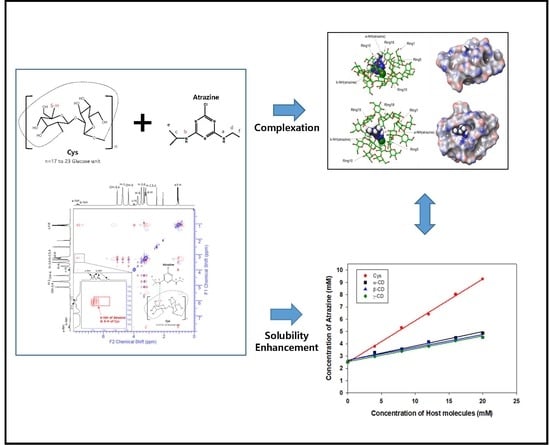
Solubility Enhancement of Atrazine by Complexation with Cyclosophoraose Isolated from Rhizobium leguminosarum biovar trifolii TA-1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cyclosophoraoses (Cys)
2.3. 1H Nuclear Magnetic Resonance (NMR) Spectroscopy
2.4. Phase Solubility Analysis
2.5. Job’s Plot Analysis
2.6. Fourier Transform Infrared (FT-IR) Spectroscopy
2.7. Differential Scanning Calorimetry (DSC)
2.8. X-ray Diffraction Analysis (XRD)
2.9. Field Emission Scanning Electron Microscopy (FE-SEM)
2.10. Rotating Frame Nuclear Overhauser Spectroscopy (ROESY)
2.11. Molecular Modeling
3. Results
3.1. Characterization of Atrazine and Cys
3.2. Phase Solubility Tests
3.3. Fourier Transform Infrared (FT-IR) Spectroscopic Analysis
3.4. Differential Scanning Calorimetry (DSC) Analysis
3.5. X-ray Diffraction (XRD) Analysis
3.6. Field Emission Scanning Electron Microscopy (FE-SEM) Analysis
3.7. ROESY Spectroscopy of Atrazine/Cys Inclusion Complexes
3.8. Molecular Modeling of Atrazine/Cys Inclusion Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abe, M.; Amemura, A.; Higashi, S. Studies on cyclic beta-l,2-glucan obtained from periplasmic space of Rhizobium trifolii cells. Plant Soil 1982, 64, 315–324. [Google Scholar] [CrossRef]
- Amemura, A.; Hisamatsu, M.; Mitani, H.; Harada, T. Cyclic (1,2)-b-D-glucan and the octasaccharide repeating-units of extracellular acidic polysaccharides produced by Rhizobium. Carbohydr. Res. 1983, 114, 277–285. [Google Scholar] [CrossRef]
- Miller, K.J.; Kennedy, E.P.; Reinhold, V.N. Osmotic adaptation by gram-negative bacteria: Possible role for periplasmic oligosaccharides. Science 1986, 231, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Zorreguieta, A.; Cavaignac, S.; Geremia, R.A.; Ugalde, R.A. Osmotic regulation of beta(1–2) glucan synthesis in members of the family Rhizobiaceae. J. Bacteriol. 1990, 172, 4701–4704. [Google Scholar] [CrossRef] [PubMed]
- Leighand, J.L.; Coplin, D.L. Exopolysaccharides in plant-bacterial interactions. Annu. Rev. Microbiol. 1992, 46, 307–346. [Google Scholar]
- André, I.; Mazeau, K.; Taravel, F.R.; Tvaroska, I. Conformation and dynamicsof a cyclic (1→2)-β-d-glucan. Int. J. Biol. Macromol. 1995, 17, 189–198. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, C.; Choi, J.; Seo, D.; Kim, H.W.; Jung, S. Inclusion complexationof a family of cyclosophoraoses with indomethacin. J. Microbiol. 2001, 11, 463–468. [Google Scholar]
- Lee, S.; Seo, D.H.; Kim, H.W.; Jung, S. Investigation of inclusion complexation of paclitaxel by cyclohenicosakis-(1→2)-(β-D-glucopyranosyl), by cyclic-(1→2)-β-D-glucans (cyclosophoraoses), and by cyclomaltoheptaoses (β-cyclodextrins). Carbohydr. Res. 2001, 334, 119–126. [Google Scholar] [CrossRef]
- Lee, S.; Seo, D.; Park, H.; Choi, Y.; Jung, S. Solubility enhancement of ahydrophobic flavonoid, luteolin by the complexation with cyclosophoraosesisolated from Rhizobium meliloti. Antonie Van Leeuwenhoek 2003, 84, 201–207. [Google Scholar] [CrossRef]
- Kwon, Y.; Cho, E.; Lee, I.S.; Jung, S. Synthesis and characterization of butyrylcyclosophoraose, and its inclusion complexation behavior for some flavonoids. Bull. Korean Chem. Soc. 2011, 32, 2779–2782. [Google Scholar] [CrossRef]
- Lee, S.; Park, H.; Seo, D.; Choi, Y.; Jung, S. Synthesis and characterization ofcarboxymethylated cyclosophoraose, and its inclusion complexation behavior. Carbohydr. Res. 2004, 339, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Masson, M. Cyclodextrins in topical drug formulations: Theory and practice. Int. J. Pharm. 2001, 225, 15–30. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Liu, B.; Zhao, J.; Thomas, D.S.; Hook, J.M. An investigation into the supramolecular structure, solubility, stability and antioxidant activity of rutin/cyclodextrin inclusion complex. Food Chem. 2013, 136, 186–192. [Google Scholar] [CrossRef]
