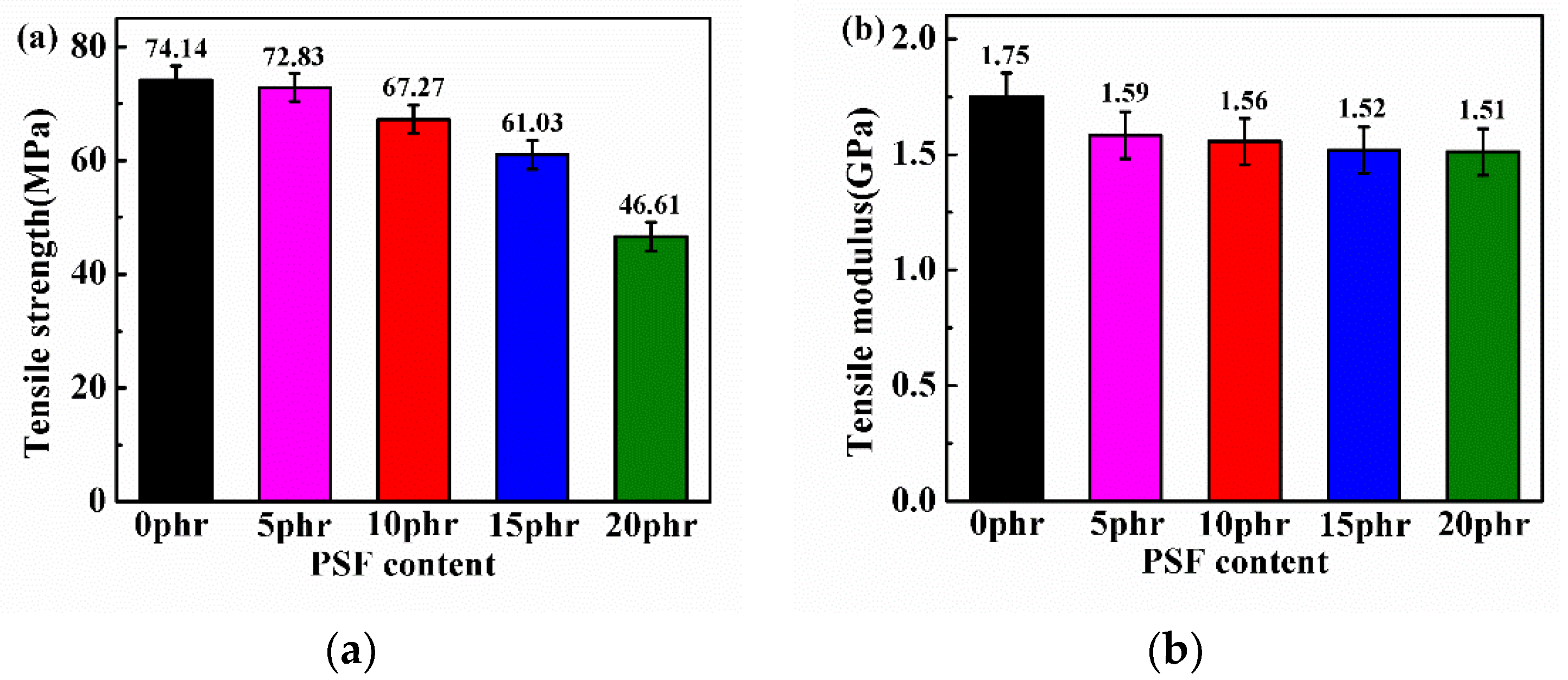
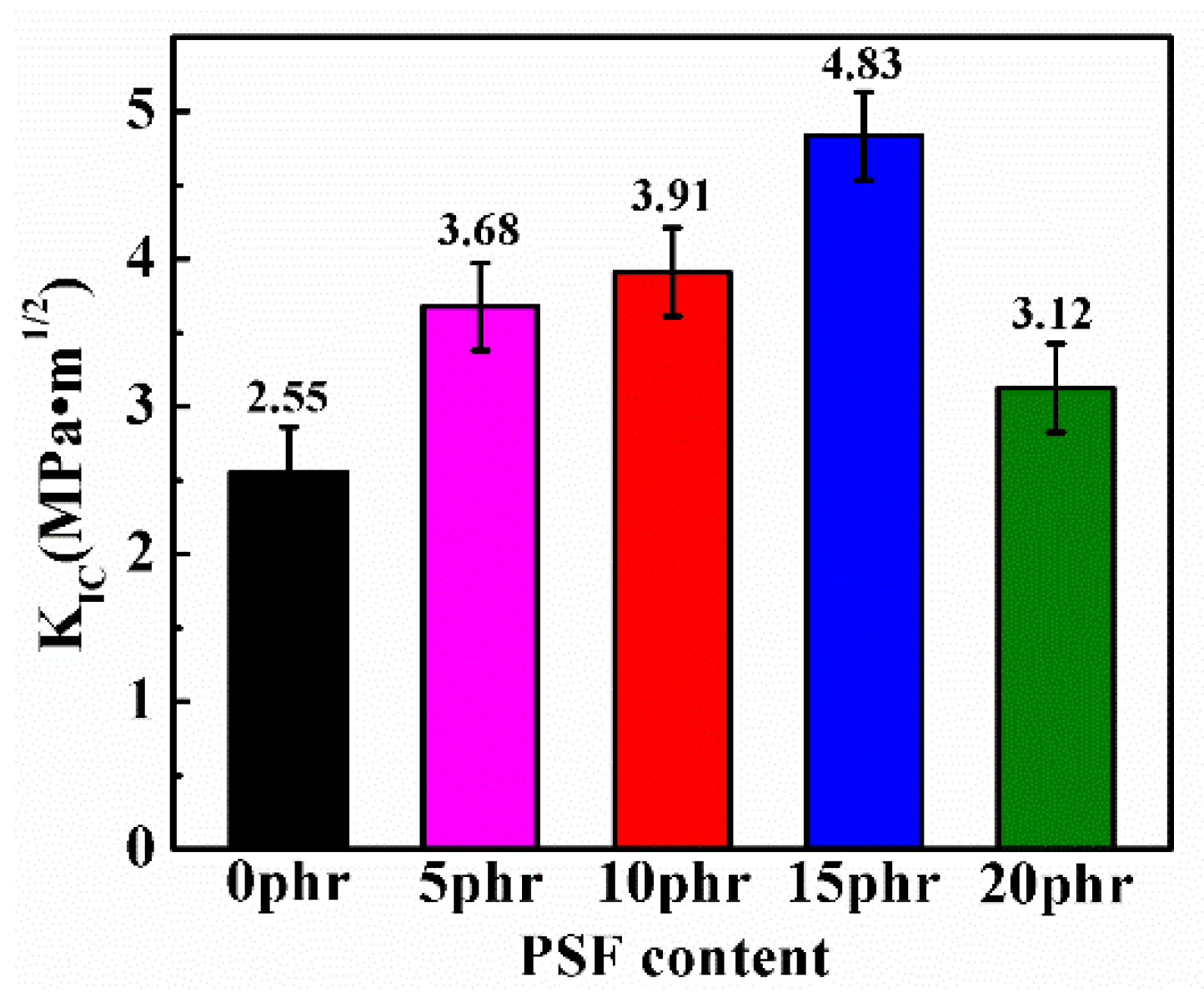
3.5. Mechanical Properties of PSF/Epoxy Blends
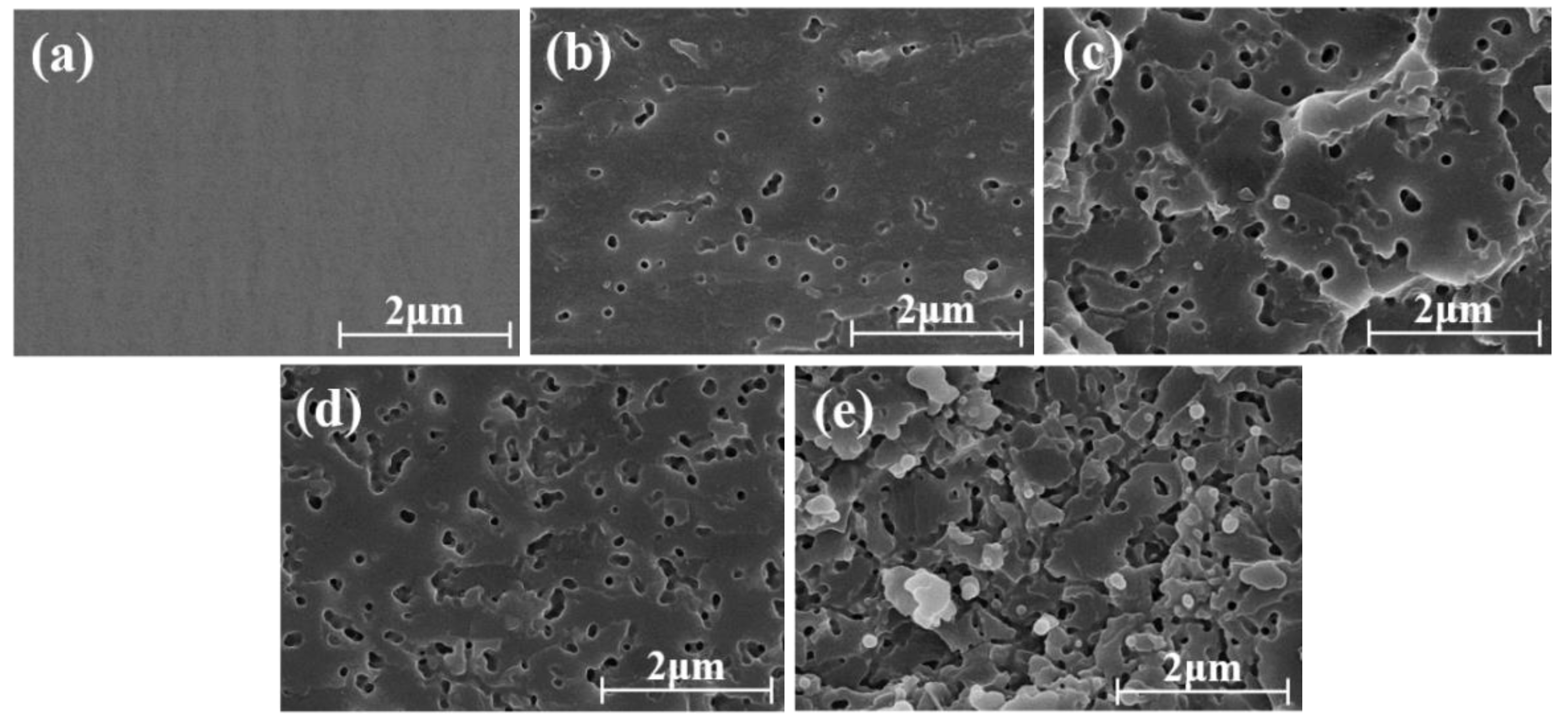
3.6. Phase Structures of the PSF/epoxy Blends
3.7. Glass Transition Temperature of PSF/Epoxy Cured Product
3.8. Thermogravimetric Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kadhim, N.; Mei, Y.; Wang, Y.; Li, Y.; Meng, F.B.; Jiang, M.; Zhou, Z.W. Remarkable improvement in the mechanical properties of epoxy composites achieved by a small amount of modified helical carbon nanotubes. Polymers 2018, 10, 1103. [Google Scholar] [CrossRef]
- Yang, X.T.; Guo, Y.Q.; Luo, X.; Zheng, N.; Ma, T.B.; Tan, J.J.; Li, C.M.; Zhang, Q.Y.; Gu, J.W. Self-healing, recoverable epoxy elastomers and their composites with desirable thermal conductivities by incorporating BN fillers via in-situ polymerization. Compos. Sci. Technol. 2018, 164, 59–64. [Google Scholar] [CrossRef]
- Sun, Z.; Xiao, J.; Tao, L.; Wei, Y.; Wang, S.; Zhang, H.; Yu, M. Preparation of High-Performance Carbon Fiber-Reinforced Epoxy Composites by Compression Resin Transfer Molding. Materials 2019, 12, e13. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.G.; Song, P.G.; Zhang, J.; Guo, Q.P.; Mai, Y.W. Epoxy nanocomposites simultaneously strengthened and toughened by hybridization with graphene oxide and block ionomer. Compos. Sci. Technol. 2018, 168, 363–370. [Google Scholar] [CrossRef]
- Cicala, G.; Recca, G.; Carciotto, S.; Restucca, C.L. Development of epoxy/hyperbranched blends for resin transfer molding and vacuum assisted resin transfer molding applications: Effect of a reactive diluent. Polym. Eng. Sci. 2009, 49, 577–584. [Google Scholar] [CrossRef]
- Vijayan, P.P.; Puglia, D.; Vijayan, P.P.; Kenny, J.M.; Thomas, S. The role of clay modifier on cure characteristics and properties of epoxy/clay/carboxyl terminated poly(butadiene-co-acrylonitrile) (CTBN) hybrid. Mater. Technol. 2017, 32, 171–177. [Google Scholar] [CrossRef]
- Zhou, L.; Fu, Y.W.; Yin, T.; Luo, Z.Y. Synergetic effect of epoxy resin and carboxylated nitrile rubber on tribological and mechanical properties of soft paper-based friction materials. Tribol. Int. 2019, 129, 314–322. [Google Scholar] [CrossRef]
- Huang, Z.F.; Tan, S.T.; Wang, X.Y. Modification of an o-cresol formaldehyde epoxy resin by the thermotropic liquid crystalline polymer. J. Appl. Polym. Sci. 2005, 97, 1626–1631. [Google Scholar] [CrossRef]
- Zhang, B.L.; Tang, G.L.; Shi, K.Y.; You, Y.C.; Du, Z.J.; Huang, J.F. A study on the properties of epoxy resin toughened by a liquid crystal-type oligomer. J. Appl. Polym. Sci. 1999, 77, 117–184. [Google Scholar] [CrossRef]
- Miyagawa, H.; Mohanty, A.; Drzal, L.T.; Misra, M. Effect of clay and alumina-nanowhisker reinforcements on the mechanical properties of nanocomposites from biobased epoxy: A comparative study. Ind. Eng. Chem. Res. 2004, 43, 7001–7009. [Google Scholar] [CrossRef]
- Mousavi, A.; Roghani-Mamaqani, H.; Salami-Kalajahi, M.; Shahi, S.; Abdollahi, A. Modification of graphene with silica nanoparticles for use in hybrid network formation from epoxy, novolac, and epoxidized novolac resins by sol-gel method: Investigation of thermal properties. Expess Polym. Lett. 2018, 12, 187–202. [Google Scholar] [CrossRef]
- Goyat, M.S.; Ray, S.; Ghosh, P.K. Innovative application of ultrasonic mixing to produce homogeneously mixed nanoparticulate-epoxy composite of improved physical properties. Compos. Part A 2011, 42, 1421–1431. [Google Scholar] [CrossRef]
- Blanco, I.; Cicala, G.; Costa, M.; Recca, A. Development of an epoxy system characterized by low water absorption and high thermomechanical performances. J. Appl. Polym. Sci. 2006, 100, 4880–4887. [Google Scholar] [CrossRef]