- Sambasevam, K.P.; Mohamad, S.; Sarih, N.M.; Ismail, N.A. Synthesis and characterization of the inclusion complex of β-cyclodextrin and azomethine. Int. J. Mol. Sci. 2013, 14, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Dandawate, P.R.; Vyas, A.; Ahmad, A.; Banerjee, S.; Deshpande, J.; Swamy, K.V.; Jamadar, A.; Dumhe-Klaire, A.C.; Padhye, S.; Sarkar, F.H. Inclusion complex of novel curcumin analogue cdf and β-cyclodextrin (1:2) and its enhanced in vivo anticancer activity against pancreatic cancer. Pharm. Res. 2012, 29, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Guo, G.; Ding, K.; Wang, L.; Liu, T.; Yang, F. Effect of bioaugmentation by bacterial consortium and methyl-β-cyclodextrin on soil functional diversity and removal of polycyclic aromatic hydrocarbons. Polycycl. Aromat. Compd. 2017, 1–10. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Yang, W.L.; He, H.J.; Yang, C.P.; Yu, J.P.; Wu, X.; Zeng, G.M.; Tarre, S.; Green, M. Preparation, performance and mechanisms of magnetic Saccharomyces cerevisiae bionanocomposites for atrazine removal. Chemosphere 2018, 200, 380–387. [Google Scholar] [CrossRef]
- Wu, S.H.; He, H.J.; Li, X.; Yang, C.P.; Zeng, G.M.; Wu, B.; He, S.Y.; Lu, L. Insights into atrazine degradation by persulfate activation using composite of nanoscale zero-valent iron and graphene: Performances and mechanisms. Chem. Eng. J. 2018, 341, 126–136. [Google Scholar] [CrossRef]
- Liu, N.; Charrua, A.B.; Weng, C.H.; Yuan, X.L.; Ding, F. Characterization of biochars derived from agriculture wastes and their adsorptive removal of atrazine from aqueous solution: A comparative study. Bioresour. Technol. 2015, 198, 55–62. [Google Scholar] [CrossRef]
- Wu, S.H.; He, H.J.; Inthapanya, X.; Yang, C.P.; Lu, L.; Zeng, G.M.; Han, Z.F. Role of biochar on composting of organic wastes and remediation of contaminated soils—A review. Environ. Sci. Pollut. Res. 2017, 24, 16560–16577. [Google Scholar] [CrossRef]
- Byer, J.D.; Struger, J.; Sverko, E.; Klawunn, P.; Todd, A. Spatial and seasonal variations in atrazine and metolachlor surface water concentrations in Ontario (Canada) using ELISA. Chemosphere 2010, 82, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Markovic, M.; Cupac, S.; Durovic, R.; Milinovic, J.; Kljajic, P. Assessment of heavy metal and pesticide levels in soil and plant products from agricultural area of Belgrade, Serbia. Arch Environ. Contam Toxicol. 2010, 58, 341–351. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission decision of 10 March 2004 concerning the non-inclusion of atrazine in Annex I to Council Directive 91/414/EEC and the withdrawal of authorisations for plant protection products containing this active substance, 2004/248/EC. OJEU 2004, 78, 53–55. [Google Scholar]
- Nodler, K.; Licha, T.; Voutsa, D. Twenty years later-Atrazine concentrations in selected coastal waters of the Mediterranean and the Baltic Sea. Mar. Pollut. Bull. 2013, 70, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Nawab, A.; Aleem, A.; Malik, A. Determination of organochlorine pesticides in agricultural soil with special reference to γ-HCH degradation by Pseudomonas strains. Bioresour. Technol. 2003, 88, 41–46. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Farombi, E.O. Atrazine induces apoptosis of SHSY5Y human neuroblastoma cells via the regulation of Bax/Bcl2 ratio and caspase-3-dependent pathway. Pestic. Biochem. Physiol. 2015, 118, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Fakhouri, W.D.; Nuñez, J.L.; Trail, F. Atrazine binds to the growth hormone-releasing hormone receptor and affects growth hormone gene expression. Environ. Health Perspect. 2010, 118, 1400–1405. [Google Scholar] [CrossRef]