- Cheng, X.L.; Wu, Q.; Morgan, S.E.; Wiggins, J.S. Morphologies and mechanical properties of polyethersulfone modified epoxy blends through multifunctional epoxy composition. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Wang, F.Z.; Drzal, L.T. Development of stiff, tough and conductive composites by the addition of graphene nanoplatelets to polyethersulfone/epoxy composites. Materials 2018, 11, 2137. [Google Scholar] [CrossRef] [PubMed]
- Rosetti, Y.; Alcouffe, P.; Pascault, J.P.; Gerard, J.F.; Lortie, F. Polyether sulfone-based epoxy toughening: From micro- to nano-phase separation via PES end-chain modification and process engineering. Materials. 2018, 10, 1960. [Google Scholar] [CrossRef] [PubMed]
- Blanco, I.; Oliveri, L.; Cicala, G.; Recca, A. Effects of novel reactive toughening agent on thermal stability of epoxy resin. J. Therm. Anal. Calorim. 2012, 108, 685–693. [Google Scholar] [CrossRef]
- Francis, B.; Rao, V.L.; Ramaswamy, R.; Jose, S.; Thomas, S.; Raju, K. Morphology, viscoelastic properties and mechanical behavior of epoxy resin modified with hydroxyl-terminated poly (ether-ether-ketone) oligomer with pendent tert-butyl groups. Polym. Eng. Sci. 2005, 45, 1645–1654. [Google Scholar] [CrossRef]
- Francis, B.; Thomas, S.; Jose, J.; Ramaswamy, R.; Rao, V.L. Hydroxyl terminated poly(ether-ether-ketone) with pendent methyl group toughened epoxy resin: Miscibility, morphology and mechanical properties. Polymer 2005, 46, 12372–12385. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, X.M. Influence of addition of silica particles on reaction-induced phase separation and properties of epoxy/PEI blends. Compos. Part B 2011, 42, 2163–2169. [Google Scholar] [CrossRef]
- Korokhin, R.A.; Solodilov, V.I.; Gorbatkina, Y.A.; Shapagin, A.V. Rheological and physicomechanical properties of epoxy-polyetherimide compositions. Mech. Compos. Mater. 2015, 51, 313–320. [Google Scholar] [CrossRef]
- Bonnaud, L.; Pascalut, J.P.; Sautereau, H.; Zhao, J.Q.; Jia, D.M. Use of reactive polyetherimide to modify epoxy thermosets. I. Synthesis of an amino-grafted polyetherimide. Eur. Polym. J. 2004, 40, 2637–2643. [Google Scholar] [CrossRef]
- Chen, Z.C.; Tian, J.; Guo, J.H.; Xie, Z.Q.; Chu, F.Y. Study on the properties of the layered-structure in the epoxy/polysulfone blends. Thermosetting Resin. 2018, 33, 15–18. [Google Scholar]
- Jin, H.; Yang, B.Q.; Jin, F.L.; Park, S.J. Fracture toughness and surface morphology of polysulfone-modified epoxy resin. J. Ind. Eng. Chem. 2015, 25, 9–11. [Google Scholar] [CrossRef]
- Oyanguren, P.A.; Galante, M.J.; Andromaque, K.; Frontini, P.M.; Williams, R.J.J. Development of bicontinuous morphologies in polysulfone epoxy blends. Polymer 1999, 40, 5249–5255. [Google Scholar] [CrossRef]
- Tanaka, N.; Iijima, T.; Fukuda, W.; Tomoi, M. Synthesis and properties of interpenetrating polymer networks composed of epoxy resins and polysulphones with cross-linkable pendant vinylbenzyl groups. Polym. Int. 1997, 42, 95–106. [Google Scholar] [CrossRef]
- Zheng, N.; Sun, W.F.; Liu, H.Y.; Huang, Y.D.; Gao, J.F.; Mai, Y.W. Effects of carboxylated carbon nanotubes on the phase separation behaviour and fracture-mechanical properties of an epoxy/polysulfone blend. Compos. Sci. Technol. 2018, 59, 180–188. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Han, J.L.; Hsieh, K.H.; Chiu, W.Y. Kinetics of curing reaction of epoxide catalyzed by tertiary amine. J. Appl. Polym. Sci. 2010, 50, 1099–1106. [Google Scholar] [CrossRef]
- Yang, L.P.; Li, P.; Xue, Z.M. Cure kinetics of a TGDDM epoxy/DDS system modifide with polysulfone nanofiber. Fiber Reinf. Plast. Compos. 2007, 5, 7–10. [Google Scholar]
- Huang, P.; Zheng, S.X.; Huang, J.Y.; Guo, Q.P.; Zhu, W. Miscibility and mechanical properties of epoxy resin/polysulfone blends. Polymer 1997, 38, 5565–5571. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, P.A.; Fu, S.Y.; Chen, F.H. Morphological structure and mechanical properties of epoxy/ polysulfone/cellulose nanofiber ternary nanocomposites. Compos. Sci. Technol. 2015, 115, 66–71. [Google Scholar] [CrossRef]