- Cavas, T. In vivo genotoxicity evaluation of atrazine and atrazine-based herbicide on fish Carassius auratus using the micronucleus test and the comet assay. Food Chem. Toxicol. 2011, 49, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.O.; Pant, A.B.; Farombi, E.O. The protective effects of quercetin on the cytotoxicity of atrazine on rat Sertoli-germ cell co-culture. Int. J. Androl. 2012, 35, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Rajkovic, V.; Kovac, R.; Koledin, I.; Matavulj, M. Atrazineinduced changes in the myocardial structure of peripubertal rats. Toxicol. Ind. Health 2014, 30, 250–258. [Google Scholar] [CrossRef]
- Wild, S.R.; Obbard, J.P.; Munn, C.I.; Berrow, M.L.; Jones, K.C. The long-term persistence of polynuclear aromatic hydrocarbons (PAHs) in an agricultural soil amended with metal-contaminated sewage sludges. Sci. Total Environ. 1991, 101, 235–253. [Google Scholar] [CrossRef]
- Boving, T.B.; Brusseau, M.L. Solubilization and removal of residual trichloroethene from porous media: Comparison of several solubilization agents. J. Contam. Hydrol. 2000, 42, 51–67. [Google Scholar] [CrossRef]
- Pennell, K.D.; Abriola, L.M.; Weber, W.J., Jr. Surfactant-enhanced solubilization of residual dodecane in soil columns. 1. Experimental investigation. Environ. Sci. Technol. 1993, 27, 2332–2340. [Google Scholar] [CrossRef]
- Boving, T.B.; McCray, J.E. Cyclodextrin-enhanced remediation of organic and metal contaminants in porous media and groundwater. Remediat. J. 2000, 10, 59–83. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Pospíšil, L.; Trsková, R.M.; Colombini, M.P.; Fuoco, R. Inclusion complexes of atrazine with α-, β-and γ-cyclodextrins. Evidence by polarographic kinetic currents. J. Incl. Phenom. Mol. Recognit. Chem. 1998, 31, 57–70. [Google Scholar] [CrossRef]
- Venceslau, A.D.; dos Santos, F.E.; de Fátima Silva, A.; Rocha, D.A.; de Abreu, A.J.; Jaime, C.; Andrade-Vieira, L.F.; Pinto, L.D. Cyclodextrins as effective tools to reduce the toxicity of atrazine. Energy Ecol. Environ. 2018, 3, 81–86. [Google Scholar] [CrossRef]
- de Carvalho, L.B.; Pinto, L.D.M.A. Formation of inclusion complexes and controlled release of atrazine using free or silica-anchored β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2012, 74, 375–381. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Crini, G. Environmental applications of water-insoluble β-cyclodextrin–epichlorohydrin polymers. Prog. Polym. Sci. 2013, 38, 344–368. [Google Scholar] [CrossRef]
- Alsbaiee, A.; Smith, B.J.; Xiao, L.; Ling, Y.; Helbling, D.E.; Dichtel, W.R. Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature 2016, 529, 190. [Google Scholar] [CrossRef] [PubMed]
- Breedveld, M.W.; Zevenhuizen, L.P.; Zehnder, A.J. Excessive excretion of cyclic b-(1,2)-glucan by Rhizobium trifolii TA-1. Appl. Environ. Microbiol. 1990, 56, 2080–2086. [Google Scholar]
- Kwon, C.; Choi, Y.; Jeong, D.; Kim, J.G.; Choi, J.M.; Chun, S.; Park, S.; Jung, S. Inclusion complexation of naproxen with cyclosophoraoses and succinylated cyclosophoraoses in different pH environments. J. Incl. Phenom. 2012, 74, 325–333. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase-solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Job, P. Job’s method of continuous variation. Ann. Chim. 1928, 9, 113–203. [Google Scholar]