- Yu, Y.F.; Wang, M.H.; Gan, W.J.; Tao, Q.S.; Li, S.J. Polymerization-induced viscoelastic phase separation in polyethersulfone-modified epoxy systems. J. Phys. Chem. B 2004, 108, 6208–6215. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Huang, Z.B.; Xin, C.L.; Li, P.; Jia, X.L.; Wang, B.H.; He, Y.D.; Ryu, S.; Yang, X.P. Morphology evolution of polysulfone nanofibrous membranes toughened epoxy resin during reaction-induced phase separation. Mater. Chem. Phys. 2009, 118, 398–404. [Google Scholar] [CrossRef]
- Varley, R.J.; Hodgkin, J.H.; Simon, G.P. Toughening of a trifunctional epoxy system - Part VI. Structure property relationships of the thermoplastic toughened system. Polymer 2001, 42, 3847–3858. [Google Scholar] [CrossRef]
- Rajasekaran, R.; Karikalchozhan, C.; Alagar, M. Preparation and characterization of 3,3’-bis (maleimidophenyl)pheny phosphine oxide (BMI)/polysulfone modified epoxy intercrosslinked matrices. Polym. Compos. 2008, 29, 773–781. [Google Scholar] [CrossRef]
- Li, Y.Q.; Gao, J.; Li, X.Y.; Xu, X.; Lu, S.R. High mechanical and thermal properties of epoxy composites with liquid crystalline polyurethane modified graphene. Polymers 2018, 10, 485. [Google Scholar] [CrossRef]
- Mimura, K.; Ito, H.; Fujioka, H. Improvement of thermal and mechanical properties by control of morphologies in PES-modified epoxy resins. Polymer 2000, 41, 4451–4459. [Google Scholar] [CrossRef]
- Jiang, M.Q.; Liu, Y.; Cheng, C.; Zhou, J.L.; Liu, B.H.; Yu, M.H.; Zhang, H. Enhanced mechanical and thermal properties of monocomponent high performance epoxy resin by blending with hydroxyl terminated polyethersulfone. Polym. Test. 2018, 69, 302–309. [Google Scholar] [CrossRef]
- Rajasekaran, R.; Alagar, M. Mechanical properties of bismaleimides modified polysulfone epoxy matrices. Int. J. Polym. Mater. 2007, 56, 911–927. [Google Scholar] [CrossRef]