- Jeong, D.; Choi, J.M.; Choi, Y.; Jeong, K.; Cho, E.; Jung, S. Complexation of fisetin with novel cyclosophoroase dimer to improve solubility and bioavailability. Carbohydr. Polym. 2013, 97, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Dindulkar, S.D.; Jeong, D.; Cho, E.; Kim, D.; Jung, S. Microbial cyclosophoraose as a catalyst for the synthesis of diversified indolyl 4 H-chromenes via one-pot three component reactions in water. Green Chem. 2016, 18, 3620–3627. [Google Scholar] [CrossRef]
- Welhouse, G.J.; Bleam, W.F. NMR spectroscopic investigation of hydrogen bonding in atrazine. Environ. Sci. Technol. 1992, 26, 959–964. [Google Scholar] [CrossRef]
- Park, K.H.; Choi, J.M.; Cho, E.; Jeong, D.; Shinde, V.V.; Kim, H.; Choi, Y.; Jung, S. Enhancement of Solubility and Bioavailability of Quercetin by Inclusion Complexation with the Cavity of Mono-6-deoxy-6-aminoethylamino-β-cyclodextrin. Bull. Korean Chem. Soc. 2017, 38, 880–889. [Google Scholar] [CrossRef]
- Novoa, G.A.; Heinämäki, J.; Mirza, S.; Antikainen, O.; Colarte, A.I.; Paz, A.S.; Yliruusi, J. Physical solid-state properties and dissolution of sustained-release matrices of polyvinylacetate. Eur. J. Pharm. Biopharm. 2005, 59, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Bertacche, V.; Lorenzi, N.; Nava, D.; Pini, E.; Sinico, C. Host–guest interaction study of resveratrol with natural and modified cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2006, 55, 279–287. [Google Scholar] [CrossRef]
- Nalluri, B.N.; Chowdary, K.P.; Murthy, K.V.; Hayman, A.R.; Becket, G. Physicochemical characterization and dissolution properties of nimesulide-cyclodextrin binary systems. AAPs PharmSciTech 2003, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Forgo, P.; D’Souza, V.T. The application of selective ROE experiments to study solution structures of cyclomaltooligosacharide derivatives and complexes. Carbohydr. Res. 1998, 306, 473–478. [Google Scholar] [PubMed]









| Assignment | Atrazine (cm−1) | Cys (cm−1) | Atrazine/Cys Physical Mixture (cm−1) | Atrazine/Cys Inclusion Complex (cm−1) |
|---|---|---|---|---|
| N–H | 3252 | 3251 | ||
| C–H | 2971 | 2885 | 2843 | 2888 |
| Triazine group | 1541 | 1545 | ||
| Semicircle ring | 1401 | 1412 | ||
| C–N | 1167 | 1176 | ||
| Aromatic chlorine | 1056 | 1058 | ||
| Triazine ring sextant | 804 | 800 | ||
| C–H stretching of aromatic rings | 730 | 729 | ||
| O–H stretching, bending | 3200–3600, 1618 | 3200–3700, 1626 | ||
| C–O | 1056 | 1068 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Shinde, V.V.; Jeong, D.; Choi, Y.; Jung, S. Solubility Enhancement of Atrazine by Complexation with Cyclosophoraose Isolated from Rhizobium leguminosarum biovar trifolii TA-1. Polymers 2019, 11, 474. https://doi.org/10.3390/polym11030474
Kim Y, Shinde VV, Jeong D, Choi Y, Jung S. Solubility Enhancement of Atrazine by Complexation with Cyclosophoraose Isolated from Rhizobium leguminosarum biovar trifolii TA-1. Polymers. 2019; 11(3):474. https://doi.org/10.3390/polym11030474
Chicago/Turabian StyleKim, Yohan, Vijay Vilas Shinde, Daham Jeong, Youngjin Choi, and Seunho Jung. 2019. "Solubility Enhancement of Atrazine by Complexation with Cyclosophoraose Isolated from Rhizobium leguminosarum biovar trifolii TA-1" Polymers 11, no. 3: 474. https://doi.org/10.3390/polym11030474
APA StyleKim, Y., Shinde, V. V., Jeong, D., Choi, Y., & Jung, S. (2019). Solubility Enhancement of Atrazine by Complexation with Cyclosophoraose Isolated from Rhizobium leguminosarum biovar trifolii TA-1. Polymers, 11(3), 474. https://doi.org/10.3390/polym11030474