- Doyle, C.D. Estimating thermal stability of experimental polymers by empirical thermogravimetric analysis. Anal. Chem. 1961, 33, 77–79. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, H.C. Thermal stability and toughening of epoxy resin with polysulfone resin. J. Polym. Sci. Pol. Phys. 2001, 39, 121–128. [Google Scholar] [CrossRef]











| Sample | 0 phr | 5 phr | 10 phr | 15 phr | 20 phr |
|---|---|---|---|---|---|
| ΔE (kJ/mol) | 58.969 | 57.009 | 57.997 | 54.730 | 55.043 |
| R12 | 0.995 | 0.998 | 0.998 | 0.998 | 0.999 |
| n | 0.871 | 0.878 | 0.88 | 0.874 | 0.875 |
| R22 | 0.996 | 0.999 | 0.999 | 0.998 | 0.999 |
| PSF Content | IDT (°C) | IPDT (°C) | A*K* | Tmax (°C) |
|---|---|---|---|---|
| 0 phr | 386.2 | 364.6 | 0.370 | 412.2 |
| 5 phr | 389.2 | 369.3 | 0.376 | 412.5 |
| 10 phr | 392.3 | 373.5 | 0.381 | 414.2 |
| 15 phr | 394.1 | 378.1 | 0.386 | 416.2 |
| 20 phr | 395.9 | 379.4 | 0.388 | 418.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Xu, L.; Chen, Z.; Wang, Y.; Tusiime, R.; Cheng, C.; Zhou, S.; Liu, Y.; Yu, M.; Zhang, H. Enhancing the Mechanical and Thermal Properties of Epoxy Resin via Blending with Thermoplastic Polysulfone. Polymers 2019, 11, 461. https://doi.org/10.3390/polym11030461
Sun Z, Xu L, Chen Z, Wang Y, Tusiime R, Cheng C, Zhou S, Liu Y, Yu M, Zhang H. Enhancing the Mechanical and Thermal Properties of Epoxy Resin via Blending with Thermoplastic Polysulfone. Polymers. 2019; 11(3):461. https://doi.org/10.3390/polym11030461
Chicago/Turabian StyleSun, Zeyu, Lei Xu, Zhengguo Chen, Yuhao Wang, Rogers Tusiime, Chao Cheng, Shuai Zhou, Yong Liu, Muhuo Yu, and Hui Zhang. 2019. "Enhancing the Mechanical and Thermal Properties of Epoxy Resin via Blending with Thermoplastic Polysulfone" Polymers 11, no. 3: 461. https://doi.org/10.3390/polym11030461
APA StyleSun, Z., Xu, L., Chen, Z., Wang, Y., Tusiime, R., Cheng, C., Zhou, S., Liu, Y., Yu, M., & Zhang, H. (2019). Enhancing the Mechanical and Thermal Properties of Epoxy Resin via Blending with Thermoplastic Polysulfone. Polymers, 11(3), 461. https://doi.org/10.3390/polym11030461







